Abstract
Over the last decades, the incidence of infestation by minor parasites has decreased in developed countries. Infectious agents can also suppress autoimmune and allergic disorders. Some investigations show that various protozoa and helminthes are connected with the main immune-mediated intestinal conditions including celiac disease (CD), inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS). Celiac disease is a digestive and autoimmune disorder that can damage the small intestine and characterized by a multitude gastrointestinal (GI) and extra GI symptoms. IBD (including ulcerative colitis and Crohn’s disease) is a group of inflammatory conditions of the small intestine and colon. The etiology of IBD is unknown, but it may be related to instability in the intestinal microflora that leading to an immoderate inflammatory response to commensal microbiota. Irritable bowel syndrome (IBS) is a common, long-term condition of the digestive system. Bloating, diarrhoea and/or constipation are nonspecific symptoms of IBS. Various studies have shown that some intestinal parasites can effect on immune system of infected hosts and in some cases, they are able to modify and change the host’s immune responses, particularly in autoimmune disorders like celiac disease and IBD. The main objective of this review is to investigate the relationship between intestinal parasites and different inflammatory bowel disorders.
Key Words: Celiac disease, Inflammatory bowel diseases, Irritable bowel syndrome, Intestinal parasites
Introduction
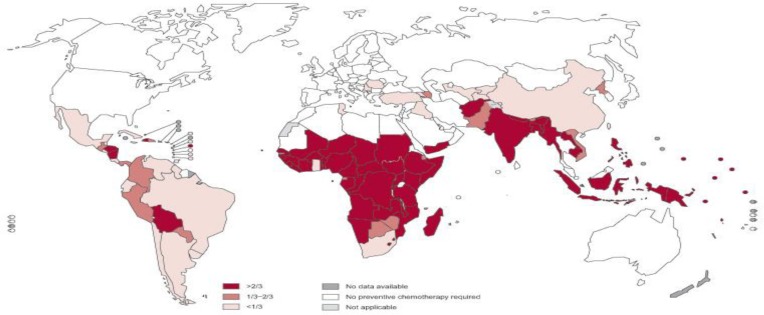
Parasites and microbes have been important for adjusting and forming the human immune system (1). Industrialized countries are actually experiencing rising in some autoimmune disorders. Loss of parasite colonization in those individuals living in developed countries has had a remarkable impact on our immune response and it is likely the chief factor contributing to the progression of autoimmune diseases (2, 3). Figure 1 shows global prevalence of soil-transmitted helminths. Celiac disease (CD), inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS) are the most important immune-mediated intestinal conditions. Celiac disease is an autoimmune disease of the small intestine typically leading to malabsorption and affects many organ systems. It can involve people of all ages from middle infancy to old age (4, 5). When someone with celiac disease consumes gluten, his or her immune system assaults the lining of the small intestine. Gluten is a mixed protein composed mainly of the gliadin and glutenin that found in wheat, barley, and rye. Consuming gluten-containing foods can initiate a range of gastrointestinal symptoms like abdominal pain, diarrhea, flatulence, bloating, weight loss, and extra intestinal signs such as anemia, osteoporosis, infertility and nervous problems (6).
Figure 1.
Global prevalence of Soil Transmitted Helminths (Source: World Health Organisation)
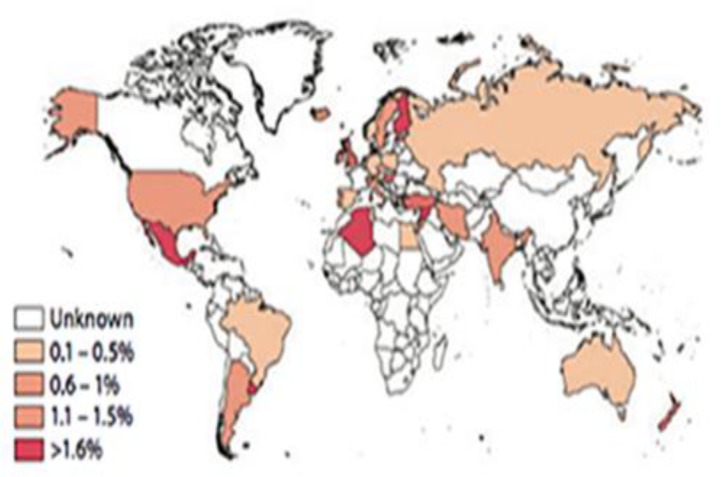
The only treatment for CD is a long life gluten free diet. Figure 2. shows the global prevalence of celiac disease.
