Abstract
Experimental evidence suggested that sodium ferulate (SF) and oxymatrine (OMT) combination had synergistic anti-inflammatory and antioxidant effects. We hypothesized that SF and OMT combination treatment might have protective effects on paraquat-induced acute lung injury. In our study, the Swiss mice were randomly divided into seven groups, including control, paraquat (PQ), SF (6.2 mg/Kg/day); OMT (13.8 mg/Kg/day) and three SF+OMT groups (3.1 + 6.9; 6.2 + 13.8 and 12.3 + 27.7 mg/Kg/day). The mortality and death time were monitored. Sprague-Dawley rats were randomly divided into seven groups including control, PQ, SF (3.1 mg/Kg/day); OMT (6.9 mg/Kg/day) and three SF+OMT groups (1.6 + 3.4; 3.1 + 6.9 and 6.2 + 13.8 mg/Kg/day). The lung wet/dry weight (W/D) ratio, lung histopathologic changes, C-reactive protein (CRP), interleukin-6 (IL-6), nuclear factor κB (NF-κB), malondialdehyde (MDA) and superoxidase dismutase (SOD) were analysed. Compared with PQ group, the mortality significantly decreased and the death time prolonged in SF and OMT combination treatment groups of mice. Also in SF and OMT combination treatment groups of rats, the increased lung W/D ratio and histopathological score induced by PQ injection were significantly decreased; the levels of CRP, IL-6, NF-κB and MDA in serum and lung homogenate were significantly decreased; the SOD activities in serum and lung homogenate were improved. These results suggested that SF and OMT combination had an obvious protective effect on PQ-induced lung injury. The anti-inflammatory and antioxidant effect might be involved in the mechanism.
Key Words: Sodium ferulate, Oxymatrine, Paraquat, Lung, Inflammation
Introduction
It is well known that the lung is one of the friable organs which can be injured by many factors, such as virus, bacteria, Lipopolysaccharide (LPS) and some chemical substances. Paraquat (1,1’-dimethyl-4,4’-bipyridinium dichloride, PQ) is a nonselective herbicide widely used for its high efficiency and low residues in agriculture worldwide. PQ intoxication, after intentional or accidental ingestion, usually led to lung injury (1, 2) and importantly, the death was mainly caused by irreversible respiratory failure (3, 4). It was reported that the mortality of PQ intoxication patients was as high as 55.2% (5). Many researchers (6, 7) believed that the production of superoxide radicals and its triggered oxidative damage, oxidative stress-related cell damage, and followed inflammatory reaction were mainly associated with the mechanisms of PQ-induced lung injury. The pathophysiologic progress including inflammatory infiltration into the alveolar spaces, excessive collagen deposition and proliferation of the pulmonary edema fluid ultimately lead to pulmonary fibrosis and respiratory failure. Now, some anti-inflammatory and antioxidant drugs had been demonstrated curative effects for PQ intoxication, such as dexamethasone and methylprednisolone which could reduce the inflammatory reaction, including reducing pulmonary vascular permeability and pulmonary fibrosis (8, 9).
In our previous studies, we found that sodium ferulate (SF) and oxymatrine (OMT) combination had remarkable synergetic anti-inflammatory and antioxidant effects (10). So we hypothesized that SF and OMT combination treatment might alleviate PQ-induced lung injury. In this study, the mice and rats PQ intoxication models were duplicated to observe the protective effect of SF and OMT combination, and it’s anti-inflammatory and antioxidant mechanism were also investigated. We hoped these data would provide experimental evidence in a new lung injury protection drug development.
Experimental
Drugs and chemicals
SF (molecular formula, C10H9NaO4·2H2O; molecular weight, 252.20; CAS, 24276-84-4; HPLC purity > 99%), OMT (molecular formula, C15H24N2O2·H2O; molecular weight, 282.38; CAS, 16837-52-8; HPLC purity > 98%) were provided by Beijing SL Pharmaceutical Co., Ltd. (Beijing, China). PQ was produced by Shandong Green Abundant Pesticide Co., (Weifang, China). MDA and SOD kits were produced by Jiancheng Bioengineering Institute (Nanjing, China). Protein test kits were produced by Shanghai Beyotime Institute of Biotechnology. Enzyme linked immunosorbent Assay (ELISA) kits for determination of IL-6, CRP and NF-κB were produced by Groundwork Biotechnology Diagnosticate Ltd. (San Diego, CA, USA) and imported by Yantai Dite Trade Co., Ltd. (Yantai, China).
Animals
Swiss mice (18–20 g) and Sprague–Dawley rats (120–150 g) were purchased from the Shandong Luye Pharmaceutical Co., Ltd (Quality Certificated Number: Lu 20090013). The animals were kept under standard conditions (temperature, 23 ± 2 °C; humidity, 55 ± 5%, 12 h light/dark cycle) and acclimatized to the laboratory environment for 3-7 days. Only water was provided to the animals within 12 h before the experiment and then the animals were fed regularly after the PQ injection. Experimental procedures in this study were approved by the Experimental Animal Management Center of Yantai University and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals. The duration of the experiments was as short as possible.
