Abstract
Anti-angiogenic therapy has increased the progression-free survival of many cancer patients but has had little effect on overall survival, even in colon cancer (average 6–8 weeks) due to resistance. The current licensed targeted therapies all inhibit VEGF signalling (Table1). Many mechanisms of resistance to anti-VEGF therapy have been identified that enable cancers to bypass the angiogenic blockade. In addition, over the last decade, there has been increasing evidence for the role that the hypoxic and metabolic responses play in tumour adaptation to anti-angiogenic therapy. The hypoxic tumour response, through the transcription factor hypoxia-inducible factors (HIFs), induces major gene expression, metabolic and phenotypic changes, including increased invasion and metastasis. Pre-clinical studies combining anti-angiogenics with inhibitors of tumour hypoxic and metabolic adaptation have shown great promise, and combination clinical trials have been instigated. Understanding individual patient response and the response timing, given the opposing effects of vascular normalisation versus reduced perfusion seen with anti-angiogenics, provides a further hurdle in the paradigm of personalised therapeutic intervention. Additional approaches for targeting the hypoxic tumour microenvironment are being investigated in pre-clinical and clinical studies that have potential for producing synthetic lethality in combination with anti-angiogenic therapy as a future therapeutic strategy.
Keywords: angiogenesis, anti-VEGF therapy, combination therapy, hypoxia, metabolism
Introduction
The original version of the ‘Hallmarks of Cancer’ highlighted the role of neo-angiogenesis in tumour progression (Hanahan & Weinberg, 2000). Targeting angiogenesis as a tumour therapy was first hypothesised over four decades ago (Folkman, 1971). In the updated ‘Hallmarks of Cancer: The Next Generation,’ the ‘deregulation of cellular energetics’ has also been added (Hanahan & Weinberg, 2011). This was linked to the renewed interest and substantial findings of the role of metabolic reprogramming in tumour progression. Low oxygen level (hypoxia) was overlooked in both versions of the ‘Hallmarks of Cancer’ but is inextricably linked with nearly all the hallmarks (Kroemer & Pouyssegur, 2008). In particular, the regulation of both angiogenesis and metabolism by hypoxia is well documented (Semenza, 2014). Here, we review the link between these three important aspects of tumour physiology with a particular focus on combination therapeutic approaches.
Tumour angiogenesis
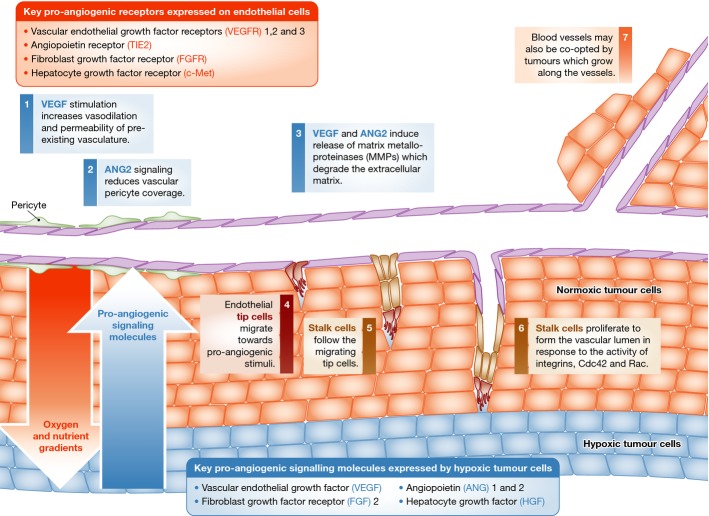
As tumours grow, they require additional nutrients and oxygen, and induce new blood vessels. The growth of new blood vessels (angiogenesis) is a process tightly regulated by a balance of pro- and anti-angiogenic signalling in normal physiology. In tumour tissues, this balance is lost resulting in a vasculature with a chaotic, leaky architecture. Angiogenesis is a multistage process regulated by numerous growth factors and their receptors (Fig1). Key growth factors (and their cognate receptors on endothelial cells shown in brackets) in the angiogenic process include VEGF, VEGFR1, 2 and 3, ANG1 and 2 (TIE2), FGF2 (FGFR1, 2, 3, 4) and HGF (c-Met) (Welti et al, 2013). These cross talk with additional signalling pathways, in particular the Notch pathway, which is required for patterning of functional vasculature (Blanco & Gerhardt, 2013; Welti et al, 2013). Vascular endothelial growth factor (VEGF), one of the main drivers of angiogenesis, increases vasodilation and permeability of pre-existing vasculature (Welti et al, 2013). TIE2 and angiopoietin 2 (ANG2) signalling reduce vascular pericyte coverage (Augustin et al, 2009). These induce the release of proteases including matrix metalloproteinases, resulting in degradation of the extracellular matrix (Bridges & Harris, 2011). Endothelial tip cells migrate towards the pro-angiogenic stimuli, and are followed by stalk cells, which proliferate to form the vascular lumen, in response to activity of integrins, Cdc42 and Rac (Bridges & Harris, 2011; Welti et al, 2013). Blood vessels may also be co-opted by tumours. Tumour vessel co-option (Fig1) is the utilisation of pre-existing vasculature by growing or migrating along these vessels. It has mostly been identified in well-vascularised tissues of the lung, liver and brain (Donnem et al, 2013; Pezzella & Harris, 2014). Metabolically, vessel-co-opted tumours have higher expression of genes encoding mitochondrial proteins, suggesting a greater use of TCA cycle (Donnem et al, 2013). Distinguishing co-opted vessels from neo-angiogenic vessels is difficult and a mixed phenotype of vascularisation is often identified (Donnem et al, 2013), suggesting combined approaches for targeting both types of vasculature may be required for some tumours (Pezzella & Harris, 2014). Many of the factors that regulate angiogenesis are increased in the hypoxic conditions found in tumours (Semenza, 2014). However, tumour anti-angiogenic anti-VEGF therapy increases hypoxia (Franco et al, 2006; De Bock et al, 2011).
Figure 1. Angiogenesis into the hypoxic tumour microenvironment.
This figure highlights the role of hypoxia-regulated proteins in the angiogenic process.
Hypoxia
Hypoxia arises from the combination of high metabolic and proliferative rates, that is consumption and aberrant tumour vascularisation with poor oxygen delivery (Semenza, 2014). Clinically, tumour hypoxia is associated with poor patient prognosis and resistance to chemotherapy (Rebucci & Michiels, 2013) and radiotherapy (Multhoff et al, 2014) and poor outcome as an independent factor, regardless of treatment modality. Hypoxia regulates the expression of genes under the transcriptional control of hypoxia-inducible factor-1α and hypoxia-inducible factor-2α (HIF1α and HIF2α), which heterodimerise with HIF1β and bind the hypoxia response element (HRE) in the promoter of many genes (Choudhry et al, 2014). HIF1α and HIF2α mRNAs are constitutively expressed, but the proteins produced are constantly targeted for degradation in normoxic conditions by prolyl-hydroxylases, which require O2 as a cofactor (Shen & Kaelin, 2013). Upon hydroxylation, HIF1α and HIF2α interact with VHL, which mediates HIF ubiquitination and degradation. Inactivating mutations in VHL lead to HIF stabilisation in normoxia and are often found in renal cell carcinoma (RCC) (Shen & Kaelin, 2013). Growth factor signalling cascades PI3K/AKT/mTOR and MAPK also regulate the expression and phosphorylation of HIF1α and HIF2α (Agani & Jiang, 2013). Many HIF-regulated genes trigger more aggressive growth and survival, and contribute to the major hallmarks of cancer. HIF regulates genes in key processes of tumour growth in particular metabolism and angiogenesis (Favaro et al, 2011; Semenza, 2014). Metabolic genes increased by HIF1 in response to hypoxia include many of those involved in glycolysis: GLUT1, GLUT3, PDK1, PKM2, PFKFB3, GYS1, ENO1, LDHA, HK2 and GAPDH (Favaro et al, 2011). Angiogenesis genes increased by HIF in response to hypoxia include VEGF, FLT-1, ANG1, 2, TIE2, PDGF, MMP2, 9 and FLK1 (Favaro et al, 2011; Semenza, 2014) (Fig1).