Figure 2.
Global prevalence of celiac disease (Source: Annual Review of Immunology Vol. 29)
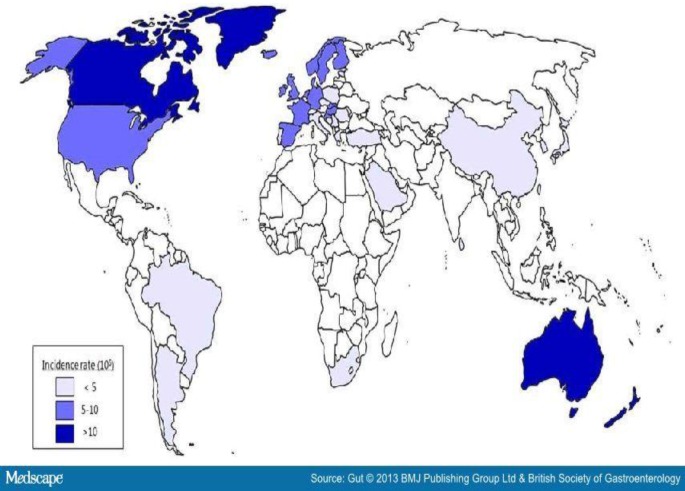
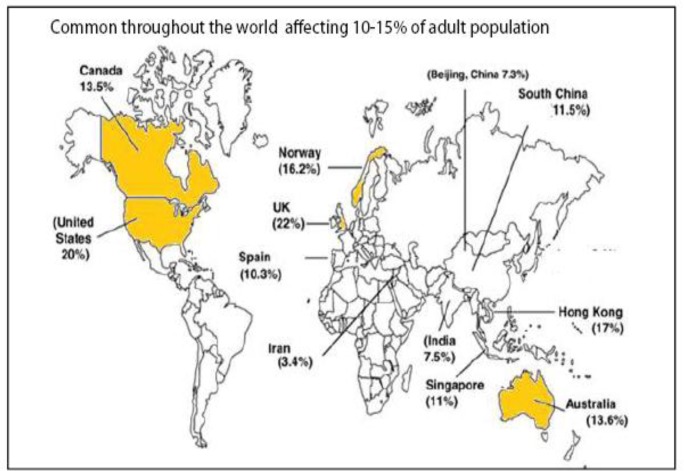
IBD is an idiopathic, chronic, and recurring inflammatory disease of the gastrointestinal tract, which is represented principally by ulcerative colitis (UC) and Crohn's disease. Lately, the intestinal microbiota have been considered to be a significant factor in their etiology (7). UC is a worldwide chronic inflammatory disorder of the colon that causes typical ulcers in the mucosa of the rectum and colon (8). On the other hand, Crohn's disease is a chronic inflammatory condition that can influence any part of the gut from mouth to anus (9). Methylated thiopurine metabolites, like 6-methyl mercaptopurine, are frequently used for the treatment of IBD (10). Global prevalence of Inflammatory Bowel Disease has been shown in figure 3. IBS is a gastrointestinal disorder typically present with chronic abdominal pain and changed bowel habits (11). Recent investigation presented that IBS is characterized by meaningful alterations in the gut microflora (12). Many studies have shown that gastrointestinal infection is an important risk factor for the development of IBS (13, 14). IBS prevalence varied according to diagnostic criteria and geographic regions (figure 4).
