Abstract
Background and Purpose
Vision depends on retinoid exchange between the retinal pigment epithelium (RPE) and photoreceptors. Defects in any step of the canonical visual cycle can lead to retinal degenerations. All-trans-retinol (atROL) plays an important role in visual signal transduction. However, how atROL enters human RPE from the apical membrane remains unclear. This study investigated the role of human organic anion transporting polypeptide 1A2 (OATP1A2) in atROL uptake in human RPE.
Experimental Approach
Immunoblotting and immunostaining elucidated the expression and localization of OATP1A2 in human RPE. Transporter functional studies were conducted to assess the interaction of OATP1A2 with atROL.
Key Results
Our study revealed OATP1A2 is expressed in human RPE, mainly at the apical membrane. Our data also indicated atROL inhibited the uptake of the typical OATP1A2 substrate, oestrone-3-sulfate (E3S), in over-expressing cells. Studies on the uptake of 3H-atROL in these over-expressing cells revealed atROL is a substrate of OATP1A2. We confirmed these findings in human primary RPE cells. The transport of E3S and atROL was significantly reduced in human primary RPE cells with OATP1A2 siRNA silencing.
Conclusion and Implications
Our data provides the first evidence of OATP1A2 expression in human RPE and more importantly, its novel role in the cellular uptake of atROL, which might be essential to the proper functioning of the canonical visual cycle. Our findings contribute to the understanding of the molecular mechanisms involved in retinoid transport between the RPE and photoreceptors and provide novel insights into potential pharmaceutical interventions for visual cycle disruption associated with retinal degenerations.
Tables of Links
| TARGETS |
|---|
| Transporters |
| OATP1A2 (SLCO1A2) |
| OATP2B1 (SLCO2B1) |
| LIGANDS |
|---|
| atROL, all-trans-retinol |
| E3S, oestrone-3-sulfate |
| Imatinib |
| Methotrexate |
| Ouabain |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Retinoids are important vitamin A compounds that are critical for human vision (Travis et al., 2007; Wang et al., 2012). Vision in all vertebrates is dependent on the exchange of retinoids to produce rhodopsin that is required to convert light energy into an electrical signal in the retina (Saari, 2012). The two main types of photoreceptor cells in the vertebrate retina are rods and cones (Travis et al., 2007). The canonical visual cycle takes place between the photoreceptors and the retinal pigment epithelium (RPE) monolayer (Travis et al., 2007; Wang et al., 2012). The RPE monolayer is firmly attached to the choroid and provides support to the photoreceptors. Apart from the canonical visual cycle, an alternative cone-dominant visual cycle has also been proposed, which may take place between the cones and the Müller cells (the principal glial cells of the retina). However, less is known about this cycle (Travis et al., 2007).
Defects in any step of the visual cycle may lead to retinal degeneration. The accumulation of cytotoxic products resulting from the derangement of the visual cycle is one of the primary causes of many blinding diseases (Sparrow et al., 2003). Deficits of the genes involved in the visual cycle have been implicated as risk factors in retinal degenerations including age-related macular degeneration (AMD), Leber's congenital amaurosis, Stargardt's disease, congenital cone-rod dystrophy and retinitis pigmentosa (Allikmets, 1997; Mata et al., 2000; Thompson et al., 2000; Janecke et al., 2004; Perrault et al., 2004; Travis et al., 2007). Gene therapies and pharmaceutical inhibition of retinoid supply to eyes are under investigation in preclinical and clinical studies to treat these diseases.
Within the canonical visual cycle, all-trans-retinol (atROL) is the specific retinoid that traverses from photoreceptors to the RPE cells across the interphotoreceptor matrix (IPM). Under pathological conditions, blockage of the visual cycle may lead to the accumulation of prominent components of the retinal ‘waste product’ lipofuscin, which is a major component of soft drusen (Delori et al., 2000). Accumulation of soft drusen external to the RPE basement membrane is considered a major risk factor for vision loss associated with the progression of AMD (Sparrow et al., 2003). Therefore, reducing the toxic accumulation of retinoids involved in the visual cycle is one of the leading therapeutic strategies proposed for dry AMD as well as a range of other retinal disorders.
atROL is thought to be delivered into the RPE cells by two means: (i) within the canonical visual cycle, the secreted atROL from the photoreceptors is taken up from the IPM into the RPE through the apical membrane, and (ii) from the blood circulation through the basolateral membrane of the RPE (Travis et al., 2007). A receptor-mediated mechanism has been reported to be responsible for the uptake of atROL from the basolateral membrane of RPE (Kawaguchi et al., 2007). The soluble interphotoreceptor retinoid-binding protein (IRBP or RBP3) has been reported to facilitate atROL traveling through the IPM to the RPE cells (Pepperberg et al., 1993). However, whether the large IRBP protein (∼140 kDa) is involved with atROL moving across the apical membrane of RPE remains unknown. To date, it is yet to be explored how atROL is taken up into RPE cells from the IPM through the apical RPE membrane.
Membrane transporters regulate the movement of molecules into and out of cells. Their primary function is to transport endogenous or exogenous substrates. Influx transporters, principally the solute carrier transporters (SLCs), determine the rate at which substances enter cells. Organic anion transporting polypeptides (OATPs) are important SLC transporter proteins that are responsible for the cellular influx of endogenous substances such as hormones and exogenous substances including a wide range of clinically important drugs (Zhou and You, 2007; Kalliokoski and Niemi, 2009). They have been found to be expressed in various human key organs, such as the kidney and liver (Anzai et al., 2006). OATPs are also well recognized to contribute to therapeutic toxicities when drugs compete with endogenous or exogenous substances for the same OATP protein(s) in specific tissues (Gao et al., 2014; Taylor-Wells and Meredith, 2014).