Hypoxia increases invasion and metastasis, and HIF1 regulates the expression of key genes (c-Met, CXCR4, RIOK3 and LOX), of these processes (De Bock et al, 2011; Singleton et al, 2014). Hypoxia also increases autophagy, promoting survival of hypoxic tumour cells (Brahimi-Horn et al, 2011). High numbers of tumour-associated macrophages (TAMs) accumulate in hypoxic areas of tumours (Gilkes et al, 2014). TAMs are attracted to the hypoxic microenvironment by increased expression of monocytic chemotactic proteins, VEGF, semaphorin 3A and interleukin 1 (Gilkes et al, 2014).
The hypoxic tumour extracellular microenvironment is also acidic because of increased production of metabolic acids, CO2 and lactic acid (from glycolysis) (Parks et al, 2013) and longer diffusion distances to functional blood capillaries. The optimal range of intracellular pH (pHi) is narrow, such that only a fraction of a pHi-unit change can lead to aberrant function or even death. Regulation of pHi is required to maintain optimum conditions for signalling such as mTOR (Balgi et al, 2011). Many pH regulatory proteins have increased expression and/or activity in hypoxia including monocarboxylate transporters 1 and 4 (MCT1, 4) that export lactate, and carbonic anhydrase 9 (CA9) (Parks et al, 2013) that hydrates extracellular CO2 to produce a steeper efflux gradient (Swietach et al, 2014).
Tumour metabolism
The metabolic profiles of tumour cells are dramatically different from normal adult cells as their metabolic requirements shift based on phenotypic changes, including increased proliferation and survival in the acidic- and nutrient-depleted tumour microenvironment. Energy generation, anabolism and maintenance of redox potential are promoted in normoxia and hypoxia as the need for biomass precursors (nucleotides, lipids and proteins) and ATP increase (Schulze & Harris, 2012). Tumour metabolism is regulated by a number of different factors: most notably HIF (hypoxia), AMP-activated protein kinase (AMPK) (nutrient sensing), growth factor signalling and oncogenes (RAS, MYC, etc.) (Schulze & Harris, 2012). AMPK is activated in response to increased AMP/ATP ratios and hypoxia. It induces a metabolic shift away from glycolysis to oxidative phosphorylation and autophagy (Liang & Mills, 2013).
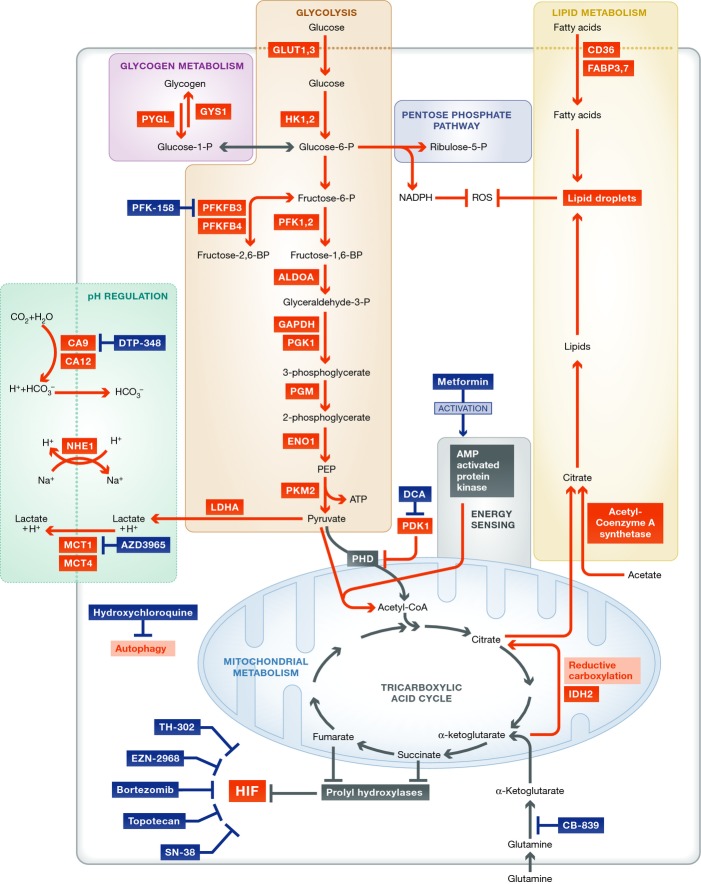
Here, we focus on the metabolism of hypoxic cells (Fig2). Hypoxia reduces oxidative phosphorylation by preventing pyruvate from entering the tricarboxylic acid (TCA) cycle, via up-regulation of pyruvate dehydrogenase kinase (PDK1) and then inhibition of pyruvate dehydrogenase (PDH). Pyruvate is instead broken down to lactic acid and extruded via MCT4 (Brahimi-Horn et al, 2011). However, mitochondria remain active in the hypoxic microenvironment (Sun & Denko, 2014). Hypoxia increases glutamine uptake and metabolism. Glutamine-derived α-ketoglutarate is used to replenish intermediates of the tricarboxylic acid (TCA) cycle as an alternative to pyruvate. This includes citrate produced via reductive carboxylation of α-ketoglutarate, a process promoted by hypoxia. The reductive carboxylation-derived citrate can be used in lipid synthesis, which is required for tumour growth (Sun & Denko, 2014).
Figure 2. Metabolic reprogramming in the hypoxic microenvironment.
This figure shows the metabolic processes that are upregulated in response to hypoxia and the therapeutic drugs that target these processes, which are in clinical trials (highlighted in white boxes). Proteins in red have increased expression or activity in hypoxia. Arrows in red denote increased flux in hypoxia. ALDOA, aldolase A; CA, carbonic anhydrase; CD36, fatty acid translocase; DCA, dichloroacetate; ENO1, enolase 1; FABP, fatty acid binding protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GLUT, glucose transporter; GYS1, glycogen synthase; HK, hexokinase; HIF, hypoxia-inducible factor; IDH2, isocitrate dehydrogenase 2; LDHA, lactate dehydrogenase A; MCT, monocarboxylate transporter; NHE1, sodium hydrogen antiporter 1; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; PFK, phosphofructokinase; PFKFBP, phosphofructokinase bisphosphatase; PGK1, phosphoglycerate kinase 1; PGM, phosphoglycerate mutase; PKM2, pyruvate kinase M2; PYGL, liver glycogen phosphorylase.
The pentose phosphate pathway (PPP) is up-regulated in cancer, and stabilisation of HIF1α increases expression of genes involved in the PPP (Riganti et al, 2012). The PPP generates nucleotides and also NADPH, an important reducing agent required for lipid, nucleotide and amino acid synthesis, and, in particular, glutathione and ROS protection (Favaro et al, 2012). Hypoxia increases expression of glycogen synthase 1 (GYS1) in a HIF-dependent manner, which increases glycogen stores. HIF-dependent increased expression of PYGL breaks down the glycogen stores, and knock-down of this enzyme was shown to reduce entry of glucose into the PPP and induce ROS-dependent senescence (Favaro et al, 2012), highlighting the importance of glycogen metabolism in protection from free radicals (Favaro et al, 2012). Similarly, hypoxia-regulated 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4) is essential for regulating entry to glycolysis or PPP for NADPH production (Ros & Schulze, 2013). Hypoxia-inducible PFKFB3 conversely increases glycolytic flux due to its greater kinase activity (Ros & Schulze, 2013).
