Abstract
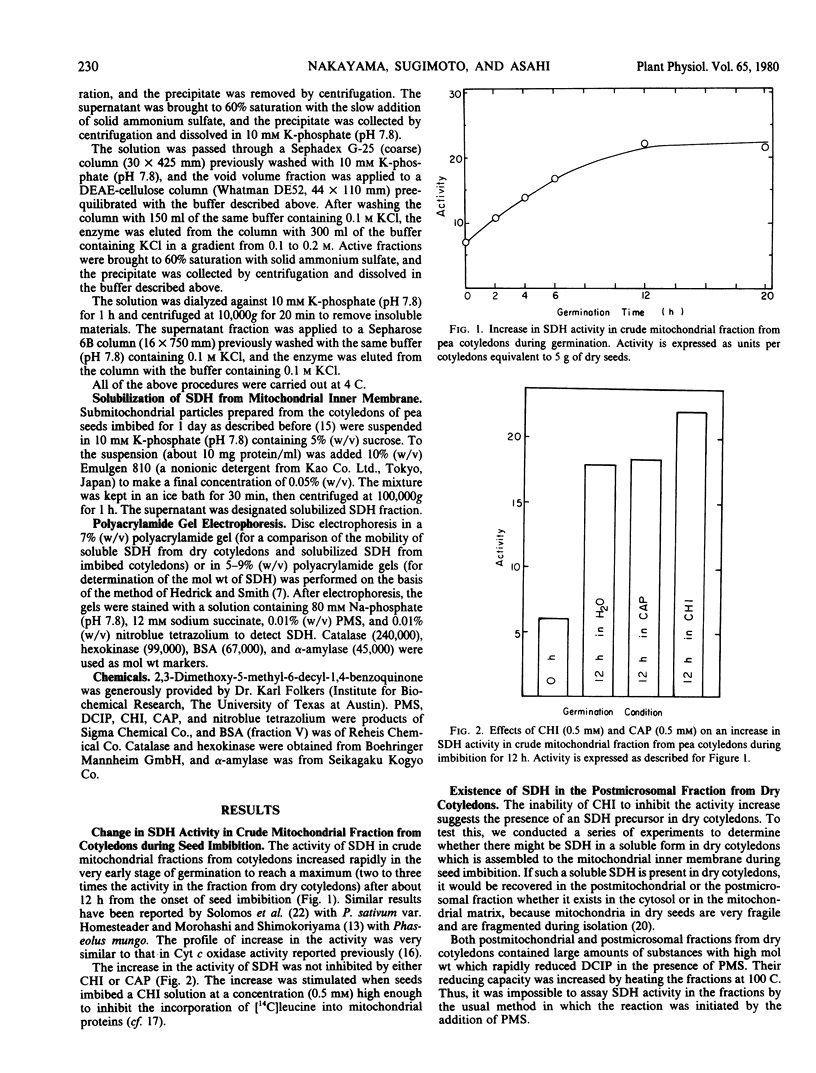
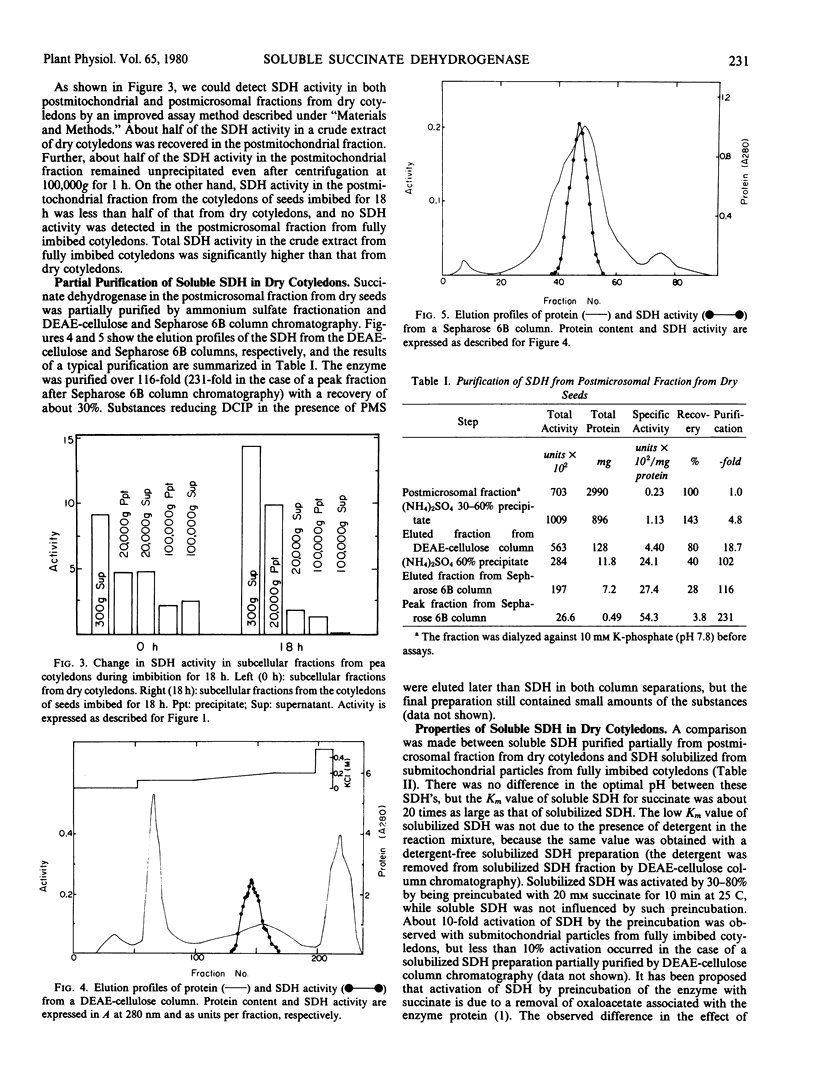
Succinate dehydrogenase (succinate: phenazine methosulfate oxidoreductase, EC 1.3.99.1) activity in crude mitochondrial fraction from pea (var. Alaska) cotyledons increased during seed imbibition to reach a maximum after about 12 hours. The increase was not inhibited by either cycloheximide or d(-)threo-chloramphenicol. The postmicrosomal fraction from dry cotyledons, but not that from fully imbibed ones, contained a soluble form of succinate dehydrogenase. The soluble enzyme was partially purified by ammonium sulfate fractionation and diethylaminoethyl-cellulose and Sepharose 6B column chromatography. The enzyme showed no succinate-coenzyme Q oxidoreductase activity and had a molecular mass of about 100,000 daltons. The soluble enzyme seemed to differ only slightly from succinate dehydrogenase solubilized from the mitochondrial inner membrane from fully imbibed cotyledons by a detergent. It is proposed that the soluble succinate dehydrogenase is associated with an inert mitochondrial inner membrane in dry cotyledons to form an active one during seed imbibition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Kearney E. B., Mayr M. Role 3f oxalacetate in the regulation of mammalian succinate dehydrogenase. J Biol Chem. 1974 Apr 10;249(7):2021–2027. [PubMed] [Google Scholar]
- Cherry J. H. Nucleic Acid, Mitochondria, & Enzyme Changes in Cotyledons of Peanut Seeds during Germination. Plant Physiol. 1963 Jul;38(4):440–446. doi: 10.1104/pp.38.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. Resolution of complex II and isolation of succinate dehydrogenase (EC 1.3.99.1). Methods Enzymol. 1978;53:27–35. doi: 10.1016/s0076-6879(78)53009-7. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stiggall D. L. Preparation and properties of succinate: ubiquinone oxidoreductase (complex II). Methods Enzymol. 1978;53:21–27. doi: 10.1016/s0076-6879(78)53008-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahler H. R., Perlman P. S. [Mitochondriogenesis analyzed by blocks on mitochondrial translation and transcription]. Biochemistry. 