Mortality and death time of mice
The animals were randomly divided into seven groups: (1) control (saline), (2) PQ (saline), (3) SF (6.2 mg/Kg/day), (4) OMT (13.8 mg/Kg/day), (5) SF+OMT (low dose group, 3.1 + 6.9 mg/Kg/day), (6) SF+OMT (middle dose group, 6.2 + 13.8 mg/Kg/day), (7) SF+OMT (high dose group, 12.3 + 27.7 mg/Kg/day). There were ten animals in each group, male and female in half. Optimal molar ratio of SF and OMT (1:2) combination was obtained from the tests of pharmacology and pharmaceutical. We found that when the molar ratio of SF and OMT was 1:2, the combination had the best pharmacological activity and the system was the most stable with pH value of 7.0 (will be published in another paper). PQ (20 mg/Kg) was administered to the animals by intraperitoneal injection except the animals in control group (saline was injected). Thirty minutes later, saline or corresponding drug was hypodermic injected, and followed two times administration daily, respectively at 8:00 AM and 4:00 PM until the third day. The animals were observed carefully at the first 8 hours and every four hour observation was followed until 52 hours after PQ administration. The death time was recorded as the following principle: if the animal died during 8 hours to 12 hours period, the death time was recorded as 12 hours, and so on. If the animal did not die at 52 hours point, the death time was recorded as 52 hours.
L ung injury model in rats and drug administration
The rat PQ-induced lung injury model was duplicated referring to previous report (11). In our preliminary studies, we found that with the dose of 6.2 + 13.8 mg/Kg/day, SF and OMT combination treatment showed curative effect on PQ intoxication rats. So, we designed seven groups as following: (1) control (saline), (2) PQ (saline), (3) SF (3.1 mg/Kg/day), (4) OMT (6.9 mg/Kg/day), (5) SF+OMT (low dose group, 1.6 + 3.4 mg/Kg/day), (6) SF+OMT (middle dose group, 3.1 + 6.9 mg/Kg/day), (7) SF+OMT (high dose group, 6.2 + 13.8 mg/Kg/day). There were ten animals in each group, male and female in half. Except the animals in control group (saline was administered), all other animals were administered PQ (25 mg/Kg) by intraperitoneal injection, and 30 minutes later, the drug or saline was administered to the animals through hypodermic injection, and two times injections daily at 8:00 AM and 4:00 PM respectively until the third day.
Preparation of serum and tissue homogenates of rats
Preliminary experiment showed that the rats began to die after 60 hours of PQ injection. So, on the third day, about 56 hours later after PQ injection (when no rats died), the animal was anesthetized with diethyl ether and 5 mL blood was drawn via abdominal aortic. The blood samples were centrifuged at 2500 rpm for 10 minutes at 4 oC. The serum was separated and stored at −80 oC for biochemical analysis. Then the rat was killed and the lung was rapidly excised. The lower lobe of right lung (n = 10) was fixed by formaldehyde for histopathological analysis, and the upper lobe (n = 10) was homogenized in buffer solution, prepared for 10% lung homogenate with Vertishear tissue homogenizer (Virtis, Gardiner, NY, USA). The supernatant was stored at −80 oC for further biochemical analysis. The left lung was used to evaluate lung W/D ratio as following.
Lung W/D ratio of rats
After the animal was killed and lung was excised as mentioned above, the left lung (n = 10) was weighed and then dried in a drying oven at 60 °C for 72 h and weighed again as Oliveira described (12). The lung W/D ratio represented the degree of lung edema was calculated using follow formula:
W/D ratio = wet weight/dry weight
Biochemical analysis
CRP, IL-6 and NF-κB levels in serum and lung homogenate were measured with ELISA kits according to the manufacturers’ manual. MDA level and SOD activity in serum and lung homogenate were assayed using MDA and SOD kits as described previously (13, 14). In brief, MDA level was measured based on the thiobarbituric acid method with a maximal absorbance at 532 nm, and SOD activity was measured on the basis of SOD-mediated inhibition of nitrite formation from hydroxyammonium in the presence of superoxide radical anion (O2−) generators (xanthine/xanthine oxidase system).
Histopathological analysis
The animal's right lower lobe (n = 10) was fixed with 10% formalin for histopathological specimen. The specimens were embedded in paraffin, sectioned and stained with eosin and hematoxylin. As the report of Yuan et al. (15), the degree of inflammatory cell infiltration situation and lung injury were scored under light microscopy from 0–4 as follows: 0 (no damage); 1 (slight diffuse damage of neutrophilic in alveolar walls, no thickening of alveolar walls, no hemorrhage, < 25 % congestion of alveolar space); 2 (diffuse damage of mononuclear and neutrophilic in alveolar walls and slight thickening of the alveolar walls, at least five erythrocytes per alveolus in one to five alveoli, < 25-30 % congestion of alveolar space,); 3 (two or three times thickening of the alveolar walls, at least five erythrocytes in five to ten alveoli, 30-50 % congestion of alveolar space); 4 (alveolar wall thickening with up to 50% of lung consolidated, at least five erythrocytes in more than ten alveoli, > 60 % congestion of alveolar space). The validity of the method for evaluation of the lung injury had been well established and the tissue sections were evaluated by the independent blinded observer Dr. Yujun Li, Director of Pathological Department, Shandong Luye Drug Safety Evaluation Center (Yantai, Shandong Province; Good Laboratory Practice Lab. certified by State Food and Drug Administration of China).