Hypoxia and RAS transformation increase scavenging of serum fatty acids in particular lysophospholipids, and hypoxia reduces de novo lipogenesis (Kamphorst et al, 2013). Furthermore, fatty acid uptake and storage is increased in hypoxia via fatty acid binding proteins 3 (FABP3) and 7 (FABP7) (Bensaad et al, 2014). An increase in fatty acid storage in hypoxia protects against ROS toxicity (Bensaad et al, 2014).
Further to those already discussed, additional metabolic proteins have increased expression or activity in hypoxia including HK1, PFK1, PFK2, ALDOA, PGK1, PGM (glycolysis), NHE1 (pH regulation), CD36 and acetyl-coenzyme A synthetase (lipid metabolism) (Bensaad & Harris, 2014).
In addition to HIF and hypoxia-regulating metabolic processes, metabolic intermediates (including succinate and fumarate) can increase HIF stabilisation via inhibition of prolyl-hydroxylases (Schulze & Harris, 2012). This highlights the bidirectional regulation of hypoxia/HIF and metabolism as is also found with angiogenesis.
A likely confounding factor of targeting metabolic processes alone in tumours is the plasticity of metabolic networks. To complicate this further, the identified metabolic interactions with stromal cells (Martinez-Outschoorn et al, 2014) make our current understanding of tumour metabolism, which is mostly derived from 2D culture experiments, less conclusive with regard to intervention. In addition, metabolic reprogramming of endothelial cells plays an important role in the process of angiogenesis (Goveia et al, 2014).
Anti-angiogenic therapies
Most anti-angiogenic therapies target the key pro-angiogenic factors or their endothelial cell-expressed receptors including VEGF, VEGFR1, 2 and 3, ANG 1 and 2, TIE2, FGF2, FGFR, c-Met and HGF (Bridges & Harris, 2011). The first anti-angiogenic therapy Avastin (bevacizumab) was approved by the Food and Drug Administration (FDA) in 2003. This inhibits the main driver of angiogenesis, VEGFA. A list of FDA-approved anti-angiogenic therapies is shown in Table1. The receptor tyrosine kinase (RTK)-based inhibitor approaches to anti-angiogenic therapy are likely also effecting tumour RTK signalling cascades, which regulate tumour proliferation, survival, metabolism and HIF (Shimobayashi & Hall, 2014).
Table 1.
A list of all FDA-approved antiangiogenic therapies (2014).
| Compound | Target | Indication |
|---|---|---|
| Antibody-based therapies | ||
| Bevacizumab (Avastin) | Anti-VEGF antibody | Glioblastoma, metastatic colorectal cancer, metastatic RCC, some non-small cell lung cancers |
| Aflibercept (Eylea) | VEGF-trap recombinant fusion protein of VEGF-binding domains from VEGFR | Metastatic colorectal cancer |
| Ramucirumab (Cyrazma) | Human monoclonal VEGFR2 antibody inhibits VEGF binding | Advanced gastric or gastro-oesophageal junction adenocarcinoma |
| Small molecular inhibitors | ||
| Axitinib (Inlyta) | VEGFR1-3, PDGFRβ, and c-KIT | Advanced RCC |
| Cabozantinib (Cometriq) | VEGFR1-3, MET | Metastatic medullary thyroid cancer |
| Everolimus (Afinitor) | mTOR | RCC, neuroendocrine tumours |
| Pazopanib (Votrient) | VEGFR1-3, PDGFR, c-KIT | RCC |
| Regorafanib (Stivarga) | VEGFR1-3, PDGFRβ, TIE2 | Metastatic colorectal cancer |
| Sorafenib (Nexavar) | VEGFR1-3, PDGFR, RAF | Hepatocellular carcinoma, RCC |
| Sunitinib (Sutent) | VEGFR1-3, PDGFR, c-KIT, FLT3, RET, CSF-1R | RCC, neuroendocrine tumours |
| Vendatanib (Caprelsa) | VEGFR1-3, EGFR, RET | Medullary thyroid cancer in patients with unrespectable locally advanced or metastatic disease |
RCC, renal cell carcinoma.
In addition to the anti-angiogenic therapies described above, there is an additional class of inhibitors, the vascular disrupting agents (VDA) such as Zybrestat (combretastatin A-4 phosphate; CA4P) (Nathan et al, 2012). These work by selectively targeting immature, rapidly proliferating endothelial cells, inducing apoptosis or disrupting endothelial cytoskeleton resulting in vascular collapse (Nathan et al, 2012). CA4P has also been combined with bevacizumab in pre-clinical and clinical studies but had substantial toxicity (Nathan et al, 2012).
Three main vascular responses to anti-angiogenic therapy have been described clinically utilising MRI. These are reduced perfusion, no perfusion response and increased perfusion also referred to as vascular normalisation (Mehta et al, 2011; Van der Veldt et al, 2012; Batchelor et al, 2013). Many studies have identified reduced tumour perfusion as an early and most frequent response to anti-angiogenic therapy, these include studies where no samples with vascular normalisation were identified (Willett et al, 2004; Mehta et al, 2011; Van der Veldt et al, 2012). Consistent with the identified reduced perfusion, studies have shown robust and significant increases in hypoxia in approximately half the patients or more (Hattingen et al, 2011; Mehta et al, 2011; Yopp et al, 2011; DeLay et al, 2012). These include increased expression of a hypoxic gene signature in 10 of 21 patients (Mehta et al, 2011), increased immunohistochemical staining for CA9 and HIF averaging a threefold increase in 9 of 21 tumours (DeLay et al, 2012) and increased, relative tumour hypoxia as analysed by high-resolution T2 and T2′ mapping (Hattingen et al, 2011). Bevacizumab-induced reductions in tumour perfusion were significantly associated with increased HIF1α or CA9 expression in primary liver cancer (Yopp et al, 2011).
A few studies have identified vascular normalisation mainly at the start of therapy, in half the assessed tumours or less, but not in other tumours, which showed reduced perfusion or no perfusion response (Sorensen et al, 2012; Batchelor et al, 2013). Increased survival effects were identified in patients who responded to anti-angiogenic therapy with increased tumour perfusion, due to increased chemotherapeutic delivery (Batchelor et al, 2013).
Additional clinical studies directly examining the effects of anti-angiogenics on perfusion, hypoxia and metabolic profiles should be pursued to enable more meaningful conclusions regarding the frequency of the different responses across tumour types and anti-angiogenic therapies. However, given the opposing responses to anti-angiogenic therapy, it is clear that clinically useable biomarkers of individual longitudinal response to anti-angiogenics need to be developed and included as part of clinical practice with anti-angiogenics. This is required to personalise combination approaches, for example, to include radiotherapy and chemotherapy for the window of vascular normalisation if present and targeting of hypoxic/metabolic adaptation once reduced perfusion is identified.
Anti-angiogenic therapies increase progression-free survival in many tumour types and overall survival in a few tumours, but this is variable. For example, a phase III clinical trial in metastatic colorectal cancer identified an increase in progression-free survival from 6.2 to 10.6 months when patients were treated with bevacizumab in combination with irinotecan, fluorouracil and leucovorin. An increase in overall survival was also identified (10.6 versus 15.6 months) (Hurwitz et al, 2004; Mittal et al, 2014). Similar results are seen in other cancer types with increases in progression-free survival in most studies and increases in overall survival in some studies with bevacizumab and additional agents (Mittal et al, 2014).