Currently, little is known about the roles of OATPs in the human eye. Several Oatp isoforms have been detected in rat eyes, with Oatp-2, Oatp-3, Oatp1c1 and Oatp-E specifically localized at the RPE, however, the physiological functions of these rodent transporter proteins in the RPE remains unclear (Gao et al., 2002; Ito et al., 2002; 2003,; Akanuma et al., 2013). Previous studies have demonstrated that two of the human classic OATP isoforms, human organic anion transporting polypeptide 1A2 (OATP1A2) and OATP2B1, were expressed in the retina/choroid at the mRNA level (Zhang et al., 2008; Kadam et al., 2013). In a recent study, Gao et al. identified broad OATP1A2 immunoreactivity in various retinal cell layers including the outer nuclear layer and inner nuclear layer (INL). However, the presence of OATP1A2 in the RPE cell layer was not clearly identified due to autofluorescence of the RPE cells (Gao et al., 2014). Similarly, tissue staining of OATP2B1 was observed in the INL and inner plexiform layer, but the authors were also unable to conclude whether this transporter was expressed in the RPE cells (Gao et al., 2014). Our study provides evidence of OATP protein expression in human RPE cells. More importantly, our study suggests a novel role of OATP1A2 in the cellular transport of atROL. Our findings may have clinical significance in understanding the contribution of OATP1A2 to the canonical visual cycle and the pathophysiology of many retinal disorders.
Methods
Generation of OATP1A2 expressing HEK293 cells
The OATP1A2 cDNA was purchased from GeneCopoeia (Cat. No: GC-Q0577). The cDNAs were then subcloned into the PCI vector (Promega, Alexandria, NSW, Australia) as described previously (Zhou et al., 2011; 2013,). All sequences were confirmed by the dideoxy chain termination method (Ramaciotti Centre, University of New South Wales, Randwick, NSW, Australia).
Isolation of human eye tissue and RPE primary cells
Four post-mortem human eyes (age range, 38–69 years, post-mortem delay <16 h) were obtained from the Lions NSW Eye Bank, with consent and ethics approval from The University of Sydney Human Research Ethics Committee in accordance with the tenets of the Declaration of Helsinki. Primary RPE cells were isolated from these eyes as described previously (McKay and Burke, 1994; Zhu et al., 1998; Munoz-Erazo et al., 2012). Briefly, after removing the anterior segment from the eyes, the neurosensory retina and vitreous was gently removed from the underlying RPE. The eyecups were rinsed with phosphate buffered saline (PBS, pH 7.4) and then filled with 0.25% trypsin and 0.01% EDTA; the eyecup was incubated at 37°C for 45 min. The RPE was gently removed from the underlying Bruch's membrane with pipetting, and collected in DMEM with 4.5 g·L−1 glucose, 2 mM L-glutamine and 20% FBS. Cells were pelleted by centrifugation (163× g for 5 min), resuspended in fresh medium and initially grown in 35 mm2, dishes. After reaching confluency, cells were trypsinized and subsequently grown in 25 cm2 flasks maintained at 37°C with 5% CO2. RPE cells were used between passage 2 and passage 5.
Subcultures of primary RPE cells were also pelleted and lysed for immunoblotting in lysis buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, that contained the protease inhibitors phenylmethylsulfonyl fluoride, 200 mg·mL−1, and leupeptin, 3 mg·mL−1, pH 7.4).
Transfection of cells
HEK-293 and human primary RPE cells were maintained at 37°C and 5% CO2 in DMEM supplemented with 10% fetal calf serum. HEK293 cells were transfected with OATP1A2 plasmid DNA using Lipofectamine 2000 reagent (Invitrogen, Mount Waverley, Vic., Australia) as we previously described (Zhou et al., 2010; 2011; 2013,,). Twenty-four hours after transfection, substrate uptake activities or transporter protein expression were measured. Human primary RPE cells were transfected with scrambled siRNAs (VWR, Murarrie, Qld., Australia; Cat. No: sc-37007) or each of the three OATP1A2-specific siRNAs (Invitrogen; Cat. No: 4392420, ID: s13099, s13100, s13101) using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. Forty-eight hours after transfection, substrate uptake activities or transporter protein expression were measured.
Transport studies
Cellular uptake of [3H]-E3S (final concentration 0.3 μM by mixing 0.01 μM [3H]-E3S and 0.29 μM E3S, 150 μL uptake solution per well, 67 nCi per well) in HEK-293 was conducted as described previously (Zhou et al., 2011; 2013,). Uptake was initiated at 37°C in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4) containing 1 mM CaCl2, and 1 mM MgCl2, and was terminated by rapidly washing the cells in buffer at 4°C. Preliminary experiments indicated that initial rates of OATP1A2-mediated substrate uptake in HEK293 cells were linear over at least 8 min, which was the time selected for subsequent experiments. The cells were then solubilized in 0.2 M NaOH, neutralized with 0.2 M HCl, and aliquoted for liquid scintillation counting. The uptake of [3H]-atROL (final concentration 0.1 μM by mixing 0.02 μM [3H]-atROL and 0.08 μM atROL, 150 μL uptake solution/well, 0.15 μCi per well) was undertaken in analogous fashion except that the buffer pH was adjusted to 5.0. Uptake was standardized to the amount of protein in each well. Kinetic studies were performed with varying concentrations of atROL (0.02–500 μM) added to the uptake buffer for a 3 min incubation in cells and apparent Km and Vmax values for transporter activity were then calculated where possible (GraphPad Prism 5.0; GraphPad Inc, La Jolla, CA, USA).
Electrophoresis and immunoblotting
After removing medium, the HEK-293 or human primary RPE cells were lysed with lysis buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% SDS and 1% Triton X-100, which contained the protease inhibitors phenylmethylsulfonyl fluoride, 200 mg·mL−1, and leupeptin, 3 mg·mL−1, pH 7.4). Unlysed cells were removed by centrifugation at 14 000× g at 4°C. Protein concentration of supernatant was measured with Bradford assay. Protein samples were denatured, loaded onto 7.5% polyacrylamide minigels and electrophoresed using a mini cell (Bio-Rad, Gladesville, NSW, Australia). Proteins were transferred to polyvinylidene fluoride membranes (Merck Millipore, Kilsyth, Victoria, Australia) in an electroelution cell (Bio-Rad, Gladesville, NSW, Australia) and blocked for 1 h with 5% non-fat dry milk in PBS-Tween (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 and 0.05% Tween 20; pH 7.5), washed and then incubated overnight at 4°C with anti-OATP1A2 antibody (1 μg·mL−1; VWR; Cat. No: sc-48744). The membranes were washed, incubated with goat anti-rabbit IgG conjugated to HRP (1:5000; VWR; Cat. No: sc-2004), and signals were detected using the Immobilon Western Chemiluminescent HRP Substrate (Merck Millipore, Kilsyth, Vic., Australia).