Statistical analysis
The results were expressed as means ± SEM and data were analysed by one-way ANOVA, with the Statistical Product and Service Solutions (SPSS 17.0, USA). The differences of the mortality between two groups was analysed by the Chi-squared test (X2 test). A value of P < 0.05 was taken as statistical significant (*P < 0.05; **P < 0.01).
Results
Effects of SF and OMT combination or the drug used alone on mortality and death time of mice
After PQ injection, all animals died within 52 hours in PQ group and no animal died in control group. Compared with the PQ group, treatment with SF and OMT combination could decrease the mortality (Table 1) and prolonged the death time (Figure 1). As showed in the Table 1, the mice mortality was 80%, 50%, 30% at 48 hours, and 90%, 80%, 60% at 52 hours respectively in low dose group, middle dose group and high dose group of the combination treatment. Compared with the PQ group, treatment with either SF (6.2 mg/Kg/day) or OMT (13.8 mg/Kg/day) alone could not significantly decrease the mortality (100% and 90% respectively at 52 hours), or prolong the death time.
Table 1.
Effects of the combination of SF and OMT on the mortality of PQ-intoxication mice.
| Group | Dose (mg/Kg/day) |
Mice
number |
Mortality within 52 h
|
|
|---|---|---|---|---|
| 48 h | 52 h | |||
| Con | -- | 10 | 0% | 0% |
| PQ | -- | 10 | 90%## | 100% ## |
| SF | 6.2 | 10 | 80% | 100% |
| OMT | 13.8 | 10 | 70% | 90% |
| SF+OMT (low dose) | 3.1+6.9 | 10 | 80% | 90% |
| SF+OMT(middle dose) | 6.2+13.8 | 10 | 50% | 80% |
| SF+OMT(high dose) | 12.3+27.7 | 10 | 30%** | 60%* |
CON, PQ, SF and OMT represented the control group, PQ group, SF group and OMT group, respectively. SF + OMT represented the group of the combination of SF and OMT. The statistical different between two groups was analysed by X2 test.
P < 0.01 versus the control group.
P < 0.05,
P < 0.01 versus the PQ group.
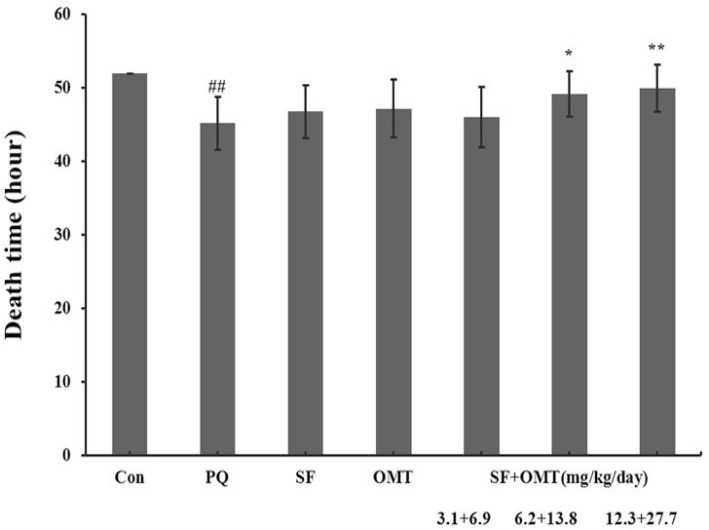
Figure 1.
Effects of SF and OMT combination on the death time of PQ-intoxication mice. CON, PQ, SF and OMT represent the control group, paraquat group, sodium ferulate group (6.2 mg/Kg/day) and oxymatrine group (13.8 mg/Kg/day), respectively. SF+OMT represents sodium ferulate and oxymatrine combination treatment groups, and the 3.1 + 6.9, 6.2 + 13.8 and 12.3 + 27.7 respectively represent drug administration doses of SF+OMT in the low dose group, middle dose group and high dose group. Data are expressed as means ± SEM. n = 10 in each group. ##P < 0.01 compared with the control group. *P < 0.05, **P < 0.01 compared with the PQ group
Effects of SF and OMT combination or the drug used alone on lung W/D ratio and histopathological changes of rats
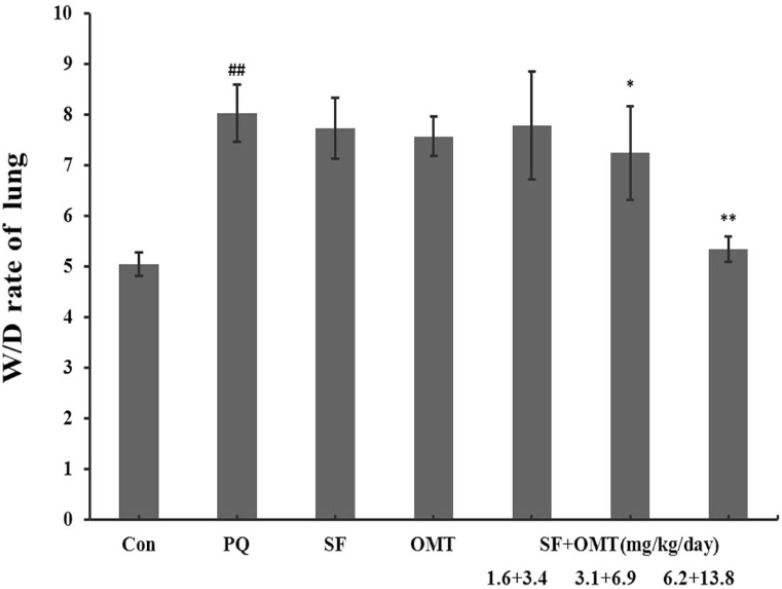
As shown in Figure 2, the lung W/D ratio increased markedly in PQ group. In the middle dose group and high dose group of the combination treatment (SF+OMT 3.1 + 6.9, 6.2 + 13.8 mg/Kg/day), the lung W/D ratio was significantly decreased compared with the PQ group. Treatment with SF (3.1 mg/Kg/day) or OMT (6.9 mg/Kg/day) alone could not significantly decrease the lung W/D ratio.