What is intriguing is that there was no effect in most adjuvant studies, suggesting the paradigm of dormancy until VEGF is activated and there is an angiogenic switch, needs revising (Maru et al, 2013). A few studies have shown improved disease-free survival whilst on treatment, an effect that is lost or reversed once anti-angiogenic treatment is stopped (Mountzios et al, 2014). However two studies with bevacizumab in ovarian cancer showed increases of disease-free survival which were much shorter than the duration of treatment (Mountzios et al, 2014) suggesting an optimal duration rather than unceasing treatment may be the best fit, this is complicated by individual vascular and tumour responses. However, most studies show that continuing with anti-angiogenic therapy is of benefit to progression-free survival. This again highlights the requirement for incorporation of a robust biomarker of response for anti-angiogenic therapy.
Although increased metastasis with anti-angiogenic therapy was found in pre-clinical models, extensive reviews of patterns of relapse on and off bevacizumab do not support this pre-clinical observation (Welti et al, 2013). One possible explanation for this is the effect of additional chemotherapeutic drugs combined with anti-angiogenics in the clinic such as doxorubicin, topotecan and gemcitabine, which counteract the sunitinib-induced metastatic dissemination of the Lewis lung carcinoma xenograft models (Rovida et al, 2013).
To explain the lack of overall patient survival, many mechanisms of resistance to anti-angiogenic therapy have been identified which can explain vascularisation despite treatment (Bridges & Harris, 2011). One can propose two main types of resistance to anti-angiogenic therapy, which are intrinsic resistance and adaptive resistance (Bergers & Hanahan, 2008).
Intrinsic resistance is due to underlying molecular differences in the tumour and its vasculature. Potential causes of intrinsic resistance include the following: differential expression of the VEGF pro- and anti-angiogenic isoforms, VEGF and VEGFR polymorphisms, limited penetration of antibodies into the tumour mass and pre-existing tumour microenvironment which results in a lack of effect such as dependence on vessel co-option, vessels that are well differentiated, or stimulated by pathways other than VEGF will also be resistant (Bergers & Hanahan, 2008; Donnem et al, 2013; Welti et al, 2013; Stapor et al, 2014). Notch signalling can down-regulate VEGF signalling and induce resistance (Li et al, 2011).
Adaptive resistance is due to changes that occur as a result of the effects of the therapy on the tumour and its vasculature. Causes of adaptive resistance may include the following: tumour expression and metabolic reprogramming by HIF after induction of hypoxia, hypoxia-induced increased invasion or vessel co-option, up-regulation of alternative pro-angiogenic factors by the tumour or by the stromal cells such as TAM recruited by hypoxia, and increased recruitment of protective pericyte coverage may also occur (Bergers & Hanahan, 2008; Welti et al, 2013; Gilkes et al, 2014).
An additional factor confounding anti-angiogenic therapy is the identification of six distinct types of vessels that coexist within tumours. These are mother vessels, capillaries, glomeruloid microvascular proliferations and vascular malformations which develop by angiogenesis from pre-existing vessels and feeder arteries and draining veins which develop by arterio-venogenesis (Nagy et al, 2010). Some of these do not require VEGF-A for maintenance, making VEGF-targeting strategies redundant (Sitohy et al, 2012). Further to the resistance mechanisms that explain the lack of increase in overall patient survival, a number of clinical factors could reduce the quantifiable effects of anti-angiogenics, including the lack of patient enrichment with predictive biomarkers, patient crossover in clinical trials and subsequent therapies post progression.
Tumour response to anti-angiogenic therapy
Tumour microenvironmental and metabolic response to anti-angiogenic therapy can play an important role in resistance. Successful therapy against VEGF induces cell death and hypoxia because of reduction in vessel perfusion and will therefore activate gene transcription programmes that respond to hypoxia. These include HIF1 and HIF2, the unfolded protein response (UPR) (Rouschop et al, 2013) and ATF4 (Pike et al, 2012). Thus, in contrast to long-term development of cytotoxic drug resistance, hypoxia can induce resistance as part of the normal physiological response to reduced perfusion rapidly. This may partly explain the few weeks of overall survival or progression-free survival generally achieved.
Approaches to understanding tumour adaptation to anti-angiogenic therapy have utilised expression arrays of treated patient samples (DeLay et al, 2012), xenografts (Kumar et al, 2013; Gokmen-Polar et al, 2014; Sounni et al, 2014), post-treatment xenografts (Sounni et al, 2014) and xenograft models of resistance (Jahangiri et al, 2013). Additional investigations have utilised magnetic resonance spectrography (MRS) to examine the tumour metabolic responses to anti-angiogenic therapy in xenografts (Keunen et al, 2011) and clinical studies (Hattingen et al, 2011).
An increase in hypoxia and the hypoxia-regulated genes CA9 and c-Met was identified in bevacizumab-resistant glioblastomas after treatment (Jahangiri et al, 2013). Expression analysis of a bevacizumab-resistant glioblastoma cell line revealed that changes in genes regulating energy metabolism were predominant and that HIF was the key driver of identified changes. Genes regulating glycolysis and the PPP were increased, whereas genes regulating oxidative phosphorylation were decreased (Kumar et al, 2013). Transcription factors that regulate UPR in response to endoplasmic reticulum (ER) stress were increased in response to bevacizumab treatment (Kumar et al, 2013). Bevacizumab treatment of a glioblastoma cell line increased expression of HIF-regulated FABP3 and 7, which were responsible for lipid accumulation in hypoxia (Bensaad et al, 2014). A recent study investigated metabolic, proteomic and transcriptomic changes in cancer xenografts after withdrawal of anti-angiogenic therapy (sunitinib and sorafenib). Post-treatment xenograft metabolism shifted away from glycolysis and towards TCA and lipid metabolism following an initial decrease in genes regulating fatty acid metabolism during anti-angiogenic therapy (Kumar et al, 2013; Sounni et al, 2014).
MRS analysis of bevacizumab-treated tumours showed increased accumulation of metabolites previously associated with brain tumour hypoxia. These included increased lactate, alanine, choline and mobile lipids (Keunen et al, 2011). Bevacizumab increased hypoxia and impaired oxidative metabolism in a 31P/1H MRSI and MRI study of 16 glioblastoma patients (Hattingen et al, 2011).
Induced essentiality
The above term reflects a variation on the term synthetic lethality (Ashworth et al, 2011). The implication is that a treatment-induced pathway then becomes essential for tumour survival. Considering that most tumours already have areas of hypoxia, anti-VEGF therapy will induce even greater dependence on hypoxia adaptations. Thus, the response to bevacizumab is dependent upon tumour susceptibility to hypoxia-induced apoptosis (Selvakumaran et al, 2008). As pre-clinical and clinical data have shown an increase in tumour hypoxia, and a metabolic adaptation driven by HIF in response to anti-angiogenic therapy, it is likely that targeting vascularisation and hypoxic adaptation in combination will yield improved therapeutic results and may be able to substantially prolong the duration of effect. However, additional unbiased functional screening of survival mechanisms in hypoxia may also provide interesting, previously unrecognised, hits. Approaches targeting HIF-driven changes in combination with anti-angiogenic therapy have shown promising results in pre-clinical models.
Dichloroacetate (DCA), a PDK inhibitor, increases oxidative phosphorylation. DCA enhanced bevacizumab treatment of glioblastoma cell lines (Kumar et al, 2013) and overcame sorafenib resistance in hepatocellular carcinoma xenografts (Shen et al, 2013). DCA treatment reduced lactate, increased ROS and ATP levels and significantly potentiated apoptosis in combination with sorafenib (Shen et al, 2013). Knock-down or inhibition of CA9, a key pH regulatory enzyme, enhanced bevacizumab therapy of colon and glioblastoma xenografts (McIntyre et al, 2012).
Knock-down of HIF1α reduced growth in combination with anti-angiogenic therapy in neuroblastoma xenografts (Hartwich et al, 2013). Similarly, combining HIF1 inhibition, by the topoisomerase inhibitor topotecan, with bevacizumab increased their anti-tumour activity synergistically in neuroblastoma xenografts (Hartwich et al, 2013).
