Abstract
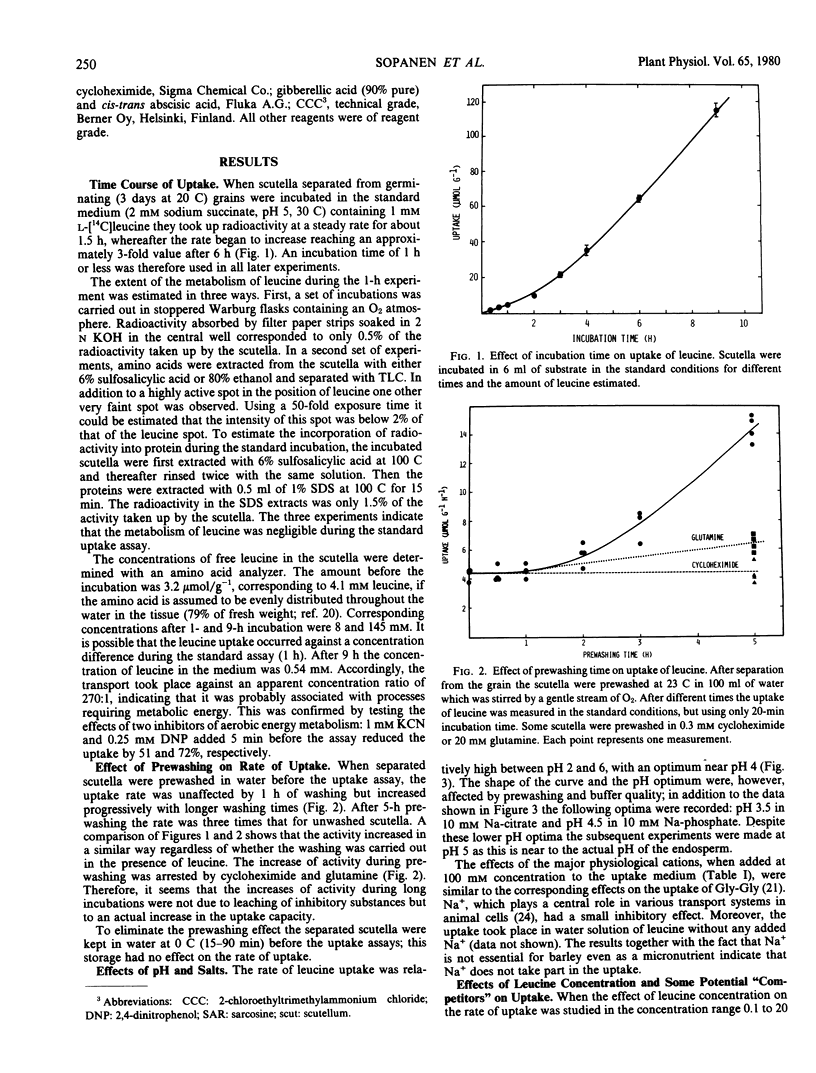
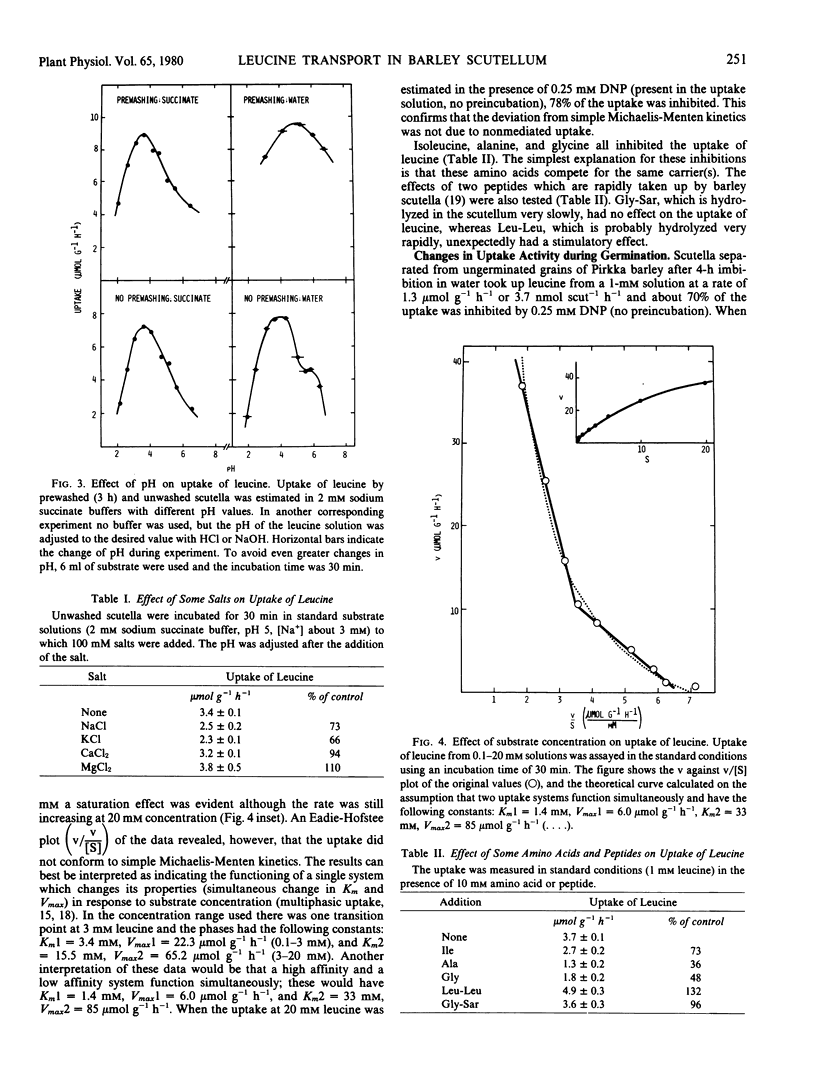
Scutella separated from grains of Himalaya barley after germination for 3 days rapidly took up l-leucine from aerated incubation media; with 1 millimolar leucine the rate varied between 4 and 14 micromoles per gram per hour and the pH optimum was at 3.5 to 5, both depending on buffer composition and prewashing time. The rate of the uptake increased with increasing concentration of leucine in a complex manner, which could be interpreted as multiphasic kinetics with apparent Km values of 3.4 and 15.5 millimolar below and above 3 millimolar leucine, respectively. The uptake took place against a concentration difference (highest estimated ratio 270: 1) and was strongly inhibited by dinitrophenol. Uptake was apparently due to active transport requiring metabolic energy.
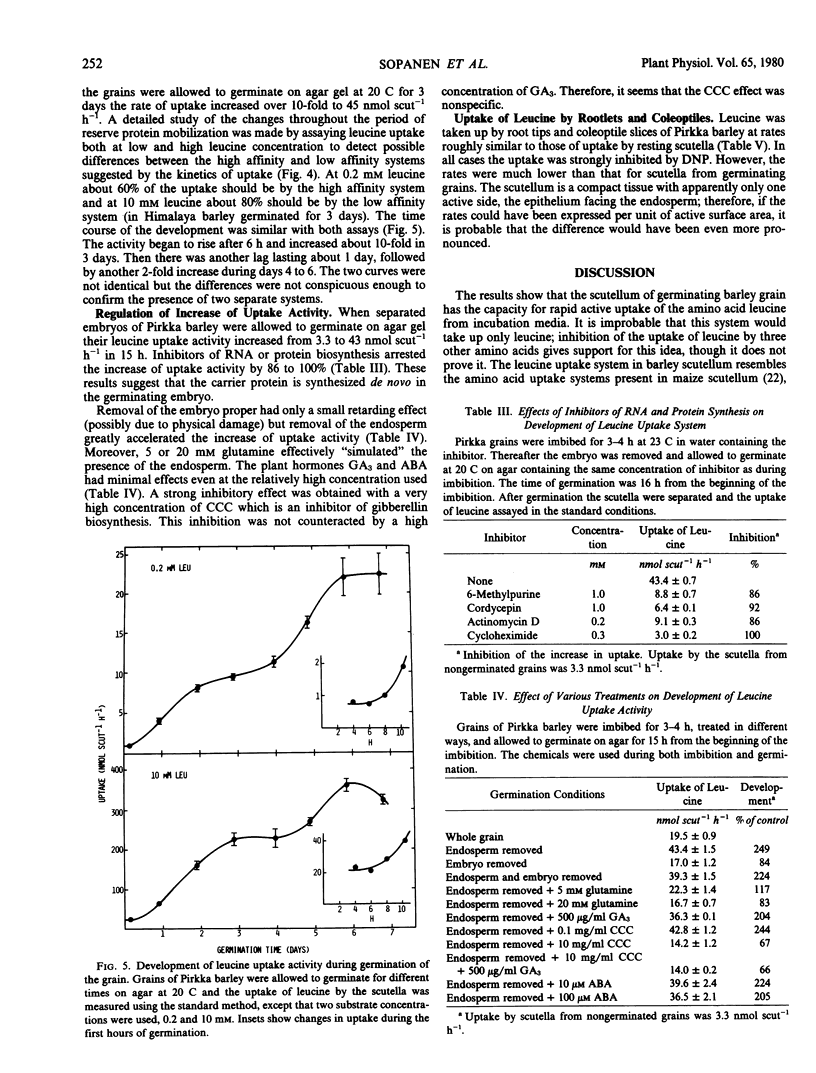
The development of the uptake activity during germination was studied using Pirkka barley. A low activity was present in the scutella of ungerminated grains. It began to increase after 6 hours imbibition, and the increase was biphasic, the major changes occurring during days 0 to 3 and 4 to 6. The total increase was about 20-fold.