Figure 3.
Global prevalence of Inflammatory Bowel Disease (Source: BMJ Publishing Ltd & British Society of Gastroenterology).
Figure 4.
Worldwide incidence of IBS
A parasite is an organism that takes its food from another organism. Parasitic diseases comprising infections that are caused by protozoa, helminths or arthropods. Some studies have shown that many parasites such as hookworm can induce not only parasite-specific immunity, but also they modified the host’s immune responses (15-17). Many parasites can imitate inflammatory bowel disorders (18) and some studies showed that infection with helminthes can improve disorders like IBD or moderate the symptoms of inflammatory bowel disorders (19). This review was focused on correlation between intestinal parasites and inflammatory bowel disorders.
Celiac disease
Hookworms and Immune system
Investigations in animal models revealed strong evidence that helminths can downregulate parasite-specific immune responses adjust autoimmune responses and enhance metabolic homoeostasis (20). Gaze, et al. (21) and Croese, et al. (22) showed experimental hookworm infection cause robust mucosal Th2, Th1, and regulatory responses, and upregulates IL-15 and ALDH1A2 (a complex recognized to elevate Th17 inflammation in celiac disease) in celiac disease patients. During hookworm infection, suppression of mucosal IL-23 and also upregulation of IL-22 was occurred. Accordingly, Th17 responses are suppressed and inflammatory in celiac disease are blocked. Contrary to both celiac and Crohn’s diseases, hookworm infection suppressed in circulating CD4+CD25+Foxp3+cells (23, 24).
Necator americanus
In the celiac disease, the responses to gluten are considerably different if N. americanus was present. Dermal immunization of N. americanus has also been used to regulate the immune response to gluten (25). Therefore, hookworm infection can decrease gluten sensitivity and can use to treat celiac disease.
Treatment
In many countries the patients intentionally infect with worms and this is considered as a possible treatment for inflammatory diseases, for example, use of the Necator americanus larvae for treatment of celiac disease (20). Long-term use of low-dose N. americanus seems to be safe in CD treatment (22), due to the changing of immune responses such as cytokine production like IL-1β, IL-15 and IL-22 (26). Slack described that extended infectious diarrhea in the visitors is usually caused by protozoal and helminth parasites, and disease symptoms/treatment should be considered from non-infectious diarrhea such as celiac disease (27).
Other parasites
Jiménez et al. (28) reported that Blastocystis hominis should be noted as an opportunistic pathogen in characteristic celiac patients with low weight and subtotal-total villous atrophy. Celiac disease and Giardia intestinalis are usual causes of dyspepsia. Fouad, et al. (29) showed that G. intestinalis genotype A demonstrated a greater connection with dyspepsia. In both children and adults, untreated celiac disease is the most common cause of malabsorption syndrome, and in these patients some pathogenic parasites like Giardia lamblia, Ancylostoma duodenale, Entamoeba histolytica/dispar, Cyclospora cayetanensis, Hymenolepis nana, Cryptosporidium, Cyclospora and Isospora belli more often colonized compared to healthy control (30). Rostami Nejad, et al. (31) reported that Toxoplasma gondii infection rate was higher among patients with CD-serology positive than among patients with negative CD serology. Lidar, et al. (32) showed that raised titers of T. gondii antibodies have been observed in celiac disease patients and individuals with inflammatory bowel disease.
Inflammatory bowel disease (IBD)
Strongyloides stercoralis
Many investigations showed the curative effects of a controlled parasitic nematode infection on autoimmune disorders like IBD (33-35), but the exact mechanism(s) of these effects are not clear. Strongyloides stercoralis, an intestinal nematode, is common intestinal parasite in tropical areas e.g. Southeast Asia, Latin America, Sub-Saharan Africa, and many European countries (36). As it can occasionally imitate IBD, particularly UC, it is important to be kept out (18).
Blastocystis
Cekin, et al. (37) reported that Blastocystosis was more frequent in patients with IBD, specifically those with UC, but no statistically differences were shown between IBD patients and control group.