Knock-down or inhibition of c-Met in bevacizumab-resistant glioblastoma xenografts increased sensitivity to bevacizumab and reduced invasion and survival in hypoxia (Jahangiri et al, 2013). Inhibition of autophagy enhanced the anti-cancer effect of bevacizumab in hepatocellular carcinoma (Guo et al, 2013).
Adaptation to anti-angiogenic therapy (sunitinib) withdrawal increases metastatic dissemination and xenograft regrowth (Sounni et al, 2014). This could be reduced with fatty acid synthase (FASN) inhibition (orlistat) or knock-down after sunitinib withdrawal (Sounni et al, 2014).
Other targets in tumour metabolism and hypoxia have been investigated, but not yet in combination with anti-angiogenic therapy. These offer additional options for future combination investigations. One study utilised macrophages, which migrate to the hypoxic area of tumours, to target a hypoxia-induced therapeutic adenovirus to prostate cancers (Muthana et al, 2011). The use of HRE promoter sequences to target induction of gene therapy approaches in the hypoxic microenvironment is a possibility, but has major issues for delivery.
Many strategies to target HIF directly have been developed. These include the use of SN-38, a camptothecin analogue that reduces transcriptional induction of HIF1α (Jeong et al, 2014b), and PX-478, which reduced HIF1α protein levels and trans-activating capacity (Lee & Kim, 2011). A recent study identified a cyclic peptide inhibitor that specifically inhibits HIF1α binding to HIF1β, preventing HIF1α trans-activating capacity (Miranda et al, 2013).
In vitro investigations of additional metabolic enzyme inhibitors have shown an effect on reducing cell viability. An inhibitor of a HIF target gene, glucose transporter GLUT1 (STF-31), was identified in a screen to target VHL-deficient RCC (Chan et al, 2011). MCT1 inhibition by AZD3965 reduced xenograft growth rate and increased sensitivity to radiotherapy (Bola et al, 2014).
It is likely that metabolic inhibitors will only work on the subset of cancers for which these pathways are critical, but inducing hypoxia may make them more essential.
Clinical trials
There are few clinical trials combining inhibitors of hypoxia or metabolism with anti-angiogenics. We identified 11 on the clinical trials database ClinicalTrials.gov (Table2). NCT000520533 combined cG250, a non-inhibiting CAIX antibody, and sunitinib in advanced RCC; however, this study was terminated due to toxicity. NCT01578551 combines metformin with bevacizumab in advanced/metastatic pulmonary adenocarcinoma. An additional clinical trial is investigating the combination of metformin and everolimus in recurrent or progressive endometrial cancer (NCT01797523). Bevacizumab has also been combined with the c-Met inhibitor tivantinib (NCT10749384).
Table 2.
A list of clinical trails which combine targeting of HIF, HIF target genes, metabolism or hypoxia with antiangiogenic therapy.
| Clinical trials identifier | Phase | Treatment | Tumour type and setting | Response |
|---|---|---|---|---|
| NCT00520533 | cG250 and sunitinib | Advanced RCC | Study terminated due to toxicity | |
| NCT01578551 | II | Metformin plus paclitaxel/carboplatin/bevacizumab | Previously untreated advanced/metastatic pulmonary adenocarcinoma | Ongoing |
| NCT01797523 | II | Metformin plus everolimus and letroxole | Recurrent or progressive endometrial cancer | Ongoing |
| NCT01749384 | I | Bevacizumab and tivantinib (c-MET inhibitor) | Solid tumours that are metastatic or cannot be removed by surgery | Ongoing |
| NCT01497444 | I/II | Sorafenib and TH-302 | Advanced kidney cancer or liver cancer | Ongoing |
| NCT01381822 | I | TH-302 in combination with sunitinib | Advanced RCC, GISTs and pancreatic neuroendocrine tumours | Ongoing |
| NCT01403610 | II | Bevacizumab followed by TH-302 | Recurrent high grade astrocytoma | Ongoing |
| NCT01485042 | I | Pazopanib plus TH-302 | Advanced solid tumours | Ongoing |
| NCT00548418 | II | Bevacizumab and topotecan with cisplatin | Recurrent/persistent cervical cancer | Ongoing |
| NCT00671112 | I | Everolimus and bortezomib | Relapsed or refractory lymphoma | Ongoing |
| NCT02142803 | I | Bevacizumab and MLN0128 (mTOR inhibitor) | Recurrent glioblastoma or advanced solid tumours | Ongoing |
RCC, renal cell carcinoma; GISTs, gastrointestinal stromal tumours.
There are four phase I/II clinical trials combining the hypoxia-activated prodrug TH-302, an alkylating agent, (Chawla et al, 2014) with an anti-angiogenic therapy in a variety of tumours (NCT01497444, NCT01381822, NCT01403610, NCT01485042). There is an additional study combining bevacizumab with cisplatin and the HIF inhibitor topotecan (NCT0054818). The proteasome inhibitor bortezomib, which represses HIF protein expression (Befani et al, 2012), is being trialed in combination with everolimus in relapsed or refractory-free lymphoma (NCT00671112). Bevacizumab is also being combined with the mTOR inhibitor, MLN0128 (NCT02142803). Targeting mTOR has dual effects, as it regulates HIF expression and is an independent metabolism regulator (Shimobayashi & Hall, 2014). A completed phase I clinical trial in solid tumours combined bevacizumab with SN-38, a camptothecin analogue which reduced expression and transcriptional activity of HIF1α (Jeong et al, 2014a).
There are a number of clinical trials investigating targeting hypoxia or metabolism without anti-angiogenic therapy. There are 20 TH-302 hypoxia prodrug entries on clinical trials.gov. An additional study targeted HIF1α directly with EZN-2968 identifying a variety of patient tumour responses from 94% reduction in HIF1α expression to increased expression (Jeong et al, 2014b).
Clinical trials are investigating metformin in recurrent ovarian, fallopian tube or primary peritoneal cancer, and pancreatic cancer (NCT02050009, NCT02122185, NCT01666730, NCT01954732). The inhibitor of autophagy hydroxychloroquine is being used before surgery as a single agent in patients with primary RCC (NCT01144169). PFK-158 an inhibitor of PFKFB3 is in a phase I clinical trial (NCT02044861). CB-839 an inhibitor of glutaminase, required for glutamine conversion to glutamate, is in a number of clinical trials including in solid tumours (NCT02071862). There is a clinical trial of the MCT1 inhibitor AZD3965 in patients with advanced cancer (NCT01791595). There is also a clinical trial of a CA9 inhibitor DTP-348 (NCT02216669). It is also important to consider the effect of any metabolic targeting therapy on endothelial cell metabolism and the impact of this on angiogenesis (Goveia et al, 2014). Clearly, once a dose is safely selected, combination studies with anti-angiogenic drugs should be a high priority.
Patient selection
Given the interrelatedness of vascular, hypoxic and metabolic tumour responses to anti-angiogenic therapy, it may be important to measure all three. It would be best to target normoxic tumour metabolism if the vasculature is normalised for the time frame of this response followed by targeting hypoxic tumour response when tumour perfusion is reduced. Imaging tumour perfusion and hypoxia over the treatment course would be required to find the best-fit treatment. Diffusion contrast-enhanced magnetic resonance imaging (DCE-MRI), with gadolinium contrast agent, enables non-invasive measurements of tumour perfusion and has been utilised to assess patient response to pre-operative chemotherapy with bevacizumab (De Bruyne et al, 2012).