Figure 2.
Effects of SF and OMT combination on the lung Wet/Dry (W/D) ratio of PQ intoxication rats. CON, PQ, SF and OMT represent the control group, paraquat group, sodium ferulate group (3.1 mg/Kg/day) and oxymatrine group (6.9 mg/Kg/day), respectively. SF+OMT represents sodium ferulate and oxymatrine combination treatment groups, and the 1.6 + 3.4, 3.1 + 6.9 and 6.2 + 13.8 respectively represent drug administration doses of SF+OMT in the low dose group, middle dose group and high dose group. The lung W/D ratio was determined at 56 h after the PQ injection. Data are expressed as means ± SEM. n = 10 in each group. ##P < 0.01 compared with the control group. *P < 0.05, **P < 0.01 compared with the PQ group
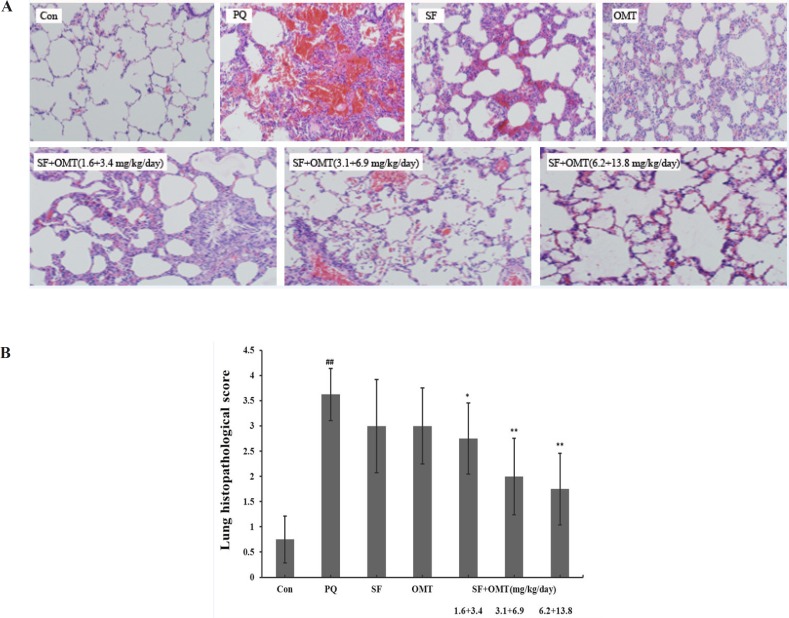
As shown in Figure 3a, the lung tissue injury or inflammation changes were almost not observed in the control group. In PQ group, light microscope showed obvious pulmonary edema sign and infiltration of lots of inflammatory cell and erythrocyte. The decreased inflammtion infiltration was observed in the SF and OMT combination treatment groups, and the effects were much stronger than the drug used alone, which were scored in Figure 3b.
Figure 3.
Effects of SF and OMT combination on the lung histopathological changes of PQ-intoxication rats. CON, PQ, SF and OMT represent the control group, paraquat group, sodium ferulate group (3.1 mg/Kg/day) and oxymatrine group (6.9 mg/Kg day), respectively. SF+OMT represents sodium ferulate and oxymatrine combination treatment groups, and the 1.6 + 3.4, 3.1 + 6.9 and 6.2 + 13.8 respectively represent drug administration doses of SF+OMT in the low dose group, middle dose group and high dose group. The degree of lung injury was scored under light microscopy from 0–4 as follow: 0 (no damage), 1 (slight diffuse damage of neutrophilic in alveolar walls, no thickening of alveolar walls, no hemorrhage, < 25 % congestion of alveolar space); 2 (diffuse damage of mononuclear and neutrophilic in alveolar walls and slight thickening of the alveolar walls, at least five erythrocytes per alveolus in one to five alveoli, < 25-30 % congestion of alveolar space,); 3 (two or three times thickening of the alveolar walls, at least five erythrocytes in five to ten alveoli, 30-50% congestion of alveolar space); 4 (alveolar wall thickening with up to 50% of lung consolidated, at least five erythrocytes in more than ten alveoli, > 60 % congestion of alveolar space). Data are expressed as means ± SEM. n = 10 in each group. ##P < 0.01 compared with the control group. *P < 0.05, **P < 0.01 compared with the PQ group.