Immunohistochemistry
Two of the post-mortem human eyes were used for immunohistochemical studies. The post-mortem delay to fixation was approximately 12 h. After removing corneas, eyecups were fixed in 4% paraformaldehyde for 4 h and rinsed with PBS followed by equilibration in 30% sucrose/PBS overnight. After dissecting the eyecups into smaller pieces, tissues (including sclera choroid and retina) were embedded in optical cutting temperature compound (ProSciTech, Kirwan, Qld., Australia) for cyrosectioning. Immunolabelling was performed as described previously (Zhu et al., 2013). Briefly, sections were incubated in anti-OATP1A2 antibody (VWR; Cat. No: sc-48744, 1:100) or anti-Na+-K+ ATPase β1 subunit antibody (VWR; Cat. No: Sc-21713, 1:100) or anti-Bestrophin antibody (Sapphire Bioscience, Waterloo, NSW, Australia; Cat. No: ab2182, 1:100) overnight at 4°C and then probed with Alexa Fluor® 488 conjugated donkey anti-mouse IgG or Alexa Fluor® 594 conjugated goat anti-rabbit IgG (1:1000 dilution; Invitrogen). Samples were treated with TrueBlack Lipofuscin Autofluorescence Quencher (Jomar Diagnostics P/L, Stepney SA, Australia) and mounted with Fluoroshield™ with DAPI histology mounting medium (Sigma-Aldrich, Castle Hill, NSW, Australia). Slides were visualized using a Leica DMI3000 B epi fluorescence microscope (Leica Microsystems, North Ryde, NSW, Australia).
Data analysis
Data are presented throughout as mean ± SEM. The Student's t-test was used to test for differences between two sets of normally distributed data. Differences in transport function of OATP1A2 were detected by one-way analysis of variance and Dunnett's testing.
Materials
[3H]Oestrone-3-sulfate (E3S; specific activity 57.3 Ci·mmol−1) and [3H]atROL (specific activity 12.5 Ci·mmol−1) were purchased from PerkinElmer (Melbourne, Vic., Australia). Culture media was obtained from Life Technologies (Mount Waverley, Vic., Australia). Human kidney total lysate (adult normal) was purchased from Sapphire Biosciences (Redfern, NSW, Australia). Unless otherwise stated, all other chemicals and biochemicals were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia).
Results
OATP1A2 expression in human RPE cells
We observed a specific protein signal for OATP1A2 at ∼95 kDa in direct immunoblots of human RPE cell lysates (Figure 1), consistent with that detected in OATP1A2 over-expressing HEK-293 cells as well as human kidney tissue lysates (Zhou et al., 2011; 2013; Zheng et al., 2014). In contrast, OATP2B1 protein was not detected in human RPE lysate by immunoblotting (data not shown).
Figure 1.
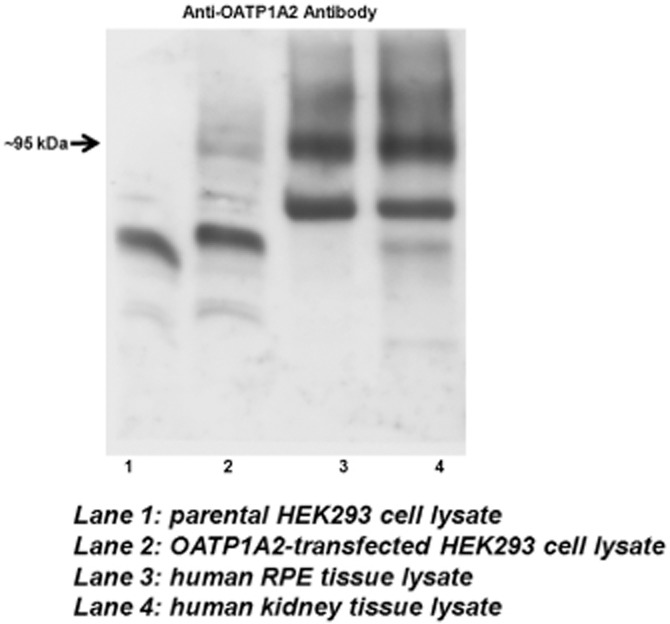
Protein expression of OATP1A2 in human RPE by immunoblot analysis. Cells and human tissues were dissolved in RIPA buffer. Protein lysate was denatured at 55°C for 30 min and loaded onto SDS-PAGE. Protein signal was detected with anti-OATP1A2 antibody (∼95 kDa). Consistent findings were made in RPE tissue lysate obtained from four independent donors, with a representative image shown in this figure.
Immunolabelling revealed that OATP1A2 was abundantly expressed in the RPE cells and, co-localized with the RPE apical membrane marker Na+-K+ ATPase (Figure 2A–D; Gundersen et al., 1991; Rizzolo and Zhou, 1995), but minimally with the basolateral marker Bestrophin (Figure 2E–H; Marmorstein et al., 2000). These observations indicate that OATP1A2 is predominantly localized at the apical membrane of human RPE cells.
Figure 2.