Enhanced glucose uptake can be monitored by imaging fluorodeoxyglucose-PET (FDG-PET). FDG-PET is used to measure drug response (Vera et al, 2014). MRS analysis of cediranib treatment of glioblastoma patient tumours showed that a high n-acetylaspartate (NAA)/choline ratio was associated with increased overall survival (Kim et al, 2011). Additional metabolic tracers, for use in PET imaging, important in hypoxic and/or anti-angiogenic therapy response such as glutamine, would also be interesting to investigate and are in development (Schulze & Harris, 2012).
Clinical trials investigating the use of markers of hypoxia in response to anti-angiogenic therapy include using 18F-FMISO-PET to provide a hypoxic index in cerebral tumours (NCT01200134). FMISO-PET and vascular MRI scans are being utilised to explore blood flow, volume and hypoxia in bevacizumab-treated glioblastoma patients (NCT02076152). It is also important to investigate HIF response, which will have an impact on changes in gene expression patterns and therefore molecular physiology. Investigation of CA9 expression by in vivo imaging with radiolabeled cG250 (Stillebroer et al, 2013), immunohistochemistry in biopsies or measurements of hypoxia-induced proteins in the blood (Takacova et al, 2013) may provide HIF response information. Imaging pH in patient samples may act as a marker for susceptibility to targeting pH regulation or for samples with high metabolic rates or high levels of hypoxia. A number of strategies for imaging pH in patients have been and are being developed (Wu et al, 2013). Analysis of metabolites in the blood or urine of patients may also enable patient selection for targeted therapies (Lodi et al, 2013). Expression analysis of hypoxic gene signatures in patient baseline biopsies and biopsies during therapy would identify tumour response (Fox et al, 2014).
As changes in DCE-MRI occur within 1 week of anti-VEGF therapy (Ferl et al, 2015) and the xenografts show rapid changes, it should be a priority to examine short-term induced adaptations to help determine the most appropriate therapy for the adapted tumour, by imaging or biopsies, or peripheral blood markers.
Future directions
It is clear that anti-angiogenic therapy, alone or with therapies targeting fast growing tumour cells, is not going to be curative in the majority of tumour patients. As with nearly all areas of cancer biology, the complexity, heterogeneity and plasticity of tumour cells and their environment were underestimated.
Given the prognostic implications of inducing the hypoxic tumour response, the utilisation of expensive and toxic drugs without selecting the correct patients can be prevented with appropriate designs for the use of precision medicine.
Targeting tumour metabolic reprogramming is a fast developing area of translational biology, and its relationship with angiogenesis and hypoxia is intertwined. It is important that the relationship between other targeting strategies and microenvironmental heterogeneity is investigated. The metabolic effects and oxygen, pH and nutritional gradients are recapitulated far better in more complex, 3-dimensional co-culture models (Thoma et al, 2014), which with analytical methods that enable more tumour-like in vitro analysis need to be more widely used. The use of pre-selection ‘window’ (between first diagnosis and tumour resection) trials for combination therapies could reduce long-term incorrect treatment strategies for patients if early response stratifying biomarkers can be developed. Furthermore, heterogeneous tumour metabolic and hypoxic responses will greatly impact the success of combination approaches and understanding fully which variations are important therapeutically and how to incorporate identification of these into treatment regimes will require further investigation.
There now exists a dichotomous future to ‘anti-angiogenic’ therapy, (perhaps better referred to as vascular adjustment therapy) focused on either increasing the effects of vascular normalisation to increase perfusion or reducing vascular perfusion and treating in combinations including hypoxia/metabolic targeting agents. Vascular normalisation has two key benefits. The first is increased delivery of chemotherapy, which has been identified to improve survival, and oxygen, which will increase the efficacy of radiotherapy. The second benefit is the reduction of hypoxic adaptation, which will reduce the negative aspects to the patient of selecting a more aggressive tumour. Reducing vascular perfusion has the advantage of the induced essentiality approaches discussed here.
Further problems remain. We cannot decipher in advance which type of vascular response patients will have. Understanding the mechanisms behind the different positive vascular responses and producing a clinically useable biomarker is a key area for progress in the paradigm of induced essentiality as one approach to personalised cancer medicine. Furthermore, there remains a subset of patients for which there will be no vascular response. Controlled clinical analysis of the effects of differing anti-angiogenics particularly given the more general tumour effects of the RTKi approaches and the impact these may have on heterogeneous subsets of patients is required.
To take advantage of the induced essentiality opportunities that arise from reduced perfusion, the field should focus on approaches that are likely to produce a kill effect rather than inhibit proliferation, as may be the case with many metabolic targeting strategies.
Utilising a hypoxia-activated prodrug such as TH-302 is an exciting approach, and the four on-going clinical trials combining this with anti-angiogenic therapies may provide strong support for the rationale. Regarding metabolic inhibitors, an example of the desired effect of apoptotic induction was identified in xenograft studies with DCA in combination with sorafenib (Shen et al, 2013). This may be due to the effects of DCA on multiple metabolic pathways. With this in mind, an area for possible exploitation in this combination approach is that of epigenetic modulation of gene expression patterns, enabling changes in a large number of ‘essential’ genes with a single inhibitor. Identification of epigenetic modulators of global hypoxic/metabolic gene expression patterns would facilitate more global hypoxia/metabolic targeting in combination with anti-angiogenics.
Pending issues
Can we better comprehend the mechanistic differences for the different vascular responses of vascular normalisation versus reduced perfusion?
Based on this understanding can early predictors of response to anti-angiogenic therapy be identified and incorporated clinically?
Will anti-angiogenic therapy and hypoxia/metabolic targeting combination approaches be as successful as pre-clinical data suggests?
Acknowledgments
This work was supported by funds from Cancer Research UK, Breast Cancer Research Foundation, Oxford NHS Biomedical Research Centre and CRUK Imaging and Cancer Centre.
Glossary
- Angiogenesis
The process of growing additional blood vessels.
- Anti-angiogenic therapies
Drugs that target the tumour blood vessels and prevent the formation of new blood vessels into the tumour.
- Hypoxia
Conditions of low oxygen and nutrients.
- Hypoxia-inducible factor (HIF)
The key regulator of the changes in gene expression that occur in conditions of low oxygen.
- Hypoxic and metabolic adaptation to anti-angiogenic therapy
Changes that occur in tumour cells in response to reduced blood flow that allow them to survive in conditions of low oxygen such as in the way they make energy and the building blocks to make more cells.
- Induced essentiality
Making tumour cells dependent on a pathway/protein for their ability to grow and survive by changing their microenvironment with a therapy.
- Patient selection
Patients do not respond in the same way to drugs including anti-angiogenic therapy. It is important to choose how to treat patients based on how they will respond.
- Tumour metabolism
Tumours change their metabolism to increase their ability to divide more rapidly by increasing the amount of cell building blocks they make and also protect them from free radical damage.