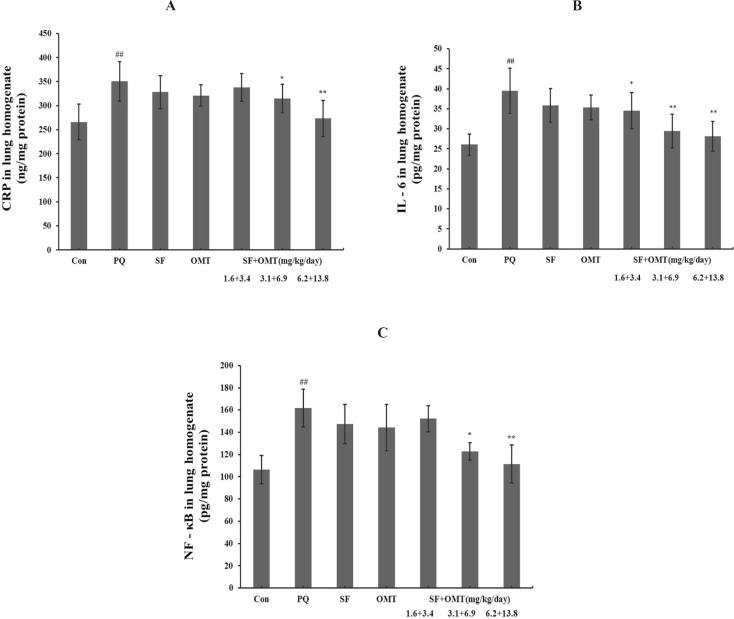
Effects of SF and OMT combination or the drug used alone on the levels of CRP, IL-6, NF-κB in serum induced by PQ of rats
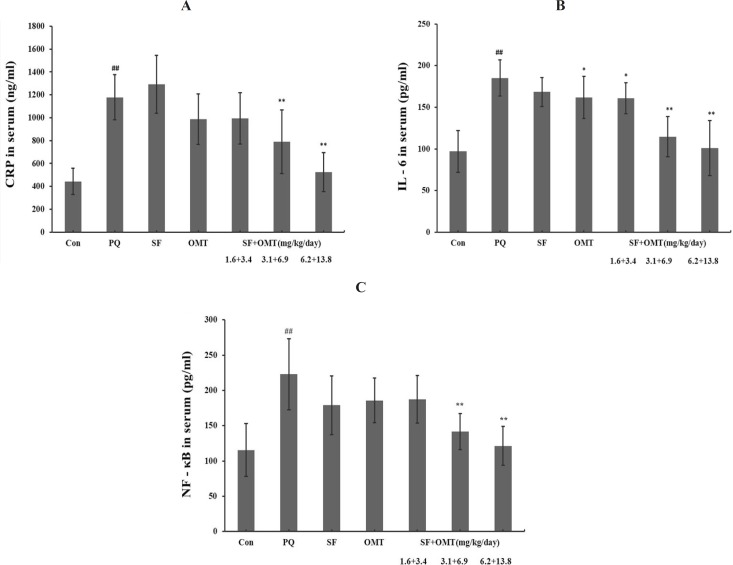
After 56 h of PQ injection, the serum CRP, IL-6, NF-κB levels all significantly increased in PQ group compared with control (Figure 4). In all of the combination treatment groups (SF+OMT 1.6 + 3.4, 3.1+6.9 and 6.2 + 13.8 mg/Kg/day), the level of IL-6 in serum was significantly decreased in a dose-dependent manner (P < 0.05 in the low dose group; P < 0.01 in the middle dose group and high dose group). The levels of CRP and NF-κB in serum significantly decreased in the middle dose group and high dose group of the combination treatment (P < 0.01). Except the level of IL-6 in serum decreased significantly (P < 0.05) in OMT (6.9 mg/Kg/day) group, other measured index did not exhibit significant change in SF or OMT alone treatment group.
Figure 4.
Effects of SF and OMT combination on the levels of CRP (a), IL-6 (b), NF-κB (c) in the serum of the PQ-intoxication rats. CON, PQ, SF and OMT represent the control group, paraquat group, sodium ferulate group (3.1 mg/Kg/day) and oxymatrine group (6.9 mg/Kg/day), respectively. SF+OMT represents sodium ferulate and oxymatrine combination treatment groups, and the 1.6 + 3.4, 3.1 + 6.9 and 6.2 + 13.8 respectively represent drug administration doses of SF+OMT in the low dose group, middle dose group and high dose group. Data are expressed as means ± SEM, n = 10 in each group. ##P < 0.01 compared with the control group. *P < 0.05, **P < 0.01 compared with the PQ group.
Effect s of SF and OMT combination on the levels of CRP, IL-6 and NF- κ B in lung homogenate of rat induced by PQ of rat s
As shown in Figure 5, after PQ injection, the lung homogenate CRP, IL-6 and NF-κB levels significantly increased in PQ group compared with the control. Compared with the PQ group, the levels of CRP, IL-6 and NF-κB significantly decreased in the middle dose group and high dose group of SF and OMT combination treatment. In the low dose group of combination treatment (SF+OMT 1.6 + 3.4 mg/Kg/day), only the level of IL-6 in lung homogenate decreased significantly (P < 0.05). Compared with PQ group, treatment with SF (3.1 mg/Kg/day) or OMT (6.9 mg/Kg/day) alone could not significantly decrease the levels of CRP, IL-6 and NF-κB in lung homogenate.