1971 Aug 3;10(16):2979–2990. doi: 10.1021/bi00792a001. [DOI] [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Biochemical Studies on Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination: Effects of Antibiotics on the Development. Plant Physiol. 1973 May;51(5):833–838. doi: 10.1104/pp.51.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971 Dec;48(6):671–674. doi: 10.1104/pp.48.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Asahi T. Biochemical Properties of Mitochondrial Membrane from Dry Pea Seeds and Changes in the Properties during Imbibition. Plant Physiol. 1975 Dec;56(6):816–820. doi: 10.1104/pp.56.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebald W. Biogenesis of mitochondrial ATPase. Biochim Biophys Acta. 1977 Jun 21;463(1):1–27. doi: 10.1016/0304-4173(77)90002-7. [DOI] [PubMed] [Google Scholar]
- Solomos T., Malhotra S. S., Prasad S., Malhotra S. K., Spencer M. Biochemical and structural changes in mitochondria and other cellular components of pea cotyledons during germination. Can J Biochem. 1972 Jul;50(7):725–737. doi: 10.1139/o72-101. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Akai A., Sierra M. F. Assembly of the mitochondrial membrane system. VII. Synthesis and integration of F 1 subunits into the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6511–6516. [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J Biol Chem. 1969 Sep 25;244(18):5027–5033. [PubMed] [Google Scholar]
- Vary M. J., Edwards C. L., Stewart P. R. The biogenesis of mitochondria. IX. Formation of the soluble mitochondrial enzymes malate dehydrogenase and fumarase in Saccharomyces cerevisiae. Arch Biochem Biophys. 1969 Mar;130(1):235–243. doi: 10.1016/0003-9861(69)90029-0. [DOI] [PubMed] [Google Scholar]
- Young J. L., Huang R. C., Vanecko S., Marks J. D., Varner J. E. Conditions Affecting Enzyme Synthesis in Cotyledons of Germinating Seeds. Plant Physiol. 1960 May;35(3):288–292. doi: 10.1104/pp.35.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]