- Vascular endothelial growth factor (VEGF)
The key regulator of angiogenesis.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13:245–251. doi: 10.2174/1568009611313030003. [DOI] [PubMed] [Google Scholar]
- Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell. 2011;145:30–38. doi: 10.1016/j.cell.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Balgi AD, Diering GH, Donohue E, Lam KK, Fonseca BD, Zimmerman C, Numata M, Roberge M. Regulation of mTORC1 signaling by pH. PLoS ONE. 2011;6:e21549. doi: 10.1371/journal.pone.0021549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, Ancukiewicz M, Polaskova P, Pinho MC, Jennings D, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befani CD, Vlachostergios PJ, Hatzidaki E, Patrikidou A, Bonanou S, Simos G, Papandreou CN, Liakos P. Bortezomib represses HIF-1alpha protein expression and nuclear accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in prostate cancer cells. J Mol Med (Berl) 2012;90:45–54. doi: 10.1007/s00109-011-0805-8. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li JL, et al. Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9:349–365. doi: 10.1016/j.celrep.2014.08.056. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Harris AL. Hypoxia and metabolism in cancer. Adv Exp Med Biol. 2014;772:1–39. doi: 10.1007/978-1-4614-5915-6_1. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bola BM, Chadwick AL, Michopoulos F, Blount KG, Telfer BA, Williams KJ, Smith PD, Critchlow SE, Stratford IJ. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Ther. 2014;13:2805–2816. doi: 10.1158/1535-7163.MCT-13-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Bellot G, Pouyssegur J. Hypoxia and energetic tumour metabolism. Curr Opin Genet Dev. 2011;21:67–72. doi: 10.1016/j.gde.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Bridges EM, Harris AL. The angiogenic process as a therapeutic target in cancer. Biochem Pharmacol. 2011;81:1183–1191. doi: 10.1016/j.bcp.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3:94ra70. doi: 10.1126/scitranslmed.3002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla SP, Cranmer LD, Van Tine BA, Reed DR, Okuno SH, Butrynski JE, Adkins DR, Hendifar AE, Kroll S, Ganjoo KN. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J Clin Oncol. 2014;32:3299–3306. doi: 10.1200/JCO.2013.54.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry H, Schodel J, Oikonomopoulos S, Camps C, Grampp S, Harris AL, Ratcliffe PJ, Ragoussis J, Mole DR. Extensive regulation of the non-coding transcriptome by hypoxia: role of HIF in releasing paused RNApol2. EMBO Rep. 2014;15:70–76. doi: 10.1002/embr.201337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K, Mazzone M, Carmeliet P. Anti-angiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8:393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- De Bruyne S, Van Damme N, Smeets P, Ferdinande L, Ceelen W, Mertens J, Van de Wiele C, Troisi R, Libbrecht L, Laurent S, et al. Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer. 2012;106:1926–1933. doi: 10.1038/bjc.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLay M, Jahangiri A, Carbonell WS, Hu YL, Tsao S, Tom MW, Paquette J, Tokuyasu TA, Aghi MK. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to anti-angiogenic therapy. Clin Cancer Res. 2012;18:2930–2942. doi: 10.1158/1078-0432.CCR-11-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnem T, Hu J, Ferguson M, Adighibe O, Snell C, Harris AL, Gatter KC, Pezzella F. Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med. 2013;2:427–436. doi: 10.1002/cam4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro E, Bensaad K, Chong MG, Tennant DA, Ferguson DJ, Snell C, Steers G, Turley H, Li JL, Gunther UL, et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012;16:751–764. doi: 10.1016/j.cmet.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Favaro E, Lord S, Harris AL, Buffa FM. Gene expression and hypoxia in breast cancer. Genome Med. 2011;3:55. doi: 10.1186/gm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferl GZ, O'Connor JP, Parker GJ, Carano RA, Acharya SJ, Jayson GC, Port RE. Mixed-effects modeling of clinical DCE-MRI data: application to colorectal liver metastases treated with bevacizumab. J Magn Reson Imaging. 2015;41:132–141. doi: 10.1002/jmri.24514. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Fox NS, Starmans MH, Haider S, Lambin P, Boutros PC. Ensemble analyses improve signatures of tumour hypoxia and reveal inter-platform differences. BMC Bioinformatics. 2014;15:170. doi: 10.1186/1471-2105-15-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Man S, Chen L, Emmenegger U, Shaked Y, Cheung AM, Brown AS, Hicklin DJ, Foster FS, Kerbel RS. Targeted anti-vascular endothelial growth factor receptor-2 therapy leads to short-term and long-term impairment of vascular function and increase in tumor hypoxia. Cancer Res. 2006;66:3639–3648. doi: 10.1158/0008-5472.CAN-05-3295. [DOI] [PubMed] [Google Scholar]
- Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokmen-Polar Y, Goswami CP, Toroni RA, Sanders KL, Mehta R, Sirimalle U, Tanasa B, Shen C, Li L, Ivan M, et al. Gene expression analysis reveals distinct pathways of resistance to bevacizumab in xenograft models of human ER-positive breast cancer. J Cancer. 2014;5:633–645. doi: 10.7150/jca.8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveia J, Stapor P, Carmeliet P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol Med. 2014;6:1105–1120. doi: 10.15252/emmm.201404156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XL, Li D, Sun K, Wang J, Liu Y, Song JR, Zhao QD, Zhang SS, Deng WJ, Zhao X, et al. Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J Mol Med (Berl) 2013;91:473–483. doi: 10.1007/s00109-012-0966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hartwich J, Orr WS, Ng CY, Spence Y, Morton C, Davidoff AM. HIF-1alpha activation mediates resistance to anti-angiogenic therapy in neuroblastoma xenografts. J Pediatr Surg. 2013;48:39–46. doi: 10.1016/j.jpedsurg.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingen E, Jurcoane A, Bahr O, Rieger J, Magerkurth J, Anti S, Steinbach JP, Pilatus U. Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro Oncol. 2011;13:1349–1363. doi: 10.1093/neuonc/nor132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Jahangiri A, De Lay M, Miller LM, Carbonell WS, Hu YL, Lu K, Tom MW, Paquette J, Tokuyasu TA, Tsao S, et al. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of anti-angiogenic therapy resistance. Clin Cancer Res. 2013;19:1773–1783. doi: 10.1158/1078-0432.CCR-12-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Park SR, Rapisarda A, Fer N, Kinders RJ, Chen A, Melillo G, Turkbey B, Steinberg SM, Choyke P, et al. Weekly EZN-2208 (PEGylated SN-38) in combination with bevacizumab in patients with refractory solid tumors. Invest New Drugs. 2014a;32:340–346. doi: 10.1007/s10637-013-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Rapisarda A, Park SR, Kinders RJ, Chen A, Melillo G, Turkbey B, Steinberg SM, Choyke P, Doroshow JH, et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2014b;73:343–348. doi: 10.1007/s00280-013-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Catana C, Ratai EM, Andronesi OC, Jennings DL, Batchelor TT, Jain RK, Sorensen AG. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71:3745–3752. doi: 10.1158/0008-5472.CAN-10-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Kumar K, Wigfield S, Gee HE, Devlin CM, Singleton D, Li JL, Buffa F, Huffman M, Sinn AL, Silver J, et al. Dichloroacetate reverses the hypoxic adaptation to bevacizumab and enhances its antitumor effects in mouse xenografts. J Mol Med (Berl) 2013;91:749–758. doi: 10.1007/s00109-013-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Kim HM. A novel approach to cancer therapy using PX-478 as a HIF-1alpha inhibitor. Arch Pharmacal Res. 2011;34:1583–1585. doi: 10.1007/s12272-011-1021-3. [DOI] [PubMed] [Google Scholar]
