Abstract
Recombinant adeno-associated virus (rAAV) vectors are rapidly becoming the first choice for human gene therapy studies, as clinical efficacy has been demonstrated in several human trials and proof-of-concept data have been demonstrated for correction of many others. When moving into human use under the auspices of an FDA Investigational New Drug (IND) application, it is necessary to demonstrate the stability of vector material under various conditions of storage, dilution, and administration when used in humans. Limited data are currently available in the literature regarding vector compatibility and stability, leading most IND sponsors to repeat all necessary studies. The current study addresses this issue with an rAAV vector (rAAV1-CB-chAATmyc) containing AAV2-inverted terminal repeat sequences packaged into an AAV1 capsid. Aliquots of vector were exposed to a variety of temperatures, diluents, container constituents, and other environmental conditions, and its functional biological activity (after these various treatments) was assessed by measuring transgene expression after intramuscular injection in mice. rAAV was found to be remarkably stable at temperatures ranging from 4°C to 55°C (with only partial loss of potency after 20 min at 70°C), at pH ranging from 5.5 to 8.5, after contact with mouse or human serum (with or without complement depletion) or with gadolinium and after contact with glass, polystyrene, polyethylene, polypropylene, and stainless steel. The only exposure resulting in near-total loss of vector activity (10,000-fold loss) was UV exposure for 10 min. The stability of rAAV1 preparations bodes well for future dissemination of this therapeutic modality.
Introduction
The remarkable success of recombinant adeno-associated virus (rAAV) vectors in recent clinical studies of conditions such as Leber congenital amaurosis1–5 and hemophilia6,7 has resulted in a dramatic increase in the interest in clinical translation of rAAV-based gene therapy. This will most likely lead to an increasing number of FDA Investigational New Drug (FDA-IND) applications for investigational human use of rAAV. Upon submission of an FDA-IND, the sponsor must provide data with the Chemistry, Manufacturing, and Control section of the IND to demonstrate the stability of the vector under the conditions of storage and its compatibility with containers, tubes, and delivery devices to be used in the production, storage, transport, and clinical administration of the vector. See Fig. 1 for a typical IND workflow. These data are also used to provide instructions for clinical investigators in the investigator's brochure about appropriate handling of the vector. Furthermore, the investigator's brochure forms the template for important sections of the product package insert (or “label”) ultimately used to guide clinicians in the approved use and administration of the biological agent. Since very limited stability and compatibility data are currently available in the literature, each IND sponsor may need to generate much of the needed data de novo for each IND.
FIG. 1.
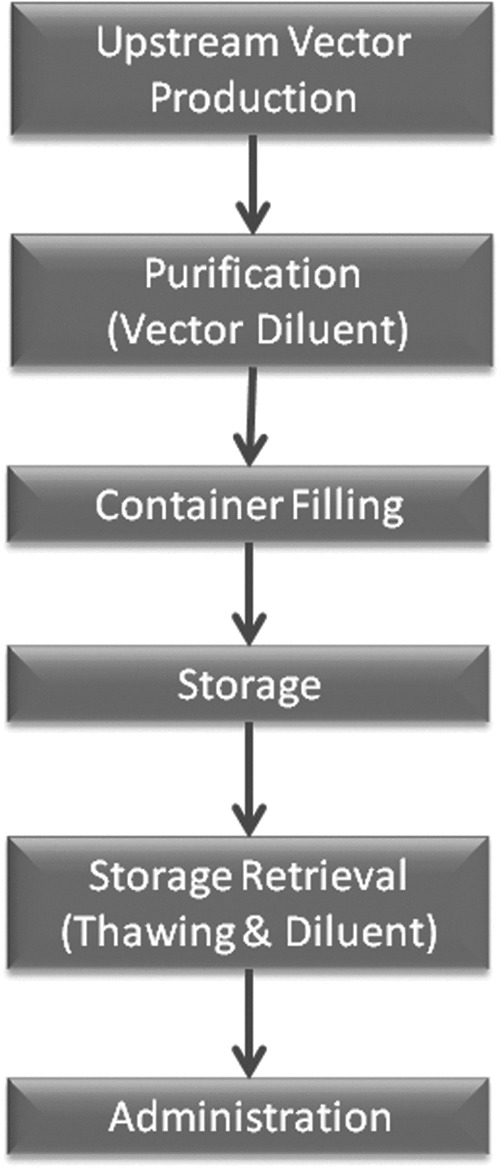
Workflow for vector production through to vector delivery. Each point is a source of potential loss and may need to be evaluated during preparation of an FDA investigational new drug.