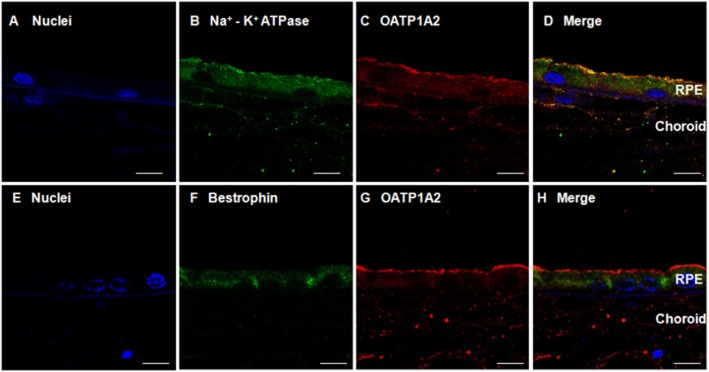
Immunofluorescent labelling of OATP1A2 in human RPE. Sections of human retina were immunolabelled with anti-Na+-K+ ATPase β1 subunit antibody or anti-bestrophin antibody and Alexa Fluor® 488 conjugate donkey anti-mouse IgG or anti-OATP1A2 antibody and Alexa Fluor® 594 conjugate goat anti-rabbit IgG. Panel A shows nuclei (blue); panel B shows immunostaining of the Na+-K+ ATPase β1 subunit (green); panel C shows the immunostaining of OATP1A2 (red); panel D shows the merged images of panels A, B and C. Panel E shows nuclei (blue); panel F shows the immunostaining of bestrophin (green); panel G shows the immunostaining of OATP1A2 (red); panel H shows the merged images of panels E, F and G. Scale bars: 50 μm in panel A–H.
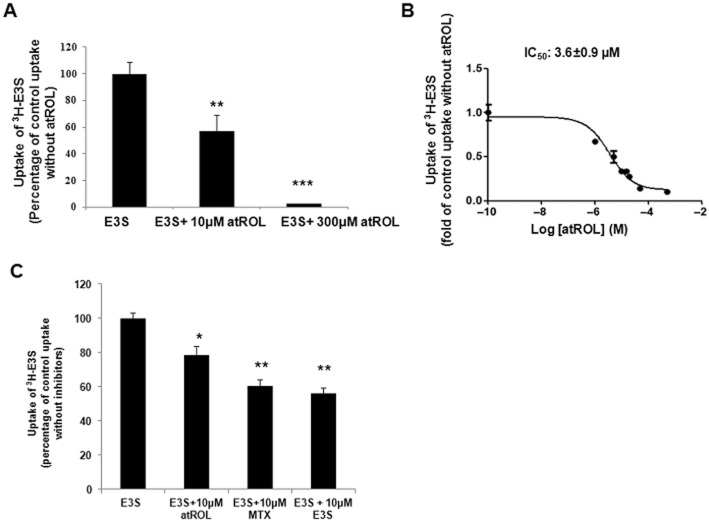
atROL is a potent inhibitor of OATP1A2-mediated E3S uptake
Given the importance of RPE uptake of atROL within the canonical visual cycle, the involvement of OATP1A2 in atROL uptake was evaluated. We first conducted a transporter inhibition study to assess the interaction of atROL with OATP1A2-mediated substrate transport. We found that atROL significantly inhibited the cellular transport of E3S [a classic substrate of OATP1A2 (Zhou et al., 2011; 2013,; Zheng et al., 2014)], mediated by HEK293 cells over-expressing OATP1A2 (Figure 3A). Furthermore, the inhibitory potency analysis indicated that atROL significantly inhibited the uptake of E3S with an IC50 value (concentration at which 50% inhibition of transporter function is obtained) of 3.6 ± 0.9 μM (Figure 3B). atROL also mildly inhibited E3S uptake by human primary RPE cells (Figure 3C) with methotrexate, another known substrate of OATP1A2 (Badagnani et al., 2006; van de Steeg et al., 2013; Zhou et al., 2013) and E3S used as controls.
Figure 3.
atROL is a potent inhibitor of OATP1A2-mediated E3S uptake. (A) atROL inhibition on E3S uptake in the OATP1A2 over-expressing HEK293 cells. Uptake of 300 nM 3H-E3S was assessed in the presence or absence of 10 μM or 300 μM atROL as described in Methods. (B) IC50 value of atROL inhibition on the uptake of 300 nM 3H-E3S was assessed in the absence or presence of a range of atROL (0.1 nM to 80 μM) in the OATP1A2 over-expressing HEK293 cells. (C) atROL inhibition on E3S uptake in the human primary RPE cells. Uptake of 300 nM 3H-E3S was assessed in the presence or absence of 10 μM atROL or methotrexate (MTX) or E3S. Data are from primary human RPE cell cultures isolated from four independent human donors. Values are mean ± SE (triplicate in each experiment, each experiment was repeated three times. *P < 0.05; **P < 0.01; ***P < 0.001; significantly different from uptake without inhibitors).