- Li JL, Sainson RC, Oon CE, Turley H, Leek R, Sheldon H, Bridges E, Shi W, Snell C, Bowden ET, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71:6073–6083. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi A, Tiziani S, Khanim FL, Gunther UL, Viant MR, Morgan GJ, Bunce CM, Drayson MT. Proton NMR-based metabolite analyses of archived serial paired serum and urine samples from myeloma patients at different stages of disease activity identifies acetylcarnitine as a novel marker of active disease. PLoS ONE. 2013;8:e56422. doi: 10.1371/journal.pone.0056422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Metabolic asymmetry in cancer: a “balancing act” that promotes tumor growth. Cancer Cell. 2014;26:5–7. doi: 10.1016/j.ccr.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Maru D, Venook AP, Ellis LM. Predictive biomarkers for bevacizumab: are we there yet? Clin Cancer Res. 2013;19:2824–2827. doi: 10.1158/1078-0432.CCR-12-3409. [DOI] [PubMed] [Google Scholar]
- McIntyre A, Patiar S, Wigfield S, Li JL, Ledaki I, Turley H, Leek R, Snell C, Gatter K, Sly WS, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res. 2012;18:3100–3111. doi: 10.1158/1078-0432.CCR-11-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Hughes NP, Buffa FM, Li SP, Adams RF, Adwani A, Taylor NJ, Levitt NC, Padhani AR, Makris A, et al. Assessing early therapeutic response to bevacizumab in primary breast cancer using magnetic resonance imaging and gene expression profiles. J Natl Cancer Inst Monogr. 2011;2011:71–74. doi: 10.1093/jncimonographs/lgr027. [DOI] [PubMed] [Google Scholar]
- Miranda E, Nordgren IK, Male AL, Lawrence CE, Hoakwie F, Cuda F, Court W, Fox KR, Townsend PA, Packham GK, et al. A cyclic peptide inhibitor of HIF-1 heterodimerization that inhibits hypoxia signaling in cancer cells. J Am Chem Soc. 2013;135:10418–10425. doi: 10.1021/ja402993u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41:235–251. doi: 10.1053/j.seminoncol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Mountzios G, Pentheroudakis G, Carmeliet P. Bevacizumab and micrometastases: revisiting the preclinical and clinical rollercoaster. Pharmacol Ther. 2014;141:117–124. doi: 10.1016/j.pharmthera.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Radons J, Vaupel P. Critical role of aberrant angiogenesis in the development of tumor hypoxia and associated radioresistance. Cancers. 2014;6:813–828. doi: 10.3390/cancers6020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthana M, Giannoudis A, Scott SD, Fang HY, Coffelt SB, Morrow FJ, Murdoch C, Burton J, Cross N, Burke B, et al. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 2011;71:1805–1815. doi: 10.1158/0008-5472.CAN-10-2349. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Semin Thromb Hemost. 2010;36:321–331. doi: 10.1055/s-0030-1253454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan P, Zweifel M, Padhani AR, Koh DM, Ng M, Collins DJ, Harris A, Carden C, Smythe J, Fisher N, et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2012;18:3428–3439. doi: 10.1158/1078-0432.CCR-11-3376. [DOI] [PubMed] [Google Scholar]
- Parks SK, Chiche J, Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–623. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- Pezzella F, Harris AL. When cancer co-opts the vasculature. N Engl J Med. 2014;370:2146–2147. doi: 10.1056/NEJMcibr1402407. [DOI] [PubMed] [Google Scholar]
- Pike LRG, Phadwal K, Simon AK, Harris AL. ATF4 orchestrates a program of BH3-only protein expression in severe hypoxia. Mol Biol Rep. 2012;39:10811–10822. doi: 10.1007/s11033-012-1975-3. [DOI] [PubMed] [Google Scholar]
- Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol. 2013;85:1219–1226. doi: 10.1016/j.bcp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Ros S, Schulze A. Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases in cancer metabolism. Cancer Metab. 2013;1:8. doi: 10.1186/2049-3002-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J, van der Kogel AJ, Koritzinsky M, Wouters BG. PERK/eIF2 alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc Natl Acad Sci U S A. 2013;110:4622–4627. doi: 10.1073/pnas.1210633110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovida A, Castiglioni V, Decio A, Scarlato V, Scanziani E, Giavazzi R, Cesca M. Chemotherapy counteracts metastatic dissemination induced by anti-angiogenic treatment in mice. Mol Cancer Ther. 2013;12:2237–2247. doi: 10.1158/1535-7163.MCT-13-0244. [DOI] [PubMed] [Google Scholar]
- Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- Selvakumaran M, Yao KS, Feldman MD, O'Dwyer PJ. Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxia-induced apoptosis. Biochem Pharmacol. 2008;75:627–638. doi: 10.1016/j.bcp.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- Shen C, Kaelin WG., Jr The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2013;23:18–25. doi: 10.1016/j.semcancer.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YC, Ou DL, Hsu C, Lin KL, Chang CY, Lin CY, Liu SH, Cheng AL. Activating oxidative phosphorylation by a pyruvate dehydrogenase kinase inhibitor overcomes sorafenib resistance of hepatocellular carcinoma. Br J Cancer. 2013;108:72–81. doi: 10.1038/bjc.2012.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- Singleton DC, Rouhi P, Zois CE, Haider S, Li JL, Kessler BM, Cao Y, Harris AL. Hypoxic regulation of RIOK3 is a major mechanism for cancer cell invasion and metastasis. Oncogene. 2014 doi: 10.1038/onc.2014.396. , doi: 10.1038/onc.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen AG, Emblem KE, Polaskova P, Jennings D, Kim H, Ancukiewicz M, Wang M, Wen PY, Ivy P, Batchelor TT, et al. Increased survival of glioblastoma patients who respond to anti-angiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE, Cimino J, Blacher S, Primac I, Truong A, Mazzucchelli G, Paye A, Calligaris D, Debois D, De Tullio P, et al. Blocking lipid synthesis overcomes tumor regrowth and metastasis after anti-angiogenic therapy withdrawal. Cell Metab. 2014;20:280–294. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Stapor P, Wang X, Goveia J, Moens S, Carmeliet P. Angiogenesis revisited – role and therapeutic potential of targeting endothelial metabolism. J Cell Sci. 2014;127:4331–4341. doi: 10.1242/jcs.153908. [DOI] [PubMed] [Google Scholar]
- Stillebroer AB, Franssen GM, Mulders PF, Oyen WJ, van Dongen GA, Laverman P, Oosterwijk E, Boerman OC. ImmunoPET imaging of renal cell carcinoma with (124)I- and (89)Zr-labeled anti-CAIX monoclonal antibody cG250 in mice. Cancer Biother Radiopharm. 2013;28:510–515. doi: 10.1089/cbr.2013.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RC, Denko NC. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 2014;19:285–292. doi: 10.1016/j.cmet.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130099. doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacova M, Bartosova M, Skvarkova L, Zatovicova M, Vidlickova I, Csaderova L, Barathova M, Breza J, Bujdak P, Pastorek J, et al. Carbonic anhydrase IX is a clinically significant tissue and serum biomarker associated with renal cell carcinoma. Oncol Lett. 2013;5:191–197. doi: 10.3892/ol.2012.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma CR, Zimmermann M, Agarkova I, Kelm JM, Krek W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv Drug Deliv Rev. 2014;69–70:29–41. doi: 10.1016/j.addr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, Hendrikse NH, Eriksson J, Windhorst AD, Postmus PE, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21:82–91. doi: 10.1016/j.ccr.2011.11.023. [DOI] [PubMed] [Google Scholar]
- Vera P, Dubray B, Palie O, Buvat I, Hapdey S, Modzelewski R, Benyoucef A. Rousseau C, Meyer ME, Bardet S, et al. Monitoring tumour response during chemo-radiotherapy: a parametric method using FDG-PET/CT images in patients with oesophageal cancer. EJNMMI Res. 2014;4:12. doi: 10.1186/2191-219X-4-12. , . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and anti-angiogenic therapies in cancer. J Clin Invest. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer (vol 10, pg 145, 2004) Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang W, Li J, Zhang Y. Optical imaging of tumor microenvironment. Am J Nucl Med Mol Imaging. 2013;3:1–15. [PMC free article] [PubMed] [Google Scholar]
- Yopp AC, Schwartz LH, Kemeny N, Gultekin DH, Gonen M, Bamboat Z, Shia J, Haviland D, D'Angelica MI, Fong Y, et al. Anti-angiogenic therapy for primary liver cancer: correlation of changes in dynamic contrast-enhanced magnetic resonance imaging with tissue hypoxia markers and clinical response. Ann Surg Oncol. 2011;18:2192–2199. doi: 10.1245/s10434-011-1570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]