Figure 5.
Effects of SF and OMT combination on the levels of CRP (a), IL-6 (b), NF-κB (c) in the lung homogenate of the PQ-intoxication rats. CON, PQ, SF and OMT represent the control group, paraquat group, sodium ferulate group (3.1 mg/Kg/day) and oxymatrine group (6.9 mg/Kg/day), respectively. SF+OMT represents sodium ferulate and oxymatrine combination treatment groups, and the 1.6 + 3.4, 3.1 + 6.9 and 6.2 + 13.8 respectively represent drug administration doses of SF + OMT in the low dose group, middle dose group and high dose group. Data are expressed as means ± SEM, n = 10 in each group. ##P < 0.01 compared with the control group. *P < 0.05, **P < 0.01 compared with the PQ group.
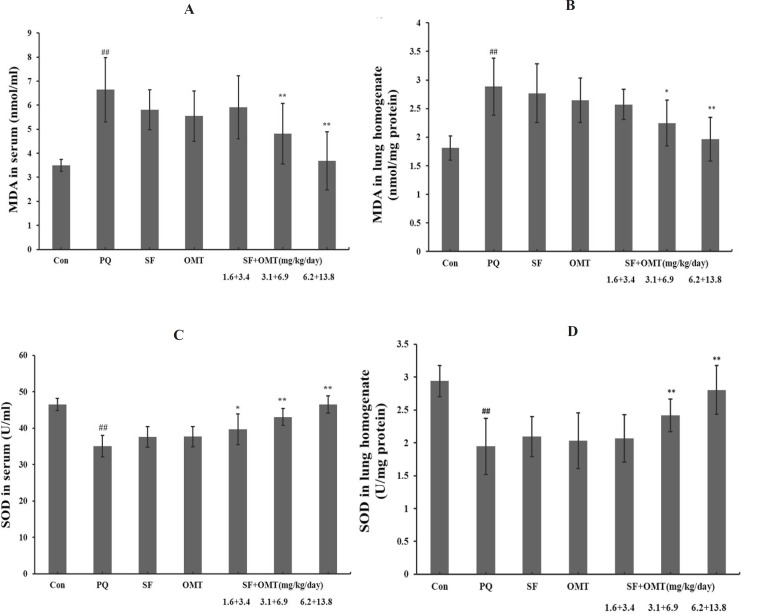
Effects of SF and OMT combination on MDA level and SOD activity in serum and lung homogenate of rats
As shown in Figure 6, a remarkable increase in MDA levels and decrease in SOD activities in serum and lung homogenate were observed in PQ group. Compared with the PQ group, in the combination treatment groups, the level of MDA significantly decreased in serum and lung homogenate with a dose-dependent manner, and the SOD activity significantly increased in serum and in lung homogenate. In the group of treatment with SF (3.1 mg/Kg/day) or OMT (6.9 mg/Kg/day) alone, the MDA level and SOD activity in serum and in lung homogenate did not exhibit significant efficacy.
Figure 6.
Effects of SF and OMT combination on MDA levels and SOD activities of the PQ-intoxication rats. a: MDA levels in the serum; b: MDA levels in the lung homogenate; c: SOD activities in the serum; d: SOD activities in the lung homogenate. CON, PQ, SF and OMT represent the control group, paraquat group, sodium ferulate group (3.1 mg/Kg/day) and oxymatrine group (6.9 mg/Kg/day), respectively. SF+OMT represents sodium ferulate and oxymatrine combination treatment groups, and the 1.6 + 3.4, 3.1 + 6.9 and 6.2 + 13.8 respectively represent drug administration doses of SF+OMT in the low dose group, middle dose group and high dose group. Data are expressed as means ± SEM. n = 10 in each group. ##P < 0.01 compared with the control group. *P < 0.05, **P < 0.01 compared with the PQ group.
Discussion
In recent years, it had become a popular research topic to find a better therapeutic efficacy of multiple drugs combination. Many academics had been involved in this field. As a compound extracted from Radix Angelicae sinensis (named Danggui in Chinese), sodium ferulate (SF) had been used to treat cerebrovascular and cardiovascular diseases because of its effects on anti-inflammation, antioxidant and platelet aggregation inhibition (16, 17). As the active component of Chinese herb Radix Sophora flavescens Ait (named Kushen in Chinese), OMT had been reported with the following pharmacological effects, anti-inflammation, anti-apoptotic, protecting hepatocytes, and inhibiting immune reaction (18). The traditional Chinese recipe composed of Danggui and Kushen had been used to treat inflammation diseases for many years. Such as the Danggui Kushen pill was used to treat the acne of youth and the effects were accepted by the doctors and the patients. In China, there were many reports about the anti-inflammatory effect of SF or OMT alone. But the experimental results confirmed that its anti-inflammatory effect was not strong, so SF or OMT alone was not used in the clinic as anti-inflammatory drugs. In our previous studies, the synergistic anti-inflammatory and antioxidant effects of the SF and OMT combination had been demonstrated respectively on the xylene-induced ear edema (10), LPS induced pulmonary edema (15) and sepsis induced lung injury (19) of mice. So, we used SF and OMT combination to treat the PQ-induced lung injury and hope to find an effective drug for the PQ intoxication treatment.
