Abstract
Background and Purpose
Recently, we demonstrated that the nucleus accumbens (NAC) is required for the acquisition and expression of relief memory. The purpose of this study was to investigate the role of NMDA receptors within the NAC in relief learning.
Experimental Approach
The NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (AP-5) was injected into the NAC. The effects of these injections on the acquisition and expression of relief memory, as well as on the reactivity to aversive electric stimuli, were tested.
Key Results
Intra-accumbal AP-5 injections blocked the acquisition but not the expression of relief memory. Furthermore, reactivity to aversive electric stimuli was not affected by the AP-5 injections.
Conclusion and Implication
The present data indicate that NMDA-dependent plasticity within the NAC is crucial for the acquisition of relief memory.
Tables of Links
| LIGANDS |
|---|
| Dopamine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b,).
Introduction
Learning about relations between stimuli and events is essential for adaptive behaviour (McNally and Westbrook, 2006). A well-investigated example for such learning is fear conditioning in which animals or humans learn that a particular cue predicts an aversive event (Fendt and Fanselow, 1999; LeDoux, 2012). This cue then becomes a conditioned fear stimulus that prepares the brain and body for future environmental dangers. Less well-known cues that are presented after an aversive event can also lead to associations. As the to-be-learned stimulus is presented in the moment of relief from the aversive event, this learning was called relief learning (Gerber et al., 2014). In contrast to conditioned fear stimuli that induce aversive behaviour (avoidance or startle potentiation), conditioned relief stimuli trigger appetitive-like behaviour such as approach behaviour or startle attenuation (Tanimoto et al., 2004; Andreatta et al., 2012). Initial research on relief learning was performed in fruit flies (Tanimoto et al., 2004; Yarali et al., 2008) but relief learning has also been demonstrated in humans and laboratory rodents (Andreatta et al., 2010; 2012,; Mohammadi et al., 2014).
Due to the appetitive-like nature of relief learning, it was suggested that the brain's reward system is involved in relief learning. Indeed, a human imaging study showed an activation of the nucleus accumbens (NAC), a crucial part of the reward system (Ikemoto, 2007), during expression of conditioned relief (Andreatta et al., 2012). Furthermore, temporary inactivation of the NAC in rats blocks both the acquisition and expression of relief memory (Andreatta et al., 2012; Mohammadi et al., 2014). Because the plasticity within the NAC is responsible for reward learning and response-reinforcement learning (Kelley, 2004; Miller and Marshall, 2005), these data suggest that the NAC is also the brain site of relief learning.
Most forms of associative learning are dependent on NMDA receptors (Maren, 2000; Martin et al., 2000; Chapman, 2001). In line with this general observation, it was repeatedly demonstrated that reward learning and response-reinforcement learning are mediated by NMDA receptors within the NAC (Kelley et al., 1997; Smith-Roe and Kelley, 2000; Di Ciano et al., 2001; Kelley, 2004). These findings suggest that relief learning may also be mediated by accumbal NDMA receptors.
The present study aims to address this hypothesis. Therefore, rats received local injections of the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (AP-5) into the NAC and were then submitted to relief conditioning. One day later, conditioned relief was tested using the acoustic startle paradigm. In a second experiment, it was tested if AP-5 injections affect the expression of conditioned relief and the reactivity to the electric stimuli. The electric stimuli were used as an unconditioned stimulus (US) in the relief conditioning procedure.
Methods
Animals and surgery
Thirty-nine adult male Sprague Dawley rats aged between 2 and 3 months (250–350 g) at the time of the surgery were used in this experiment. Rats were bred and reared at the local animal facility (original breeding stock: Taconic, Ry, Denmark). They were kept in groups of four to six animals per cage (Makrolon Type IV; Tecniplast, Hohenpeißenberg, Germany) in temperature- and humidity-controlled rooms (22 ± 2°C, 50 ± 10%) under a light : dark cycle of 12 h:12 h (lights on 6:00 h) and had free access to water and food. All experiments and surgeries were performed during the light phase. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010) and were approved by the local ethical committee (Landesverwaltungsamt Sachsen-Anhalt, Az. 42502-2-1172 UniMD).