Toxoplasma gondii
Macrophage migration inhibitory factor (MIF) is an important mediator for controlling parasitic infections such as T. gondii (38-40). Cavalcanti, et al. (41) showed that T. gondii infection influences small intestine necrosis and death in sensitive patients with inflammatory bowel disease. They suggested that MIF contributed to the inflammatory response caused by oral infection with T. gondii.
Helminths
The result of clinical trial showed that infection with helminthes can improve IBD (19). On the other hand, an increasing prevalence of IBD in western countries could be associated whith the lower prevalence of intestinal helminthes (42). Moreels, et al. (43) illustrated that Trichuris suis can moderate IBD symptoms. Weerasekara, et al. (44) reported that patients with mild IBD symptoms might have been exposed to helminths like N. americanus, Trichuris trichiura, Ascaris lumbrcoides and Enterobius vermicularis, in childhood. Elliott, et al. (45) showed that helminths colonization change Th2 and regulatory immune responses such as IL-4, IL-5, IL-10, and IL-13 production, and save animals from progressing immune-mediated diseases like ulcerative colitis or Crohn's disease. Some evidences demonstrated that rodent nematodes like Trichuris muris, Nippostrongylus brasiliensis, and Trichinella spiralis, shift the gastrointestinal immune status toward Th2 production (43, 46). Motomura, et al. (47) revealed that previous treatment with T. spiralis antigens in mice decreased the intensity of colitis and the mortality rate significantly. They explicated that up-regulation of transforming growth factor-β (TGF- β) and IL-13 and down-regulation of interleukin (IL)-1β production, myeloperoxidase (MPO) activity, and nitric oxide synthase (iNOS) expression in colon are related to decreasing disease mortality. Khan, et al. (48) showed that Trichinella spiralis protected mice from colitis and IBD. Moreover, schistosome eggs provide a defensive effect on TNBS-induced colitis in mice (49). Infection with Heligomosomoides polygyrus or Trichuris muris can prevent or invert the chronic Th1-type colitis in IL-10 deficient mice (50). Helminths and their products can modulate the innate and adaptive immune system (51). Summers, et al. (52, 53) showed T. suis is well tolerated and appears effective for Crohn’s and ulcerative colitis.
Irritable bowel syndrome (IBS)
Various parasites comprising B. hominis, Giardia spp., E. histolytica, Dientamoeba fragilis and Trichinella spp. have been considered as contributing factors to the progress of IBS (54-56), although the correlation is not confirmed.
Blastocystis
Dugroman, et al. (54) reported that Blastocystis infection was found in 67% of the patients with IBS and it could be a serious problem for diagnosis of IBS. In a comprehensive study was performed on 357 parasite carriage among IBS cases in Nicaragua, Morgan, et al. reported that statistically difference was not noticed in the prevalence of intestinal parasite infection including B. hominis, G. lamblia, E. histolytica/dispar, E. nana, A. lumbricoides, and H. nana among patients with IBS compared to the healthy controls (57).
Giardia Spp.
Dizdar, et al. (58) found that post-infectious bowel dysfunction following Giardia infection is associated with increased duodenal mucosal in IBS patients.
Dientamoeba fragilis
The first time, Borody, et al. reported the relationship between IBS and Dientamoeba fragilis (59). Epidemiological studies have shown the range of 2–4% of D. fragilis in IBS patients (60, 61). Among 25 D. fragilis-positive IBS patients, Engsbro, et al. (62) found no relationship between D. fragilis and IBS. D. fragilis can cause IBS-like symptoms. Therefore, it is not unexpected that many patients with D. fragilis are misdiagnosed as having IBS.
Haplorchis taichui
H. taichui is a fluke that belongs to the family Heterophyidae. It can live in the small intestines of mammals and birds and is found in Southeast Asia (63). Humans become infected with eating the metacercariae in infected cyprinoid fish (64). Watthanakulpanich, et al. (65) showed that H. taichui could be a possible etiologic agent for IBS-like symptoms.