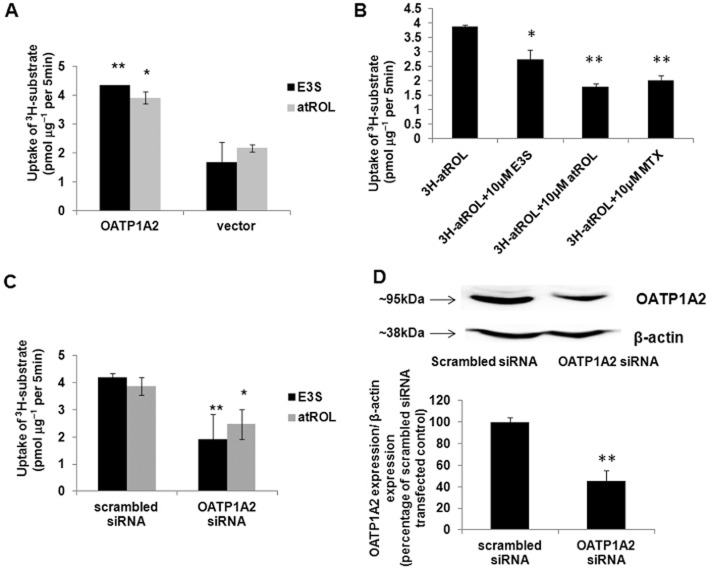
atROL is a novel substrate of OATP1A2
An inhibitor may or may not be a substrate of a transporter. In order to elucidate whether atROL is a substrate of OATP1A2, direct uptake of atROL was assessed with a commercially available 3H-atROL. The uptake of 3H-atROL was ∼1.8-fold higher than the vector-transfected control in the OATP1A2-expressing HEK-293 cells (Figure 4A). Primary RPE cells were used to further confirm the uptake of atROL via OATP1A2 using chemical inhibitors and siRNA silencing techniques. E3S or methotrexate (10 μM) both significantly decreased 3H-atROL uptake by human primary RPE cells (Figure 4B), suggesting that passive diffusion was not the sole mechanism of atROL moving into RPE cells and that a carrier-mediated mechanism is also involved. Furthermore, when OATP1A2-specific siRNAs were transiently transfected into primary RPE cultures to elucidate the role of OATP1A2 in the influx of atROL, impaired transport of both E3S and atROL was observed in all four primary RPE cultures with OATP1A2 gene silencing. We tested three specific siRNAs targeting at different coding regions of OATP1A2 gene, which all achieved comparable efficacy of gene silencing in the primary RPE culture derived from each donor. However, under the same experimental condition, the gene-silencing efficacy varies from ∼40 to ∼90% across the four primary RPE cell lines derived from different donors (data not shown), which was possibly due to the variable susceptibility of each primary culture to siRNA transfection as well as the different expression level of OATP1A2 in individual primary culture. In pooled data from the four RPE primary cultures, uptake of both E3S and atROL was reduced to ∼45 and ∼64%, respectively, of control (Figure 4C). As an example, the impaired OATP1A2 protein expression resulted from siRNA silencing was illustrated in Figure 4D with the immunoblot obtained from the primary RPE culture with moderate gene-silencing efficacy (∼55%).This in vitro finding further confirms the contribution of OATP1A2 to the cellular transport of atROL in human RPE cells.
Figure 4.
atROL is a novel substrate of OATP1A2. (A) Transport of 3H-E3S and 3H-atROL in the HEK 293 cells transiently transfected with OATP1A2. The parental and transporter expressing cells were incubated with 0.3 μM of 3H-E3S (in PBS of pH 7.4) or 0.1 μM of 3H-atROL (in PBS pH 5.0) for 5 min and excessive radio-labelled compound was removed by washing with cold-PBS for three times. Cells were lysed in 0.2M NaOH and then neutralized with 0.2M HCl. Cell lysates were then counted by scintillation counter. Values are mean ± SE (triplicate in each experiment; each experiment was repeated three times). *P < 0.05; **P < 0.01; significantly different from uptake of vector transfected control. (B) E3S and methotrexate (MTX) inhibition of atROL uptake in the human primary RPE cells. Uptake of 0.1 μM 3H-atROL was assessed in the presence or absence of 10 μM E3S or atROL or MTX. The data presented shows findings from human primary RPE cells isolated from four independent human donors. Values are mean ± SE (triplicate in each experiment; each experiment was repeated three times. *P < 0.05; **P < 0.01; significantly different from uptake without inhibitors. (C) Uptake of 3H-E3S and 3H-atROL in primary human RPE cell cultures transiently transfected with scrambled siRNA or OATP1A2-specific siRNA. The siRNA transfected cells were incubated with 0.3 μM of 3H-E3S (in PBS pH 7.4) or 0.1 μM of 3H-atROL (in PBS pH 5.0) for 5 min. The data presented shows findings from human primary RPE cells isolated from four independent human donors. Values are mean ± SE (triplicate in each experiment; each experiment was repeated three times. *P < 0.05; **P < 0.01; significantly different from uptake of scrambled siRNA transfected control). (D) Representative image of the protein expression of OATP1A2 in siRNA transfected human RPE primary cells with moderate gene-silencing efficacy. Human primary RPE cells transfected with scrambled or OATP1A2-specific siRNA were dissolved in RIPA buffer. Protein lysate was denatured and loaded onto SDS-PAGE gels. Protein signal was detected with anti-OATP1A2 and anti-β-actin antibodies. Upper panel: OATP1A2 expression; lower panel: β-actin expression. The same analysis was conducted for human primary RPE cells isolated from four independent donors with the representative blot shown here. Bottom graph: densitometry analysis of OATP1A2 expression normalized to β-actin expression (data from human primary RPE cells isolated from four different donors. **P < 0.01 significantly different from uptake of scrambled siRNA transfected control).
Further kinetic analysis of atROL uptake in both over-expressing HEK293 and human primary RPE cells revealed that the Km of atROL influx via OATP1A2 is ∼89 μM in the over-expressing HEK293 cells and ∼94 μM in the primary RPE cells (Figure 5).
Figure 5.
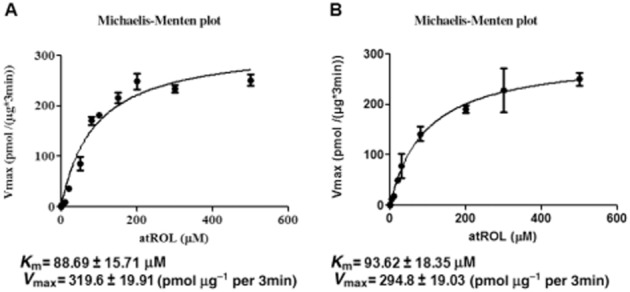
The kinetic parameters of atROL uptake via OATP1A2. (A) The kinetic parameters of 3H-atROL uptake were derived in the HEK293 cells transiently transfected with OATP1A2. Uptake of 3H-atROL was assessed with various concentrations of 3H-atROL (range from 0.02 to 500 μM) subtracting that of vector transfected control. (B) The kinetic parameters of 3H-atROL derived from human primary RPE cells transiently transfected with scrambled or OATP1A2-specific siRNA. Uptake of 3H-atROL was assessed with various concentrations of 3H-atROL (range from 0.02 to 500 μM) in the human primary RPE cells (with ∼90% siRNA gene-silencing efficacy) transfected with scrambled or OATP1A2-specific siRNA. The uptake of atROL was calculated by subtracting OATP1A2-specific siRNA transfected cells from that in cells transfected with scrambled siRNA (triplicate in each experiment; each experiment was repeated two times). Km and Vmax values of atROL uptake were derived by Graphpad Prism 5.0 software. Values are mean ± SEM (n = 3).