The animals were anaesthetized with isoflurane (Baxter Germany GmbH; Unterschleißheim, Germany) mixed with pure oxygen (5% isoflurane for induction, then 2.0–2.5%). The depth of anaesthesia was assessed by testing reflexes to a hind-paw pinch. Then, the animals were fixed in a rodent stereotaxic apparatus. The skull was exposed and stainless steel guide cannulas (custom-made; diameter: 0.7 mm, length: 8.0 mm) were bilaterally implanted aiming at the NAC: 1.2 mm rostral, ± 1.5 mm lateral and 7.4 mm ventral to the bregma (Paxinos and Watson, 1997). Cannulas were fixed with dental cement to the skull and three anchoring screws. After the surgery, the animal was housed on its own and supervised for 4 h and then returned to the colony. After the surgery, there was a recovery period of 5–7 days.
Apparatus
We used a startle system with eight chambers (35 cm × 35 cm × 35 cm; SR-LAB, San Diego Instruments, San Diego, CA, USA). Each chamber consisted of a stable platform holding a horizontal, cylindrical, transparent animal enclosure with an inner diameter of 9 cm and an inner length of 16 cm. Underneath the platform, a piezoelectric motion sensor was mounted for measuring animal movements. The output signal of this sensor was digitalized with a sampling rate of 1 kHz and sent to the computer. Beginning at startle stimulus or electric stimulus onset, consecutive 1 ms readings were recorded to obtain the magnitude of the animal's response to the startle stimulus or electric stimulus (arbitrary units). The startle magnitude, average readout in the ‘startle response peak window’, was taken every 10–30 ms after startle stimulus onset.
For relief conditioning, aversive electric stimuli (US) and light stimuli (conditioned stimulus, CS) were used. The light stimulus was produced by a 10 W bulb, had an intensity of ∼1000 lux and duration of 5 s. The electric stimuli were administered via a floor grid (six bars with 5 mm diameter, distance: 10 mm), had an intensity of 0.4 mA and a duration of 0.5 s. For the application of acoustic stimuli, a loudspeaker mounted on the ceiling of the box was used. During all tests, a background noise with an intensity of 50 dB sound pressure level (SPL) was presented to mask environmental noises. The acoustic startle stimulus was a noise burst with an intensity of 96 dB SPL for a duration of 40 ms. For testing the reactivity to electric stimuli, stimulus intensities of 0.0, 0.1., 0.2, 0.3 and 0.4 mA were used. As a readout for reactivity to electric stimuli, the summed readout of the piezoelectric motion sensor during the electric stimuli (500 ms) was used.
Behavioural protocol
Experiment 1: effects of intra-NAC AP-5 injections on acquisition of relief memory
Day 1 (baseline session): animals (n = 31) were put in the chambers and after 5 min of acclimatization, 10 startle stimuli were delivered with an inter-trial interval of 30 s (see also Figure 1). Then, the animals were put back into their home cages. Based on the mean startle amplitude of this session, the animals were distributed into groups with balanced mean baseline startle amplitude.
Figure 1.
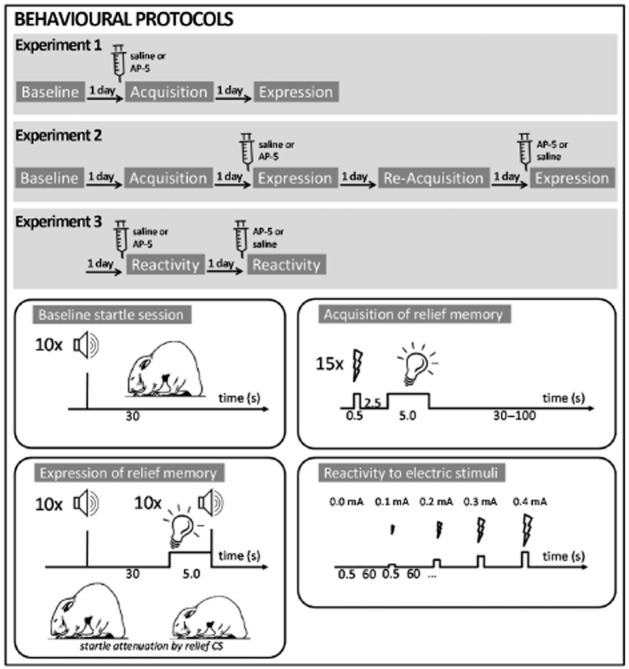
Behavioural protocols of the present study. Upper panels depict the treatment schedule and the test sessions performed. Lower panels give detailed information on the different test sessions.