In the current study, we sought to more systematically investigate the effects on rAAV of varying temperatures (including stable temperature storage and freeze–thaw cycles), diluents (including serum and solutes with varying pH), container constituents (plastics, glass, and steel), and other environmental exposures. We utilized in vivo biological efficacy of the vector for production of a readily measurable transgene product (alpha-1 antitrypsin, AAT) that appears in the serum of mice after intramuscular injection of the vector. These data provide strong evidence of the preservation of functional vector under a wide range of such conditions.
Materials and Methods
Vector
The recombinant AAV1 vectors encoding the chimpanzee alpha-1 anti-trypsin gene (ChimpAAT) with a c-myc tag used for this study were packaged, purified, and titered by the University of Massachusetts Medical School Vector Core by a previously published protocol.8 Vector particle concentration was determined using silver staining. After production, all vector preparations were stored at −80°C until use, unless otherwise noted. The chimpAAT gene was under the control of a chicken beta-actin promoter with a cytomegalo virus enhancer element and had an SV-40 polyadenylation sequence. The total size of the genome (including inverted terminal repeats) was 3705 base pairs (∼79% of the wild-type AAV genome).
Mouse work
Mice were housed under specific pathogen-free conditions and all experiments were approved by the University of Massachusetts Institutional Animal Care and Use Committee. Because the reporter transgene used in this study (chimpAAT) produces a protein foreign to the murine immune system, all studies in this article were done in B6.129S7-Rag1tm1Mom/J (002216; The Jackson Laboratory). These mice do not produce mature B or T cells, stopping the immune response to the chimpAAT protein. Vector was delivered by intramuscular injection, 1×1011 viral particles/mouse, in a volume of 50 μl to the right hind limb as described previously.9 The vector was loaded into the syringe just before injection. The same investigator performed all injections. The dose was determined in a pilot where mice were administered the chimpAAT vector at doses from 5×1010 viral particles/mouse to 1×1012 viral particles/mouse. An amount of 1×1011 viral particles/mouse was chosen because it resulted in serum levels of chimpAAT that were easily measurable but below the level of saturation. For injection, 14 μl of vector was mixed with 36 μl of either phosphate buffered saline (PBS) or the diluent being tested. All groups contain five mice.
AAT ELISA
An AAT ELISA was performed as previously described.10 We determined that the humanAAT antibody fully detects the chimpAAT protein by comparing humanAAT ELISA results to c-myc ELISA results (data not shown).
Quantitative real-time PCR
Vector genomes were determined by TaqMan quantitative PCR as described previously.10 Briefly, primers and probes designed to target the SV40 polyadenylation region of the construct were used. Data were analyzed using Eppendorf Mastercycler EP Realplex 2.2 Software, and vector genome copy number was determined using a standard curve and normalized to genome copy number per 200 ng of genomic DNA.
Statistics
Each perturbation to the vector was compared with the “Thawed Just Prior” group using a two-way ANOVA. Vector copy numbers were analyzed using an unpaired t-test. p-Values <0.05 were considered significant.
Results
Vector storage and temperature stability
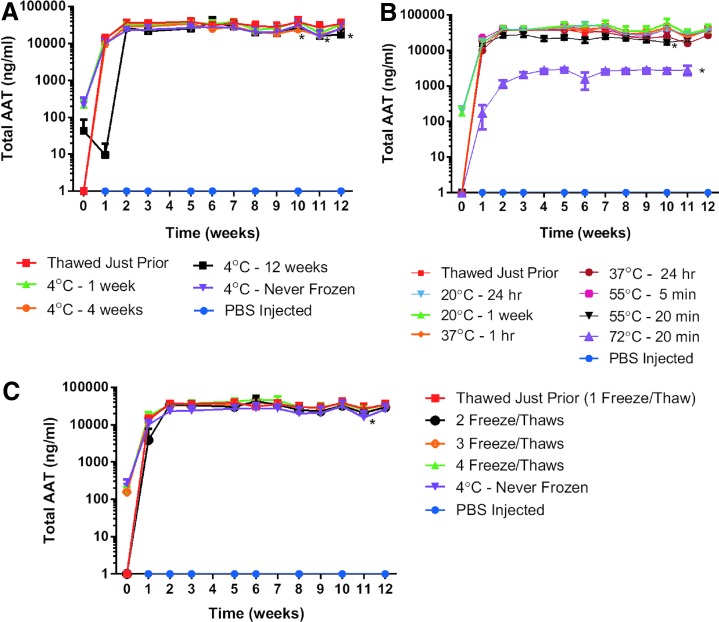
We compared vector stored at −80°C to vector kept for 1 week, 4 weeks, 12 weeks, and >1 year (never frozen) at 4°C (Fig. 2A). The storage of vector at 4°C for 4 weeks (p=0.005), 12 weeks (p=0.0071), and >1 year (p=0.0153) resulted in a statistically significant decrease in chimpAAT serum levels, although the absolute serum AAT concentrations were still relatively similar to the vector kept at −80°C. The vector was then exposed to higher temperatures ranging from room temperature (20°C) for 24 hr to 72°C for 20 min (Fig. 2B). The only temperatures that resulted in a statistically significant drop in serum chimpAAT levels were 55°C for 20 min (p=0.0001) and 72°C for 20 min (p<0.0001) when compared with vector thawed just before administration (Fig. 2B). However, the serum levels for the 55°C for 20 min group are still in the same range as the control group. When the serum chimpAAT levels were analyzed with vector injected after 2–4 freeze–thaw cycles, there was no significant difference from the vector thawed just prior (1 freeze–thaw cycle) (Fig. 2C).