Discussion and conclusions
Impaired cellular influx of atROL into RPE cells may lead to the accumulation of retinoids due to the disrupted canonical visual cycle, which consequently increases production of the retinal ‘waste product’ lipofuscin (Clarke and Gulbis, 2012; Gong and Kim, 2013; van de Steeg et al., 2013). Increased production of lipofuscin may contribute to the formation of soft drusen, which are associated with an increased risk of progression for AMD (Sparrow et al., 2003). Although the basolateral uptake of atROL in RPE cells has been well characterized as a receptor-mediated process (Kawaguchi et al., 2007), little is known about transport mechanism of atROL into the RPE cells via the apical membrane as part of the canonical visual cycle.
For the first time, we have demonstrated expression of the human OATP1A2 protein in sections of human RPE and in primary cultured RPE cells (Figure 1). The molecular size of the OATP1A2 protein we detected in RPE cell lysates was comparable with that of OATP1A2 over-expressing HEK293 cells that have been validated in several previous studies (Zhou et al., 2011; 2013; Zheng et al., 2014) and in human kidney tissue lysate, where OATP1A2 was abundantly expressed (Lee et al., 2005). Immunofluorescence microscopy demonstrated that OATP1A2 was predominantly expressed at the apical membrane of RPE cells (Figure 2).
We then explored the functional role of OATP1A2 in the RPE cells. E3S is the classic substrate of OATP1A2 (Lee et al., 2005; Badagnani et al., 2006; Zhou et al., 2011; 2013,; Zheng et al., 2014). We found that atROL was a potent inhibitor of E3S uptake via OATP1A2, with an IC50 of 3.6 ± 0.9 μM (Figure 3). Furthermore, studies on the uptake of radio-labelled atROL in over-expressing HEK293 cells demonstrated that this endogenous compound was a novel substrate of OATP1A2 (Figure 4). Transporter analysis conducted in the primary RPE cells provided more direct evidence that OATP1A2 contributes to the cellular uptake of atROL, and our findings indicated that atROL and E3S could each inhibit uptake of the other substrate (Figures 3C and 4B). More importantly, gene silencing of OATP1A2 also efficiently down-regulated the uptake of both substrates (Figure 4C and D), suggesting that OATP1A2 is at least partially responsible for the uptake of atROL into human RPE cells. Passive diffusion of molecules across biological membranes driven by a concentration gradient occurs for many substances; however, active uptake of molecules via membrane proteins, particularly transporters, may be more common than is usually assumed (Dobson and Kell, 2008). Noteworthy, carrier-mediated transport might be more clinically important than passive diffusion because it is more susceptible to modulation. For instance, co-administrating transporter inhibitors can impair drug uptakes, while inducing the molecular regulators of transporters can increase transporter expression and thus substrate influx. Therefore, OATP1A2 might become a novel therapeutic target proposed for retinal degenerations especially dry AMD in reducing toxic retinoids accumulation within the visual cycle.
Retinols, which are derivatives of vitamin A, are also found in many mammalian tissues where they are important for inter alia, skin health, tooth remineralization and bone growth (Zhong et al., 2012). Studies in enterocytes demonstrated that membrane proteins such as transporters play critical roles in the cellular transport of vitamins including vitamin A and its derivatives (Reboul and Borel, 2011; Reboul, 2013). Our study is the first to show the contribution of influx transporters, particularly OATPs, to the transepithelial movement of such compounds. OATP1A2 was previously found to be expressed in the intestine, kidney, brain and biliary cholangiocytes (Gao et al., 2000; Su et al., 2004; Lee et al., 2005; Badagnani et al., 2006; Glaeser et al., 2007; Yang et al., 2010; Roth et al., 2012). Therefore, our findings may also apply to retinol transport in other tissues and to the disposition of retinols throughout the body. Further studies are warranted to define the role of OATP1A2 in atROL influx in other human tissues.
OATPs have been recognized to be widely involved with therapeutic toxicity due to drugs competing for the OATPs with endogenous/exogenous substances that are also taken up by them (Gao et al., 2014; Taylor-Wells and Meredith, 2014). We propose that the novel transporter-mediated apical uptake of atROL across human RPE identified in the present study is not only essential to maintaining the integrity of the visual cycle and thus vision (Figure 6), but also provides novel insights into retinal dysfunction induced by certain drugs. For instance, the cardiac glycoside ouabain, a known inhibitor of OATP1A2 (Delori et al., 2000), has been reported to induce retinopathy (Marmorstein et al., 2000; Alexander et al., 2013), as may the front line anti-cancer agent imatinib mesylate, which is a substrate of OATP1A2 (Hu et al., 2008; Kitzmann et al., 2008; Zhou et al., 2013). The retinal toxic effects of these drugs could plausibly be due to their inhibition of atROL uptake into the RPE cells via OATP1A2, resulting in dysfunction of the canonical visual cycle and toxic accumulation of retinoids. OATP1A2 may thus be a novel target for pharmacological intervention in certain drug-induced retinal diseases.
Figure 6.