Day 2 (training session): the animals were injected with either the vehicle or AP-5 (2.5 or 5 nmol·0.3 μL−1, Sigma Aldrich, Munich, Germany). To do this, the animals were gently held in place by hand to insert the injection cannulas (custom-made; diameter: 0.3 mm, 12 mm long), which were connected via a tube to a 10 μL syringe into the guide cannulas. Then a volume of 0.3 μL was injected with a speed of 0.2 μL·min−1 using a microinjection pump (CMA/100, Microdialysis AB, Stockholm, Sweden). The cannulas were left in place for 1 additional minute. Ten minutes later, the animals were put into the startle chambers. After 5 min of acclimatization, the relief conditioning protocol was performed. Fifteen electric stimuli followed by a light stimulus (fixed inter-stimulus interval: 3 s from onset electric stimulus to onset light stimulus) were delivered to the animals. The inter-trial interval (onset electric stimulus to onset next electric stimulus) was pseudo-randomized and varied between 30 and 100 s. No startle stimuli were delivered to the animals during the conditioning session.
Day 3 (retention test): the animals were put into the startle chamber. Following 5 min of acclimatization, 10 startle stimuli were presented to habituate the animal, followed by 20 startle stimuli, 10 of them without the light CS and 10 of them upon presentation of the light CS (CS and startle stimuli co-terminated). The order of the trials with and without the light CS was pseudo-randomized.
Experiment 2: effects of intra-NAC AP-5 injections on expression of relief memory
Day 1 and 2: eight animals were used for this experiment. The baseline and training session was identical to those of experiment 1 except that no injections were performed before or during the relief conditioning session.
Day 3: half of the animals were injected with the vehicle and the other half with 5 nmol·0.3 μL−1 AP-5. The animals were put into the chambers immediately after injection and the retention test was run.
Day 4: the animals were reconditioned with relief conditioning protocol from day 2.
Day 5: the test of day 3 was repeated. However, animals that received injections of saline on day 3 were now injected with AP-5 and vice versa.
Experiment 3: effects of intra-NAC AP-5 injections on locomotor reactivity to electric stimuli
For this experiment, the animals of experiment 2 were used. One day after completion of experiment 2, the animals were injected with either saline or 5 nmol·0.3 μL−1 AP-5. Then they were put into the startle chambers. After an acclimatization time of 5 min, five electric stimuli with increasing intensities (0.0, 0.1, 0.2, 0.3 and 0.4 mA) were administered with an inter-stimulus interval of 30 s. One day later, the same procedure was repeated. However, rats that received saline on the day before now received injections of AP-5 and vice versa.
We used doses of 2.5 and 5 nmol AP-5·0.3 μL−1. These doses are based on the effective doses found in several published studies (Miserendino et al., 1990; Kelley et al., 1997; Schauz and Koch, 2000; Palencia and Ragozzino, 2004; Hsu and Packard, 2008).
Histology
At the end of the behavioural experiments, the animals were killed by CO2. The brains were removed and put into 30% sucrose and 4% formalin solution for fixation. The brains were sectioned by a cryostat in 60 μm slices. The slides were Nissl-stained with cresyl violet, examined with light microscopy and the injection sites (injection cannula trace) were located and marked on sections of a brain atlas (Paxinos and Watson, 1997).