The regulation of the development was studied by allowing separated embryos to germinate on agar gel. The increase of uptake activity was strongly inhibited by inhibitors of RNA or protein synthesis. Increase did not require the presence of the embryo proper, and was not affected by gibberellic or abscisic acid. Removal of the endosperm greatly accelerated the increase of uptake activity, and the presence of 5 or 20 millimolar glutamine counteracted the removal of the endosperm. The results suggest that the availability of glutamine or amino acids in general in the endosperm may regulate the development or the activity of the transport system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheruel J., Jullien M. Amino Acid Uptake into Cultivated Mesophyll Cells from Asparagus officinalis L. Plant Physiol. 1979 Apr;63(4):621–626. doi: 10.1104/pp.63.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y. N., Nobel P. S. Amino Acid uptake by pea leaf fragments: specificity, energy sources, and mechanism. Plant Physiol. 1973 Dec;52(6):633–637. doi: 10.1104/pp.52.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopanen T., Burston D., Matthews D. M. Uptake of small peptides by the scutellum of germinating barley. FEBS Lett. 1977 Jul 1;79(1):4–7. doi: 10.1016/0014-5793(77)80337-2. [DOI] [PubMed] [Google Scholar]
- Sopanen T., Burston D., Taylor E., Matthews D. M. Uptake of glycylglycine by the scutellum of germinating barley grain. Plant Physiol. 1978 Apr;61(4):630–633. doi: 10.1104/pp.61.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopanen T. Development of Peptide Transport Activity in Barley Scutellum during Germination. Plant Physiol. 1979 Oct;64(4):570–574. doi: 10.1104/pp.64.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R. Some characteristics of the uptake of glutamine by corn scutellum. Plant Physiol. 1971 Jan;47(1):157–161. doi: 10.1104/pp.47.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegman T., Woldring M. G., Pratt J. J. A new cocktail for liquid scintillation counting of aqueous radioimmunoassay-samples. Clin Chim Acta. 1975 Mar 24;59(3):347–356. doi: 10.1016/0009-8981(75)90010-8. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Cellular transport mechanisms. Annu Rev Biochem. 1978;47:933–965. doi: 10.1146/annurev.bi.47.070178.004441. [DOI] [PubMed] [Google Scholar]