FIG. 2.
Vector storage and temperature stability. Serum AAT ELISA results. (A) rAAV1 vector storage temperature. Statistically significant decreases in serum AAT levels were measured for the 4°C for 4 weeks (p=0.005), 12 weeks (p=0.0071), and >1 year (Never Frozen) (p=0.0153) groups. (B) Temperature stability of rAAV1 vector. Statistically significant decreases in serum AAT levels were measured for the 55°C for 20 min (p=0.0001) and 72°C for 20 min (p<0.0001) groups. (C) Freeze–thaw cycle stability of rAAV1 vector. No significant difference was seen except in the Never Frozen group (n=5). rAAV, recombinant adeno-associated virus.
Vector diluent and pH
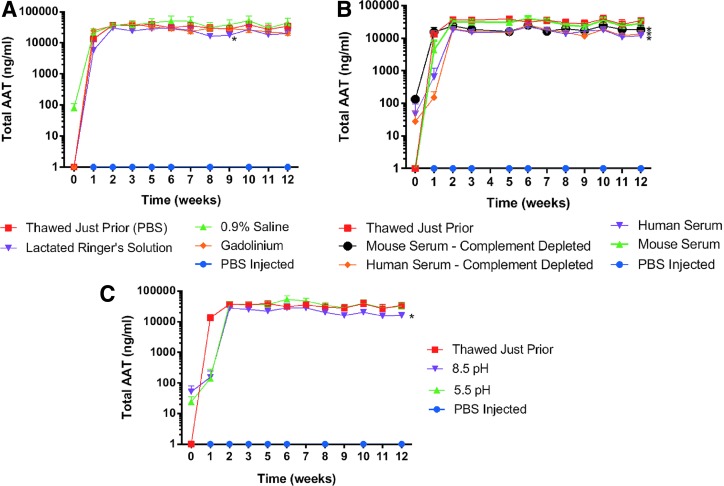
When we compared diluents that could be used for both vector administration or storage, we found that there was a significantly lower serum chimpAAT level with Lactated Ringer's Solution (p=0.0023) compared with phosphate buffered saline; however, the serum levels even in that case were still comparable (Fig. 3A). No significant difference was seen with 0.9% saline or Gadolinium (2 mM concentration) groups (Fig. 3A). We also investigated the potential effect that serum (either complete or C3 depleted) has on gene expression. The complete mouse (C57Bl/6J) and human serum were not screened for AAV1-neutralizing antibodies. Complement component C3-depleted human serum was obtained commercially, and C3-depleted mouse serum was purchased from The Jackson Laboratory (003641 mouse strain). The serum replaced the PBS diluents in these experiments. We saw a minor but significant decrease in chimpAAT serum levels in the human serum (p<0.0001) diluted as well as both the mouse (p<0.0001) and human (p<0.0001) C3-depleted serum groups (Fig. 3B). When the expression levels following pH changes to the diluents were investigated, we found a small but significant drop in chimpAAT levels with dilution in a saline solution with a pH of 8.5 (p<0.0001) but not a pH of 5.5 (p=0.5493) (Fig. 3C).
FIG. 3.
Vector diluent and pH. Serum AAT ELISA results. (A) rAAV1 diluent affect. Lactated Ringer's solution (p=0.0023) was statistically lower than the phosphate buffered saline (PBS) control. (B) rAAV1 serum dilution (with or without complement component C3). Human serum (p<0.0001). Mouse (p<0.0001) and human (p<0.0001) C3-depleted serum groups. (C) rAAV1 effect of diluent pH. Significant only at pH of 8.5 (p<0.0001) (n=5).