Data analysis
For each animal, the mean response to the electric stimuli, the mean startle amplitudes with and without the light CS (peak amplitudes within the 100 ms after the startle stimulus onset) and their difference were calculated. Because all data were normally distributed (D'Agostino and Pearson's omnibus normality test), means and SEM were shown in the figures and parametric statistical tests were used for analysis (Prism 6.0, GraphPad Software Inc., La Jolla, CA, USA). A significance level of P < 0.05 was used for all tests.
Results
Histology
Histological analyses of the injection sites revealed that 35 animals received bilateral injections of saline or AP-5 into the NAC (ns = 7–11 per group). Most of the injection sites were localized in the core region of the NAC (Figure 2). Four animals were discarded because of misplaced injections.
Figure 2.
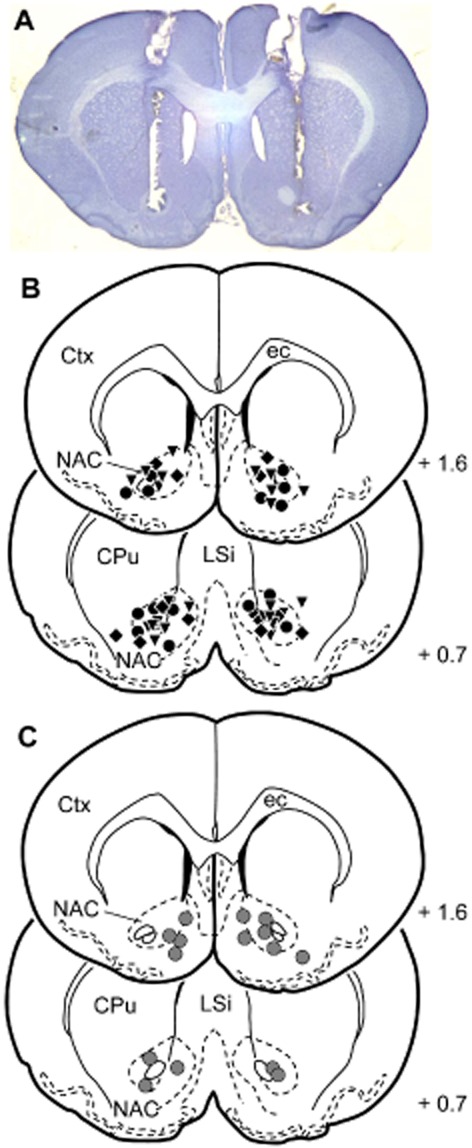
Reconstruction of the vehicle and AP-5 injection sites into the NAC on frontal brain sections (Paxinos and Watson, 1997). (A) Representative photomicrograph showing a brain slide with injection sites into the NAC. (B) Injections before relief conditioning (acquisition). Black circles, saline; inverse triangles, 2.5 nmol AP-5; diamonds, 5 nmol AP-5. (C) Injections (gray circles) before expression of relief memory and before testing on reactivity to electric stimuli. Values represent the anterior distance to bregma (mm) according to Paxinos and Watson (1997). CPu, caudate putamen; Ctx, cortex; ec, external capsule; LSi, lateral septal nucleus.
Behaviour
Experiment 1: effects of intra-NAC AP-5 injections on acquisition of relief memory
In this experiment, saline or two different doses of AP-5 (2.5 or 5 nmol·0.3 μL−1) were injected into the NAC immediately before the relief conditioning session (see Figure 1). On the following day, a retention test was performed without any injections. Figure 3A depicts the mean startle magnitudes of startle alone and CS-startle trials of the retention test as well as their difference. The data show that AP-5 injections into the NAC dose-dependently inhibit the acquisition of relief memory. This is supported by an anova with startle trial type as within-subject factor and treatment as between-subject factor. There was a main effect of trial type (F1,22 = 8.90, P < 0.0001) and significant interaction between trial type and treatment (F2,22 = 4.58, P = 0.02). There were no main effects of treatment (F2,22 = 1.13, P = 0.34) indicating that the startle response itself was not affected by AP-5 injections. Post hoc Sidak's multiple comparison tests show significant trial type effects in the saline-injected group (t = 3.94, P < 0.01) but not in the two AP-5-injected groups (ts < 0.69, Ps > 0.05). This effect of intra-NAC AP-5 is further supported by an anova on the difference scores (F2,22 = 4.50, P = 0.02) and post hoc Dunnett's tests showing significant differences between animals treated with saline and animals treated with the two AP-5 doses (Ps < 0.05).