The rodents intraperitoneal injection PQ model had been extensively used to study the intervention of PQ induced lung injury. In this study, we duplicated the PQ induced acute death model in mice, in which the animals began to die at about 40 hours after PQ injection, and died to 100% at about 52 h, but the lung injury was not obvious and the lung W/D ratio was not changed significantly (data not shown). Those results suggested us that Swiss mouse was not a good animal model to duplicate the PQ-induced acute lung injury and the main injured target organ which lead to the animals death need to be explored. In rat PQ intoxication model, the lung injury was markedly reflected by dramatically increased lung W/D ratio, lung edema, hemorrhage and histopathological scores. So the rat PQ intoxication model was fit for observation of the drug treatment efficacy on the PQ induced lung injury. In our study, we administered the drug 30 min after PQ injection with hypodermic injection, which could mimic the time of patient transferring to the hospital after PQ intoxication. In addition, it was considered that the drug was absorbed slower with hypodermic injection than the venous and intraperitoneal injection. So, the hypodermic injection method was selected to administer the drug, through which the speed of drug absorption was closer to venous drop injection in clinic. The results indicated that the PQ induced mice mortality decreased, the death time prolonged, the increased rat lung W/D ratio and lung histopathological score all attenuated after treatment with SF and OMT combination, which exhibited protective effect on PQ injury.
It was thought that anti-inflammatory effects of SF and OMT combination might be involved in the protective mechanism on PQ induce injury. In our previous study (10), we found that SF and OMT combination could synergistically down-regulated IL-6, CRP genes in LPS-stimulated RAW 264.7 cells. So, the inflammatory cytokine included CRP, IL-6, NF-κB levels were measured after 56 hours of PQ injection when there was no animals died, and the detected index all increased markedly.
CRP, as an indicator of systemic inflammatory reaction, was a kind of reactive protein in acute inflammation phrase (20), which exhibited pro-inflammatory effects by inducing inflammatory cytokines and played key role on activating the complement system and tissue factor production in monocytes (21). CRP level showed a significant positive correlation with tissue damage. In recent years, it was considered that CRP could predict the risk of the occurrence of organ damage and was known as a marker of inflammation reaction (22). IL-6, as a pro-inflammatory cytokine, had many biological features and played an important role in the process of inflammation (23). It was reported that IL-6 level was greatly up-regulated with PQ-induced lung injury (24). NF-κB, as an indicator of systemic inflammatory reaction, could induce inflammatory protein production, activate the gene transcription system and tissue factor production in nuclei pulp. As the results showed, treatment with SF and OMT combination significantly attenuated the PQ increased CRP, IL-6, NF-κB levels, which related to the acute phase of the inflammatory response. These results suggested us that the anti-inflammatory effect of SF and OMT combination involved in the protective mechanism, and further certified the anti-inflammatory effect of SF and OMT combination. Otherwise, this was the first report for the effect of SF and OMT combination on the NF-κB and the reaction pathway would be studied further in our future studies.
One of histopathological reason of PQ-induced lung injury was that too much reactive oxygen species was produced. That the intraperitoneal injection of PQ induced the generation of superoxide anions, hydroxyl radicals, and oxidant peroxynitrite which led to oxidative stress damage-related cell death and inflammation was demonstrated in many reports (2, 7, 25). MDA was one of the major secondary oxidation products derived from polyunsaturated fatty acids. As an index of structural oxidative injury of cell membrane, MDA was a well-known parameter for determining the increased free radical formation. An increase in MDA level suggested increased lipid peroxidation initiated by free radical reactions (13). SOD, as an antioxidant enzyme, had a capable of scavenging the superoxide free radical and confronting the cell damage from the free radical. The enhancement of oxidizing reaction was reflected by decrease SOD activity. In our study, the results showed that the MDA levels in serum and lung tissue were dramatically increased by PQ injection and the SOD activity was dramatically decreased. However, this increased MDA level induced by PQ injection was significantly decreased by SF and OMT combination treatment, and this SOD activity decrease was significantly improved. So that, it suggested that oxidative damage in serum and in the lung tissue were alleviated after the treatment with SF and OMT combination.
In order to further validate the synergistic effects of SF and OMT combination, we designed other two groups which were treated with the SF or OMT alone. Because the markedly protective effect was observed in middle dose group of the combination, we designed the dose of the drug SF or OMT alone was 3.1 mg/Kg/day and 6.9 mg/Kg/day which corresponded to middle dose group of the combination treatment (SF +OMT 3.1 + 6.9 mg/Kg/day). The results show that, compared with the PQ group, no obvious antioxidant or anti-inflammatory effects were observed in the SF (3.1 mg/Kg/day) or OMT (6.9 mg/Kg/day) group except the serum IL-6 level decreased in OMT (6.9 mg/Kg/day) group. The results indirectly confirmed the synergistic effect of the combination of those two drugs, and were accordance with our previous report (10, 15, 26).
Conclusions
SF and OMT combination had an obvious protective effect on PQ-induced lung injury. The anti-inflammatory and antioxidant effect might be involved in the mechanism.