Trichinella
For the first time, Soyturk, et al. (66) declared that IBS could be considered as a secondary syndrome caused by trichinellosis.
Trichuris trichiura
Diniz-Santos, et al. (67) showed that T. trichiura could be misdiagnosed due to its ability to mimic IBS symptoms. Table 1 summarizes some examples that show the relationship between intestinal parasites and inflammatory bowel disorders.
Table 1.
The association between intestinal parasites and celiac disease, inflammatory bowel disease and inflammatory bowel syndrome
| Celiac disease | IBD | IBS | |
|---|---|---|---|
| N. americanus | Down regulation of immune response to gluten | - | - |
| B. hominis | Opportunistic pathogen in CD patients with low weight and subtotal-total villous atrophy | - | - |
| Giardia spp. | It is usual causes of malabsorption and dyspepsia | - | - |
| T. gondii | - | Influences small intestine necrosis | - |
| S. stercoralis | - | Imitate IBD symptoms | - |
| T. suis | - | Can moderate IBD symptoms | - |
| T. spiralis | - | protected mice from colitis and IBD | - |
| D. fragilis | - | - | It is considered as a contributing factor to the development of IBS, can cause IBS-like symptoms |
| H. taichui | - | - | It can be a possible etiologic agent for IBS-like symptoms |
Conclusion
In many countries deliberate infection with helminthes larva considered as a possible treatment for inflammatory disorders like celiac disease due to the changing of immune responses such as cytokine production. Various surveys showed the therapeutic effects of a controlled parasitic infection on autoimmune disorders. Some studies reported that hookworm infection decrease gluten sensitivity and can employ to treat celiac disease. It is suggested that rising prevalence of inflammatory bowel disorders such as IBD could be associated with the decreased prevalence of intestinal helminthes. This review has highlighted some of these assumptions. However, the precise mechanisms of these effects remain unclear. According to these studies we think that there is a relationship between some parasitic infections such as Toxoplasma gondii, and the development of CD, helminthes infections and development of IBD, Dientamoeba fragilis and B. hominis and development of IBS. Understanding the correlation between parasitic infections and autoimmune disorders may be helpful in prediction, early identification and conceivably the prevention of these diseases.
References
- 1.Dunne DW, Cooke A. A worm's eye view of the immune system: consequences for evolution of human autoimmune disease. Nat Rev Immunol. 2005;5:420–26. doi: 10.1038/nri1601. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock JV, Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R. The possible link between de-worming and the emergence of immunological disease. J Lab Clin Med. 2002;139:334–38. doi: 10.1067/mlc.2002.124343. [DOI] [PubMed] [Google Scholar]
- 3.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 5.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 6.Bhatnagar S, Tandon N. Diagnosis of celiac disease. Indian J Pediatr. 2006;73:703–709. doi: 10.1007/BF02898449. [DOI] [PubMed] [Google Scholar]
- 7.Endo K, Shiga H, Kinouchi Y, Shimosegawa T. [Inflammatory bowel disease: IBD] Rinsho Byori. 2009;57:527–32. [PubMed] [Google Scholar]
- 8.Parray FQ, Wani ML, Malik AA, Wani SN, Bijli AH, Irshad I. Ulcerative colitis: a challenge to surgeons. Int J Prev Med. 2012;3 [PMC free article] [PubMed] [Google Scholar]