Figure 3.
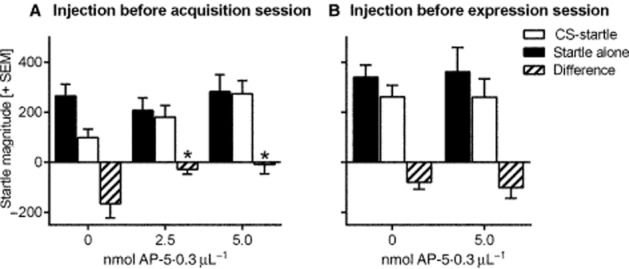
Effects of intra-NAC injections on the acquisition and expression of relief memory. Depicted is the startle magnitude (arbitrary units + SEM) without and with the presence of the relief CS (light stimulus), as well as the difference between these two trial types. (A) AP-5 injected before conditioning dose-dependently blocks acquisition of relief memory. *P < 0.05 comparison with 0 nmol (Dunnett's test) after significant effects in anova. (B) AP-5 injected before testing expression of relief memory has no effects.
Experiment 2: effects of intra-NAC AP-5 injections on expression of relief memory
Animals were relief conditioned without treatment. On the following day, they received injections of saline and AP-5 (5 nmol·0.3 μL−1) into the NAC and they were tested for their relief memory. There was apparently no effect of intra-NAC AP-5 injections on the expression of relief memory (Figure 3B). An anova with startle trial type and treatment as within-subject factors revealed a significant effect of trial type (F1,6 = 10.79, P = 0.02) but neither of treatment (F1,6 = 0.88, P = 0.88) nor an interaction between trial type and treatment (F1,6 = 0.30, P = 0.61). Furthermore, post hoc Sidak's multiple comparison tests showed significant trial type effects on both, saline- and AP-5-injected animals (ts > 2.97, Ps < 0.05). This is supported by a comparison of the difference scores showing no treatment effects (paired t-test: t = 0.59, P = 0.57). Notably, the testing order had no effect (F1,6 = 0.003, P = 0.96).
Experiment 3: effects of intra-NAC AP-5 injections on locomotor reactivity to electric stimuli
Animals were injected with either saline or AP-5 (5 nmol·0.3 μL−1) and then tested for their reactivity to electric stimuli with increasing intensities (0.0 to 0.4 mA). In Figure 4, the mean locomotor response of the animals during the 0.5 s duration of the electric stimuli is depicted. Injections of AP-5 into the NAC did not affect the reactivity to electric stimuli. An anova with intensity and treatment as within-subject factors revealed a significant effect of intensity (F4,28 = 21.26, P < 0.001) but neither of treatment (F1,28 = 0.10, P = 0.76) nor an interaction between intensity and treatment (F4,28 = 0.21, P = 0.93).
Figure 4.
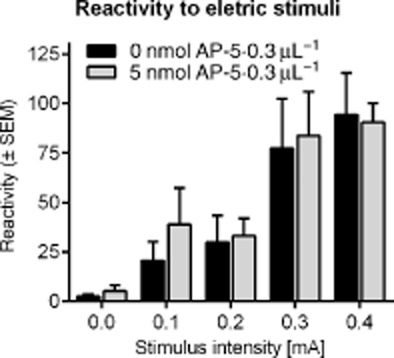
Effects of intra-NAC injections on the locomotor reactivity to electric stimuli. Shown is the mean locomotor activity (arbitrary units + SEM) during the electric stimuli (500 ms duration, different intensities). AP-5 injections did not affect the reactivity to electric stimuli.