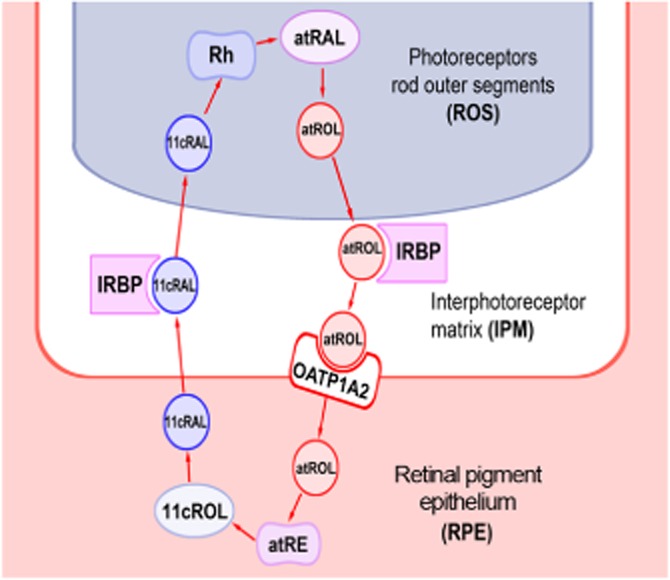
Diagram illustrating the canonical visual cycle in human retina. Photon absorption by the visual chromophore triggers the isomerization from 11-cis-retinal (11cRAL) to all-trans-retinal (atRAL). atRAL is then reduced to atROL in the rod outer segments (ROS). atROL then travels from photoreceptors to the RPE across the IPM in the presence of the IRBP or RBP3. We propose that after moving across the RPE membrane with the assistance of OATP1A2, atROL is transformed into 11cRAL, which exits the RPE and travels back to the ROS to form new functional visual pigment.
In summary, our study showed the expression of OATP1A2 protein in human RPE cells and elucidated the role of this transporter in the cellular uptake of atROL by human RPE cells, which is an essential step in the canonical visual cycle. Our study contributes to a greater understanding of the underlying molecular mechanisms of retinoid transport between RPE cells and photoreceptors. We anticipate that these novel insights may be used to develop potential pharmaceutical interventions for retinal diseases associated with visual cycle disruption for further testing in preclinical and clinical studies.
Acknowledgments
We thank Mr R. Devashayam and Dr M. Zhu, Lions New South Wales Eye Bank for coordinating access to human eye specimens. This work was supported by the University of Sydney DVCR Compact Research Innovation Challenge Research Grant to Fanfan Zhou. Mark C. Gillies is a Sydney Medical Foundation Fellow and is supported by the Australian National Health & Medical Research Council (NH&MRC) Clinical Practitioner Fellowship. Michele C. Madigan is funded by the National Foundation for Medical Research & & Innovation (NFMRI).
Glossary
- AMD
age-related macular degeneration
- atROL
all-trans-retinol
- E3S
oestrone-3-sulfate
- INL
inner nuclear layer
- IPM
interphotoreceptor matrix
- IRBP/RBP3
retinoid-binding protein
- OATP
organic anion transporting polypeptide
- RPE
retinal pigment epithelium
- SLC
solute carrier transporter
Author contributions
T. C., M. C. M. and F. Z. performed the research; L. Z., M. C. M., M. C. G. and F. Z. designed the research; L. Z., M. C. M., K. W., W. S. and F. Z. contributed to discuss and technical guidance or assistance; T. C., L. Z. and F. Z. analysed the data; L. Z., M. C. M., M. C. G. and F. Z. wrote and reviewed the paper.
Conflict of interest
The authors claim no conflicts of interest.
References
- Akanuma S, Hirose S, Tachikawa M, Hosoya K. Localization of organic anion transporting polypeptide (Oatp) 1a4 and Oatp1c1 at the rat blood-retinal barrier. Fluids Barriers CNS. 2013;10:29. doi: 10.1186/2045-8118-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;17:122. doi: 10.1038/ng0997-122a. [DOI] [PubMed] [Google Scholar]
- Anzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. J Pharmacol Sci. 2006;100:411–426. doi: 10.1254/jphs.crj06006x. [DOI] [PubMed] [Google Scholar]
- Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, et al. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther. 2006;318:521–529. doi: 10.1124/jpet.106.104364. [DOI] [PubMed] [Google Scholar]
- Clarke OB, Gulbis JM. Oligomerization at the membrane: potassium channel structure and function. Adv Exp Med Biol. 2012;747:122–136. doi: 10.1007/978-1-4614-3229-6_8. [DOI] [PubMed] [Google Scholar]
- Delori FC, Fleckner MR, Goger DG, Weiter JJ, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:496–504. [PubMed] [Google Scholar]
- Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7:205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- Gao B, Wenzel A, Grimm C, Vavricka SR, Benke D, Meier PJ, et al. Localization of organic anion transport protein 2 in the apical region of rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2002;43:510–514. [PubMed] [Google Scholar]
- Gao B, Vavricka SR, Meier PJ, Stieger B. Differential cellular expression of organic anion transporting peptides OATP1A2 and OATP2B1 in the human retina and brain: implications for carrier-mediated transport of neuropeptides and neurosteriods in the CNS. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1596-x. . [Epub 9 August 2014] [DOI] [PubMed] [Google Scholar]
- Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, et al. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–370. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- Gong IY, Kim RB. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab Pharmacokinet. 2013;28:4–18. doi: 10.2133/dmpk.dmpk-12-rv-099. [DOI] [PubMed] [Google Scholar]
- Gundersen D, Orlowski J, Rodriguez-Boulan E. Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J Cell Biol. 1991;112:863–872. doi: 10.1083/jcb.112.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- Ito A, Yamaguchi K, Onogawa T, Unno M, Suzuki T, Nishio T, et al. Distribution of organic anion-transporting polypeptide 2 (oatp2) and oatp3 in the rat retina. Invest Ophthalmol Vis Sci. 2002;43:858–863. [PubMed] [Google Scholar]
- Ito A, Yamaguchi K, Tomita H, Suzuki T, Onogawa T, Sato T, et al. Distribution of rat organic anion transporting polypeptide-E (oatp-E) in the rat eye. Invest Ophthalmol Vis Sci. 2003;44:4877–4884. doi: 10.1167/iovs.02-1108. [DOI] [PubMed] [Google Scholar]
- Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, et al. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- Kadam RS, Vooturi SK, Kompella UB. Immunohistochemical and functional characterization of peptide, organic cation, neutral and basic amino acid, and monocarboxylate drug transporters in human ocular tissues. Drug Metab Dispos. 2013;41:466–474. doi: 10.1124/dmd.112.045674. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kitzmann AS, Baratz KH, Mohney BG, Pulido JS, Cameron JD, Lee ES, et al. Histologic studies of the intraocular toxicity of imatinib mesylate in rabbits. Eye. 2008;22:712–714. doi: 10.1038/sj.eye.6703092. [DOI] [PubMed] [Google Scholar]