- 9.Lennard-Jones J. Classification of inflammatory bowel disease. Scand J Gastroenterol. 1989;24:S2–6. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 10.van Asseldonk DP, Seinen ML, de Boer NK, van Bodegraven AA, Mulder CJ. Hepatotoxicity associated with 6-methyl mercaptopurine formation during azathioprine and 6-mercaptopurine therapy does not occur on the short-term during 6-thioguanine therapy in IBD treatment. J Crohns Colitis. 2012;6:95–101. doi: 10.1016/j.crohns.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–80. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 12.Bolino CM, Bercik P. Pathogenic factors involved in the development of irritable bowel syndrome: focus on a microbial role. Infect Dis Clin North Am. 2010;24:961–75. doi: 10.1016/j.idc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez LAG, Ruigómez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ. 1999;318:565–66. doi: 10.1136/bmj.318.7183.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruigómez A, García Rodríguez LA, Panés J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: influence of comorbidities. Clin Gastroenterol Hepatol. 2007;5:465–69. doi: 10.1016/j.cgh.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 15.De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Diemert D, Loukas A. New technologies for the control of human hookworm infection. Trends Parasitol. 2006;22:327–31. doi: 10.1016/j.pt.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 17.McSorley HJ, Gaze S, Daveson J, Jones D, Anderson RP, Clouston A, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dave M, Purohit T, Razonable R, Loftus Jr EV. Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis. 2014;20:196–212. doi: 10.1097/MIB.0b013e3182a827d2. [DOI] [PubMed] [Google Scholar]
- 19.Ruyssers NE, De Winter BY, De Man JG, Loukas A, Pearson MS, Weinstock JV, et al. Therapeutic potential of helminth soluble proteins in TNBS‐induced colitis in mice. Inflamm Bowel Dis. 2009;15:491–500. doi: 10.1002/ibd.20787. [DOI] [PubMed] [Google Scholar]
- 20.Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–62. doi: 10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- 21.Gaze S, McSorley HJ, Daveson J, Jones D, Bethony JM, Oliveira LM, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croese J, Gaze ST, Loukas A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. Int J Parasitol. 2013;43:275–82. doi: 10.1016/j.ijpara.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Fantini MC, Rizzo A, Fina D, Caruso R, Sarra M, Stolfi C, et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136:1308–16. doi: 10.1053/j.gastro.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 24.Hmida NB, Ahmed MB, Moussa A, Rejeb MB, Said Y, Kourda N, et al. Impaired control of effector T cells by regulatory T cells: a clue to loss of oral tolerance and autoimmunity in celiac disease? Am J Gastroenterol. 2011;107:604–11. doi: 10.1038/ajg.2011.397. [DOI] [PubMed] [Google Scholar]
- 25.Gujral N, Freeman HJ, Thomson AB. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18 doi: 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704–12. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slack A. Parasitic causes of prolonged diarrhoea in travelers: Diagnosis and management. Aust Fam Physician. 2012;41 [PubMed] [Google Scholar]
- 28.Jiménez O, Carbonell A, García O, Rodríguez L, Triana F, Fabián L. Blastocystis hominis in symptomatic celiac patients. Acta Gastroenterol Latinoam. 2012;42:175–81. [PubMed] [Google Scholar]
- 29.Fouad SA, Esmat S, Basyoni MM, Farhan MS, Kobaisi MH. Molecular identification of Giardia intestinalis in patients with dyspepsia. Digest. 2014;90:63–71. doi: 10.1159/000362644. [DOI] [PubMed] [Google Scholar]
- 30.Behera B, Mirdha B, Makharia GK, Bhatnagar S, Dattagupta S, Samantaray J. Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig Dis Sci. 2008;53:672–79. doi: 10.1007/s10620-007-9927-9. [DOI] [PubMed] [Google Scholar]
- 31.Nejad MR, Rostami K, Cheraghipour K, Mojarad EN, Volta U, Al Dulaimi D, et al. Celiac disease increases the risk of Toxoplasma gondii infection in a large cohort of pregnant women. Am J Gastroenterol. 2011;106:548–49. doi: 10.1038/ajg.2010.425. [DOI] [PubMed] [Google Scholar]