Vector materials contact
We looked at chimpAAT protein levels following exposure of the undiluted vector to materials that may be encountered during a clinical trial (polypropylene, polystyrene, polyethylene, a syringe [polyethylene with rubber stopper], stainless steel, and glass) (Fig. 4). The vector was in contact with each material for 15 min except the syringe, where the contact time was increased to 30 min. There was a small but significant decrease in the serum chimpAAT levels for the vector that contacted stainless steel (p=0.0003), glass (p<0.0001), and polyethylene (p=0.0001) when compared with the polypropylene (microcentrifuge tube) control group (Fig. 4).
FIG. 4.
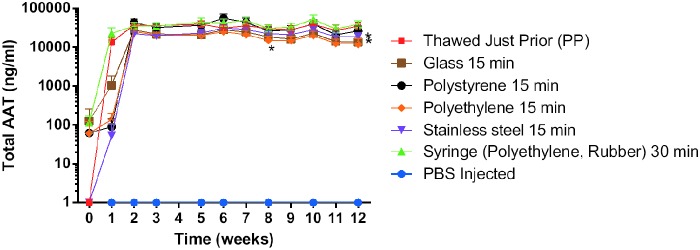
Vector materials contact. Serum AAT ELISA results. Significant decrease in the serum levels that contacted stainless steel (p=0.0003), glass (p<0.0001), and polyethylene (p=0.0001) when compared with the polypropylene (PP) control group (n=5).
Vector extreme conditions comparison
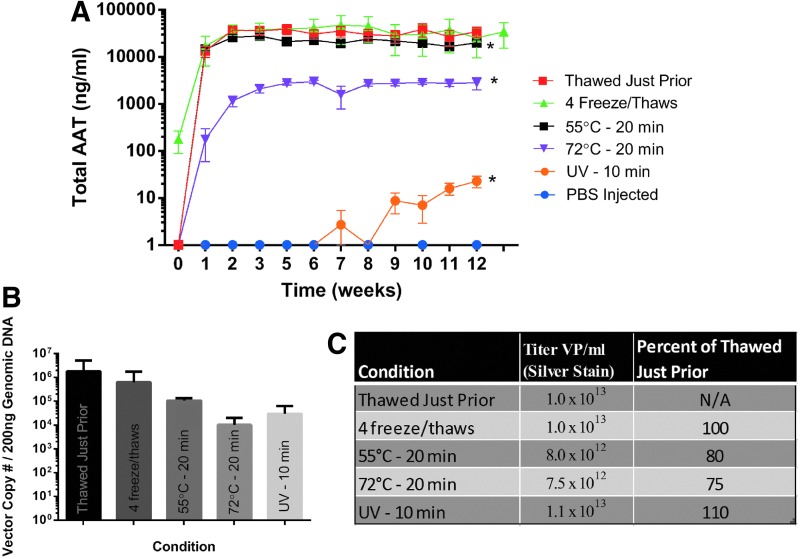
In Fig. 5A we compared the chimpAAT serum levels between some of the harshest conditions discussed in this article and added a group exposed to UV for 10 min. As mentioned before 55°C for 20 min (p=0.0001) and 72°C for 20 min (p<0.0001) did result in a significant drop in serum chimpAAT levels. We saw the most dramatic drop with UV exposure, however (p<0.0001). We compared the vector copy number in injected muscle for this group of mice, and while there was a decrease in copy number for the perturbed groups, particularly the 72°C and UV group, the difference did not reach statistical significance (Fig. 5B). When vector particle titers were compared before (thawed just prior group) and after perturbation, we did see a drop in particle concentration in the 55°C and 72°C groups, a result consistent with previously reported work (Fig. 5C).11,12
FIG. 5.
Vector extreme conditions comparison. (A) Serum AAT ELISA results: 55°C for 20 min (p=0.0001), 72°C for 20 min (p<0.0001), and UV 10 min (p<0.0001) (n=5). (B) Quantitative real-time PCR results for vector genome copy number in injected muscle. (C) Vector particle (VP) titer by silver stain for groups displayed in (A).