- Lee W, Glaeser H, Smith LH, Roberts RL, Moeckel GW, Gervasini G, et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): implications for altered drug disposition and central nervous system drug entry. J Biol Chem. 2005;280:9610–9617. doi: 10.1074/jbc.M411092200. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000;97:12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci U S A. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BS, Burke JM. Separation of phenotypically distinct subpopulations of cultured human retinal pigment epithelial cells. Exp Cell Res. 1994;213:85–92. doi: 10.1006/excr.1994.1176. [DOI] [PubMed] [Google Scholar]
- Munoz-Erazo L, Natoli R, Provis JM, Madigan MC, King NJ. Microarray analysis of gene expression in West Nile virus-infected human retinal pigment epithelium. Mol Vis. 2012;18:730–743. [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg DR, Okajima TL, Wiggert B, Ripps H, Crouch RK, Chader GJ. Interphotoreceptor retinoid-binding protein (IRBP). Molecular biology and physiological role in the visual cycle of rhodopsin. Mol Neurobiol. 1993;7:61–85. doi: 10.1007/BF02780609. [DOI] [PubMed] [Google Scholar]
- Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, et al. Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet. 2004;75:639–646. doi: 10.1086/424889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients. 2013;5:3563–3581. doi: 10.3390/nu5093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul E, Borel P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res. 2011;50:388–402. doi: 10.1016/j.plipres.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Rizzolo LJ, Zhou S. The distribution of Na+,K(+)-ATPase and 5A11 antigen in apical microvilli of the retinal pigment epithelium is unrelated to alpha-spectrin. J Cell Sci. 1995;108(Pt 11):3623–3633. doi: 10.1242/jcs.108.11.3623. [DOI] [PubMed] [Google Scholar]
- Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr. 2012;32:125–145. doi: 10.1146/annurev-nutr-071811-150748. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Fishkin N, Zhou J, Cai B, Jang YP, Krane S, et al. A2E, a byproduct of the visual cycle. Vision Res. 2003;43:2983–2990. doi: 10.1016/s0042-6989(03)00475-9. [DOI] [PubMed] [Google Scholar]
- van de Steeg E, van Esch A, Wagenaar E, Kenworthy KE, Schinkel AH. Influence of human OATP1B1, OATP1B3, and OATP1A2 on the pharmacokinetics of methotrexate and paclitaxel in humanized transgenic mice. Clin Cancer Res. 2013;19:821–832. doi: 10.1158/1078-0432.CCR-12-2080. [DOI] [PubMed] [Google Scholar]
- Su Y, Zhang X, Sinko PJ. Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of Saquinavir in Hep G2 cells. Mol Pharm. 2004;1:49–56. doi: 10.1021/mp0340136. [DOI] [PubMed] [Google Scholar]
- Taylor-Wells J, Meredith D. The signature sequence region of the human drug transporter organic anion transporting polypeptide 1B1 is important for protein surface expression. J Drug Deliv. 2014;2014:129849. doi: 10.1155/2014/129849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Gyurus P, Fleischer LL, Bingham EL, McHenry CL, Apfelstedt-Sylla E, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41:4293–4299. [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cui X, Gu Q, Chen Y, Zhou J, Kuang Y, et al. Retinol dehydrogenase 13 protects the mouse retina from acute light damage. Mol Vis. 2012;18:1021–1030. [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci. 2010;117:294–302. doi: 10.1093/toxsci/kfq219. [DOI] [PubMed] [Google Scholar]
- Zhang T, Xiang CD, Gale D, Carreiro S, Wu EY, Zhang EY. Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: implications for ocular drug disposition. Drug Metab Dispos. 2008;36:1300–1307. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- Zheng J, Chan T, Cheung FS, Zhu L, Murray M, Zhou F. PDZK1 and NHERF1 regulate the function of human organic anion transporting polypeptide 1A2 (OATP1A2) by modulating its subcellular trafficking and stability. PLoS ONE. 2014;9:e94712. doi: 10.1371/journal.pone.0094712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Kawaguchi R, Kassai M, Sun H. Retina, retinol, retinal and the natural history of vitamin A as a light sensor. Nutrients. 2012;4:2069–2096. doi: 10.3390/nu4122069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, You G. Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharm Res. 2007;24:28–36. doi: 10.1007/s11095-006-9144-9. [DOI] [PubMed] [Google Scholar]
- Zhou F, Zhu L, Cui PH, Church WB, Murray M. Functional characterization of nonsynonymous single nucleotide polymorphisms in the human organic anion transporter 4 (hOAT4) Br J Pharmacol. 2010;159:419–427. doi: 10.1111/j.1476-5381.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Lee AC, Krafczyk K, Zhu L, Murray M. Protein kinase C regulates the internalization and function of the human organic anion transporting polypeptide 1A2. Br J Pharmacol. 2011;162:1380–1388. doi: 10.1111/j.1476-5381.2010.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Zheng J, Zhu L, Jodal A, Cui PH, Wong M, et al. Functional analysis of novel polymorphisms in the human SLCO1A2 gene that encodes the transporter OATP1A2. AAPS J. 2013;15:1099–1108. doi: 10.1208/s12248-013-9515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Shen W, Zhu M, Coorey NJ, Nguyen AP, Barthelmes D, et al. Anti-retinal antibodies in patients with macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2013;54:5675–5683. doi: 10.1167/iovs.13-12050. [DOI] [PubMed] [Google Scholar]
- Zhu M, Provis JM, Penfold PL. Isolation, culture and characteristics of human foetal and adult retinal pigment epithelium. Aust N Z J Ophthalmol. 1998;26(Suppl. 1):S50–S52. doi: 10.1111/j.1442-9071.1998.tb01371.x. [DOI] [PubMed] [Google Scholar]