Discussion
The present study investigated the role of accumbal NMDA receptors in the acquisition and expression of relief memory. Therefore, local injections of the NMDA receptor antagonist AP-5 were performed either directly before relief conditioning (acquisition) or before the retention test on conditioned relief (expression). Our data clearly show that accumbal NMDA receptor blockade before conditioning, but not before the retention test, prevented conditioned relief. Furthermore, we demonstrated that NMDA receptor blockade does not affect the reactivity to electric stimuli. Taken together, this indicates that relief learning depends on NMDA receptor activation within the NAC.
To measure conditioned relief, we used the acoustic startle paradigm. In line with the data of our previous studies (Andreatta et al., 2012; Mohammadi et al., 2014), the relief CS robustly attenuated the startle magnitude under control conditions (injections of saline into the NAC). It is important to note that such an attenuation of the startle magnitude by a CS can only be observed if the CS has a positive valence. This can be obtained not only by relief conditioning (CS presented shortly after an aversive US) but also after safety conditioning (CS explicitly unpaired with an aversive US; e.g. Mohammadi et al., 2014) and after ‘pleasure conditioning’ (CS precedes an appetitive US; e.g. Schmid et al., 1995). In contrast, fear conditioning, in which the CS precedes an aversive US, induces a startle potentiation (Davis et al., 1993; Fendt and Koch, 2013). No modulation of the startle magnitude can be observed if the presentations of the CS and the US are randomized during the conditioning phase (i.e. that by chance the CS and the US can also simultaneously appear) or if the CS is presented without any US during the conditioning phase (Davis and Astrachan, 1978; Andreatta et al., 2012).
Previous studies from our group demonstrated that the NAC is crucial for the acquisition and expression of conditioned relief (Andreatta et al., 2012; Mohammadi et al., 2014). Importantly, the NAC is not involved in safety learning, that is the learning that a cue predicts the absence of the US (Josselyn et al., 2005; Mohammadi et al., 2014). In latter studies, safety learning was either induced by explicit unpairing of the US and the CS or by a conditioned inhibition procedure. (cf. Christianson et al., 2012). This demonstrates that there is a neural dissociation between relief learning and safety learning, strongly supporting the view that relief learning and safety learning represent distinct learning processes (Gerber et al., 2014). In humans and rats, we previously demonstrated a neural dissociation between fear learning and relief learning (Andreatta et al., 2012). The amygdala is crucial for fear learning but not for relief learning, whereas the NAC is crucial for relief learning but not for fear learning. Taken together, the NAC is only involved in relief learning, not in fear or safety learning. This strongly suggests that the observed effects after local injections into the NAC are specific to relief learning and not unspecific to any associative relationship between CS and US (as e.g. in safety or fear learning).
The present data now show an important role of accumbal NMDA receptors in relief learning. Both of our AP-5 doses, 2.5 and 5 nmol AP-5·0.3 μL−1, significantly blocked the acquisition of relief memory. These doses are in line with effective doses found in the literature (e.g. Kelley et al., 1997). However, it is important to note that the effects observed in the present study can also be explained by state- dependency, that is the AP-5 injections before relief conditioning induced a specific state which is necessary later to express conditioned relief. However, such a state- dependency has not been observed yet for NMDA receptor blockade in associative learning (e.g. Tzschentke and Schmidt, 1997; Bast et al., 2003). Therefore, we are confident that the observed AP-5 effects are based on a blockade of acquisition and not on state-dependency.
The NAC consists of different regions, the core and the shell regions (Zahm and Brog, 1992). Based on data from Kelley et al. (1997), it is thought that the NAC core mediates the blockade of relief conditioning observed in this study. This is supported by the fact that our injection sites were almost exclusively located within the core region (cf. Figure 2). However, we injected a volume of 0.3 μL and such a volume may diffuse ca. 0.5 μm (Martin, 1991). This means that we cannot exclude the possibility that the injected AP-5 also reaches neurons within the NAC shell. In fact, it could be that the shell region of the NAC is more important for relief learning than the core region. The shell region is the projection target of dopaminergic neurons within the ventral tegmental area which show an excitatory response to the offset of electric stimuli (Brischoux et al., 2009), that is in the moment of relief from the electric stimulus.