Discussion
The studies presented here demonstrate the retention of potency of an rAAV1 vector at temperatures ranging from 4°C to 55°C, at pH ranging from 5.5 to 8.5, and after contact with various diluents and container materials. Demonstrable loss of vector activity was observed under only two conditions, heating to 72°C and UV exposure for 10 min, all other statistically significant differences resulted in only a relatively small reduction in serum AAT levels. Some residual vector biological activity was still noted even under these extreme exposures (∼10- and 1000-fold loss, respectively). The packaged construct used in this study is approximately 79% of the size of the wild-type AAV genome. This size construct has been shown to have relative thermal stability, with only a modest loss of vector particles following exposure to temperatures around 55°C, something this study reinforced.11,12 It was somewhat remarkable that the human serum exposure as performed here did not result in neutralization from neutralizing antibodies. AAV1 seropositivity is estimated to be over 70% in the general population, but neutralization was not observed under the conditions we studied.13 Based on these results, any of the tested diluents and container constituents and storage conditions could be employed for future clinical trials.
The stability of both wild-type and recombinant AAV has been commented on in a number of previous studies. Reports describing current Good Manufacturing Practice (cGMP) approaches to rAAV production14,15 have provided limited, but very encouraging, data on stability of stored cGMP rAAV. Studies indicating instability of AAV particles generally have resorted to combinations of factors, such as high temperature with extremes of divalent cation concentration11 or high temperature with nonstandard vector genome length.12
Very little data had previously been published with regard to the compatibility of rAAV with plastics or other substances that may be found in containers, tubing, or delivery devices commonly used to inject therapeutic agents in humans or with the diluents in common clinical use. While the data presented here do not cover all possible combinations of materials and diluents, stainless steel is the most common constituent for needles in clinical use, polyethylene is commonly used in IV tubing and syringes, and other plastics and glass may be encountered in storage vials. rAAV was found to be compatible with each of these. The range of pH from 5.5 to 8.5 covers all commonly used clinical IV fluids (0.9% saline, unbuffered with lactate, is typically at pH near 5.5). The data also support medium- to long-term storage under common refrigeration (4°C) conditions and allow for heating such as may occur under a variety of transport conditions from a cold storage location to a clinic or operating room.
Taken together, the data presented here argue strongly that rAAV is a stable biological material under most conditions that are likely to be encountered clinically. This could make the preparation of INDs more straightforward and facilitate the completion of clinical trials without as much concern about creating special storage and handling conditions. The stability of the agent may well also be important in future dissemination of the therapy once it is approved for broader clinical use. This could remove a potential obstacle to widespread adoption of the therapy as human gene therapy becomes available to clinicians.
Acknowledgment
We wish to thank the UMass Medical Vector Core for all their work preparing the vector used in this study. This work was supported by grants from the NIH (R01 HL069877 and R01 DK098252).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cideciyan AV, Aleman TS, Boye SL, et al. . Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cideciyan AV, Aguirre GK, Jacobson SG, et al. . Pseudo-fovea formation after gene therapy for RPE65-LCA. Invest Ophthalmol Vis Sci 2015;56:526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bainbridge JWB, Smith AJ, Barker SS, et al. . Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 4.Hauswirth WW, Aleman TS, Kaushal S, et al. . Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire AM, Simonelli F, Pierce EA, et al. . Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathwani AC, Tuddenham EGD, Rangarajan S, et al. . Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathwani AC, Reiss UM, Tuddenham EGD, et al. . Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller C, Ratner D, Zhong L, et al. . Production and discovery of novel recombinant adeno-associated viral vectors. Curr Protoc Microbiol 2012;Chapter 14:Unit 14D.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruntman AM, Bish LT, Mueller C, et al. . Gene transfer in skeletal and cardiac muscle using recombinant adeno-associated virus. Curr Protoc Microbiol 2013;Chapter 14:Unit 14D.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller C, Tang Q, Gruntman A, et al. . Sustained miRNA-mediated knockdown of mutant AAT with simultaneous augmentation of wild-type AAT has minimal effect on global liver miRNA profiles. Mol Ther 2012;20:590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbull AE, Skulimowski A, Smythe JA, et al. . Adeno-associated virus vectors show variable dependence on divalent cations for thermostability: implications for purification and handling. Hum Gene Ther 2000;11:629–635 [DOI] [PubMed] [Google Scholar]
- 12.Horowitz ED, Rahman KS, Bower BD, et al. . Biophysical and ultrastructural characterization of adeno-associated virus capsid uncoating and genome release. J Virol 2013;87:2994–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder RO, Flotte TR. Production of clinical-grade recombinant adeno-associated virus vectors. Curr Opin Biotechnol 2002;13:418–423 [DOI] [PubMed] [Google Scholar]
- 15.Allay JA, Sleep S, Long S, et al. . Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther 2011;22:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]