- 32.Lidar M, Lipschitz N, Langevitz P, Barzilai O, Ram M, Porat‐Katz BS, et al. Infectious serologies and autoantibodies in Wegener's granulomatosis and other vasculitides: novel associations disclosed using the Rad BioPlex 2200. Ann N Y Acad Sci. 2009;1173:649–57. doi: 10.1111/j.1749-6632.2009.04641.x. [DOI] [PubMed] [Google Scholar]
- 33.Elliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune‐mediated diseases. Ann N Y Acad Sci. 2012;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ince MN, Elliott DE, Setiawan T, Metwali A, Blum A, Chen Hl, et al. Role of T cell TGF‐β signaling in intestinal cytokine responses and helminthic immune modulation. Eur J Immunol. 2009;39:1870–78. doi: 10.1002/eji.200838956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180:4265–72. doi: 10.4049/jimmunol.180.6.4265. [DOI] [PubMed] [Google Scholar]
- 36.Moghadam K, Khashayar P, Hashemi M. Gastrointestinal strongyloidiasis in immunocompromised patients: a case report. Acta Med Indones. 2011;43:191–94. [PubMed] [Google Scholar]
- 37.Cekin AH, Cekin Y, Adakan Y, Tasdemir E, Koclar FG, Yolcular BO. Blastocystosis in patients with gastrointestinal symptoms: a case-control study. BMC Gastroenterol. 2012;12 doi: 10.1186/1471-230X-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satoskar AR, Bozza M, Sosa MR, Lin G, David JR. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect Immun. 2001;69:906–11. doi: 10.1128/IAI.69.2.906-911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDevitt MA, Xie J, Shanmugasundaram G, Griffith J, Liu A, McDonald C, et al. A critical role for the host mediator macrophage migration inhibitory factor in the pathogenesis of malarial anemia. J Exp Med. 2006;203:1185–96. doi: 10.1084/jem.20052398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terrazas CA, Juarez I, Terrazas LI, Saavedra R, Calleja EA, Rodriguez-Sosa M. Toxoplasma gondii: Impaired maturation and pro-inflammatory response of dendritic cells in MIF-deficient mice favors susceptibility to infection. Exp Parasitol. 2010;26:348–58. doi: 10.1016/j.exppara.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Cavalcanti MG, Mesquita JS, Madi K, Feijó DF, Assunção-Miranda I, Souza HS, et al. MIF participates in Toxoplasma gondii-induced pathology following oral infection. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Büning J, Homann N, von Smolinski D, Borcherding F, Noack F, Stolte M, et al. Helminths as governors of inflammatory bowel disease. Gut. 2008;57:1182–83. doi: 10.1136/gut.2008.152355. [DOI] [PubMed] [Google Scholar]
- 43.Moreels TG, Pelckmans PA. Gastrointestinal parasites. Potential therapy for refractory inflammatory bowel diseases. Inflamm Bowel Dis. 2005;11:178–84. doi: 10.1097/00054725-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Weerasekara D, Fernando N, Meedin F, Holton J, Fernando D. Clinical presentation and risk factors of inflammatory bowel disease in Sri Lanka. Trop Gastroenterol. 2011;32:31–35. [PubMed] [Google Scholar]
- 45.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol. 2007;37:457–64. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 46.Brunet LR, Dunne D, Pearce E. Cytokine interaction and immune responses during Schistosoma mansoni infection. Parasitol Today. 1998;14:422–27. doi: 10.1016/s0169-4758(98)01317-9. [DOI] [PubMed] [Google Scholar]
- 47.Motomura Y, Wang H, Deng Y, El‐Sharkawy R, Verdu E, Khan W. Helminth antigen‐based strategy to ameliorate inflammation in an experimental model of colitis. Clin Exp Immunol. 2009;155:88–95. doi: 10.1111/j.1365-2249.2008.03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan W, Blennerhasset P, Varghese A, Chowdhury S, Omsted P, Deng Y, et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70:5931–37. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban Jr JF, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Renal Physiol. 2003;284:G385–G91. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 50.Wang LJ, Cao Y, Shi HN. Helminth infections and intestinal inflammation. World J Gastroenterol. 