References
- 1.Morán JM, Ortiz-Ortiz MA, Ruiz-Mesa LM, Fuentes JM. Nitric oxide in paraquat-mediated toxicity: A review. J. Biochem. Mol. Toxicol. 2010;24:402–409. doi: 10.1002/jbt.20348. [DOI] [PubMed] [Google Scholar]
- 2.Grabie V, Scemama JL, Robertson JB, Seidel ER. Paraquat uptake in the cultured gastrointestinal epithelial cell line, IEC-6. Toxicol. Appl. Pharmacol. 1993;122:95–100. doi: 10.1006/taap.1993.1176. [DOI] [PubMed] [Google Scholar]
- 3.Bismuth C, Garnier R, Dally S, Fournier PE, Scherrmann JM. Prognosis and treatment of paraquat poisoning: a review of 28 cases. J. Toxicol. Clin. Toxicol. 1982;19:461–474. doi: 10.3109/15563658208992501. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesan N. Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci. 2000;66:21–28. doi: 10.1016/s0024-3205(99)00576-7. [DOI] [PubMed] [Google Scholar]
- 5.Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med. J. 2010;51:496–500. [PubMed] [Google Scholar]
- 6.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicol. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 7.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ. Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin JL, Lin-Tan DT, Chen KH, Huang WH. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit. Care. Med. 2006;34:368–373. doi: 10.1097/01.ccm.0000195013.47004.a8. [DOI] [PubMed] [Google Scholar]
- 9.Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu CW, Hsu HH, Yen TH. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intens. Care. Med. 2011;37:1006–1013. doi: 10.1007/s00134-010-2127-7. [DOI] [PubMed] [Google Scholar]
- 10.Yuan X, Sun Y, Miao N, Sun S, Wang Y, Hu Z, Yuan J, Xu M, Liu Z. The synergistic anti-inflammatory effect of the combination of sodium ferulate and oxymatrine and its modulation on inflammation-associated mediators in RAW 264.7 cells. J. Ethnopharmacol. 2011;137:1477–1485. doi: 10.1016/j.jep.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed AA. Protective effect of montelukast on paraquat-induced lung toxicity in rats. Biosci. Trend. 2009;3:63–72. [PubMed] [Google Scholar]
- 12.Oliveira-Júnior IS, Maganhin CC, Carbonel AA, Monteiro CM, Cavassani SS, Oliveira-Filho RM. Effects of pentoxifylline on TNF-alpha and lung histopathology in HCL-induced lung injury. Clin.(Sao Paulo) 2008;63:77–84. doi: 10.1590/s1807-59322008000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Elstner EF, Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 1976;70:616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- 15.Yuan X, Wang Y, Du D, Hu Z, Xu M, Xu M, Liu Z. The effects of the combination of sodium ferulate and oxymatrine on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2012;35:1161–1168. doi: 10.1007/s10753-011-9424-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang BH, Ou-Yang JP. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc. Drug Rev. 2005;23:161–172. doi: 10.1111/j.1527-3466.2005.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu GL, Yao L, Rao SY, Gong ZN, Zhang SQ, Yu SQ. Attenuation of acute lung injury in mice by oxymatrine is associated with inhibition of phosphorylated p38 mitogen-activated protein kinase. J. Ethnopharmacol. 2005;98:177–183. doi: 10.1016/j.jep.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu MX, Wang W, Pei XK, Sun SM, Xu MB, Liu ZF. The protective effects of the combination of sodium ferulate and oxymatrine on cecal ligation and puncture induced sepsis in mice. Exp. Ther. Med. 2014;7:1297–1304. doi: 10.3892/etm.2014.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballou SP, Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992;4:361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- 21.Cermak J, Key NS, Bach RR, Balla J, Jacob HS, Vercellotti GM. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- 22.Imamura T, Tanaka S, Yoshida H, Kitamura K, Ikegami A, Takahashi A, Niikawa J, Mitamura K. Significance of measurement of high-sensitivity C-reactive protein in acute pancreatitis. J. Gastroenterol. 2002;37:935–938. doi: 10.1007/s005350200157. [DOI] [PubMed] [Google Scholar]
- 23.Tao JY, Zheng GH, Zhao L, Wu JG, Zhang XY, Zhang SL, Huang ZJ, Xiong FL, Li CM. Anti-inflammatory effects of ethyl acetate fraction from Melilotus suaveolens Ledeb on LPS-stimulated RAW 264.7 cells. J. Ethnopharmacol. 2009;123:97–105. doi: 10.1016/j.jep.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Zhi Q, Sun H, Qian X, Yang L. Edaravone, a novel antidote against lung injury and pulmonary fibrosis induced by paraquat? Int. Immunopharmacol. 2011;11:96–102. doi: 10.1016/j.intimp.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Yeh ST, Guo HR, Su YS, Lin HJ, Hou CC, Chen HM, Chang MC, Wang YJ. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicol. 2006;223:181–190. doi: 10.1016/j.tox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Sun Y, Gao Y, Chen F, Xu M, Liu Z. The analgesic effect and mechanism of the combination of sodium ferulate and oxymatrine. Neurochem. Res. 2010;35:1368–1375. doi: 10.1007/s11064-010-0193-4. [DOI] [PubMed] [Google Scholar]