2008;14 doi: 10.3748/wjg.14.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whelan RA, Hartmann S, Rausch S. Nematode modulation of inflammatory bowel disease. Protoplasma. 2012;249:871–86. doi: 10.1007/s00709-011-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Summers RW, Elliott DE, Qadir K, Urban JF, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–41. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 53.Thompson RA, Elliot DE, Urban JF, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–32. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Dogruman-Al F, Simsek Z, Boorom K, Ekici E, Sahin M, Tuncer C, et al. Comparison of methods for detection of Blastocystis infection in routinely submitted stool samples, and also in IBS/IBD Patients in Ankara, Turkey. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stark D, Van Hal S, Marriott D, Ellis J, Harkness J. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol. 2007;37:11–20. doi: 10.1016/j.ijpara.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–88. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 57.Morgan DR, Benshoff M, Cáceres M, Becker-Dreps S, Cortes L, Martin CF, et al. Irritable bowel syndrome and gastrointestinal parasite infection in a developing nation environment. Gastroenterol Res Pract. 2012;2012 doi: 10.1155/2012/343812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dizdar V, Spiller R, Singh G, Hanevik K, Gilja O, EL‐Salhy M, et al. Relative importance of abnormalities of CCK and 5‐HT (serotonin) in Giardia‐induced post‐infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–91. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 59.Borody T, Warren E, Wettstein A, Robertson G, Recabarren P, Fontella A, et al. Eradication of Dientamoeba fragilis can resolve IBS-like symptoms. J Gastroenterol Hepatol. 2002;17 [Google Scholar]
- 60.Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, et al. Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res. 2010;107:679–84. doi: 10.1007/s00436-010-1918-7. [DOI] [PubMed] [Google Scholar]
- 61.Jimenez-Gonzalez DE, Martinez-Flores WA, Reyes-Gordillo J, Ramirez-Miranda ME, Arroyo-Escalante S, Romero-Valdovinos M, et al. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol Res. 2012;110:1269–75. doi: 10.1007/s00436-011-2626-7. [DOI] [PubMed] [Google Scholar]
- 62.Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. Treatment of Dientamoeba fragilis in patients with irritable bowel syndrome. J Trop Med Hyg. 2012;87:1046–52. doi: 10.4269/ajtmh.2012.11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chai J-Y, Han E-T, Shin E-H, Sohn W-M, Yong T-S, Eom KS, et al. High prevalence of Haplorchis taichui, Phaneropsolus molenkampi, and other helminth infections among people in Khammouane Province, Lao PDR. Korean J Parasitol. 2009;47:243–47. doi: 10.3347/kjp.2009.47.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumchoo K, Wongsawad C, Chai J-Y, Vanittanakom P, Rojanapaibul A. High prevalence of Haplorchis taichui metacercariae in cyprinoid fish from Chiang Mai Province, Thailand. Southeast Asian J Trop Med Public Health. 2005;36:451–55. [PubMed] [Google Scholar]
- 65.Watthanakulpanich D, Waikagul J, Maipanich W, Nuamtanong S, Sanguankiat S, Pubampen S, et al. Haplorchis taichui as a possible etiologic agent of irritable bowel syndrome-like symptoms. Korean J Parasitol. 2010;48:225–29. doi: 10.3347/kjp.2010.48.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soyturk M, Akpinar H, Gurler O, Pozio E, Sari I, Akar S, et al. Irritable bowel syndrome in persons who acquired trichinellosis. Am J Gastroenterol. 2007;102:1064–9. doi: 10.1111/j.1572-0241.2007.01084.x. [DOI] [PubMed] [Google Scholar]
- 67.Diniz-Santos DR, Jambeiro J, Mascarenhas RR, Silva LR. Massive Trichuris trichiura infection as a cause of chronic bloody diarrhea in a child. J Trop Pediatr. 2006;52:66–68. doi: 10.1093/tropej/fmi073. [DOI] [PubMed] [Google Scholar]