In our second experiment, the animals were relief conditioned without any treatment and AP-5 was then injected immediately before the retention test. These injections clearly did not affect the expression of relief memory. This demonstrates not only that accumbal NMDA receptors are not involved in the expression of relief memory but also that the sensory processing of the visual relief CS is not disturbed by AP-5 injections into the NAC. The latter indicates that the blockade of relief learning observed in our first experiment is not due to AP-5 effects on the sensory processing of the relief CS. However, a blockade of relief learning could also be explained by disturbed sensory processing of the US. Therefore, we performed a third experiment in which we tested the effects of accumbal AP-5 injections on the locomotor reactivity to electric stimuli. Clearly, AP-5 injections did not affect this reactivity indicating that the blockade of relief learning by AP-5 injections into the NAC is also not caused by disturbed US processing.
That means the sensory processing of both the CS and the US are not impaired after AP-5 injections into the NAC. Therefore, the most obvious interpretation of the AP-5 effects in our first experiment is that AP-5 prevented the association between the CS and the US. Thus, relief learning is based on NMDA receptor-dependent plasticity within the NAC, for example long-term potentiation (Schotanus and Chergui, 2008).
Several studies have already demonstrated that accumbal NMDA receptors are involved in both appetitive Pavlovian and instrumental conditioning (Kelley et al., 1997; Smith-Roe and Kelley, 2000; Di Ciano et al., 2001; Dalley et al., 2005). In these learning processes, rewarding stimuli are used as an US, whereas in relief conditioning experiments, an aversive US is used. However, as discussed earlier and in several publications before (Gerber et al., 2014; e.g. Tanimoto et al., 2004), the timing of CS and US presentation is critical for the valence of the learned association. If the CS precedes the US in Pavlovian conditioning (forward pairing), the CS will gain negative valence and will later induce behavioural signs of fear (summarized in Fendt and Fanselow, 1999; Davis, 2006; LeDoux, 2012). The underlying plasticity of this learning occurs in the amygdala (Maren, 2005; Pape and Pare, 2010). However, if the US precedes the CS (backward pairing), the CS will gain positive valence as the relief from an aversive stimulus can be considered as a reward. In line with this idea, midbrain dopaminergic neurons were described that were phasically excited after the offset of aversive electric stimuli (Brischoux et al., 2009). These neurons were dopaminergic and located in the ventral region of the ventral tegmental area, a brain site which plays a key role in reward processing (Schultz, 1998; Wise, 2004). The ventral tegmental area projects to the NAC and is its main dopaminergic input (Fallon and Moore, 1978). For relief conditioning, it is not known whether accumbal dopamine is involved. However, as it is involved in appetitive Pavlovian and instrumental conditioning, we suggest that this is also the case in relief learning. We will address this hypothesis in future studies in our laboratory. Furthermore, appetitive conditioning depends on a coincident activation of NMDA and dopamine receptors within the NAC (Smith-Roe and Kelley, 2000; Di Ciano et al., 2001; Dalley et al., 2005). If accumbal dopamine is involved in relief learning, it is very probable that this coincident receptor activation is also the molecular mechanism underlying relief learning.
Taken together, the present results clearly demonstrate that acquisition of relief memory is dependent on NMDA receptors within the NAC. These receptors do not seem to be crucial for the expression of relief memory and the sensory processing of the CS and the US used in relief learning. Future studies should investigate the role of accumbal dopamine in relief learning and whether accumbal dopamine receptors interact with NMDA receptors during the establishment of relief memory.
Acknowledgments
The authors thank Dr. Jorge Bergado-Acosta and Evelyn Kahl for their help in performing the experiments, Kathrin Freke for animal care and Timothy French for language editing. This study was funded by the Deutsche Forschungsgemeinschaft (SFB779/B13).
Glossary
- AP-5
2-amino-5-phosphonopentanoic acid
- NAC
nucleus accumbens
Author contributions
M. M. and M. F. conceived and designed the experiments and wrote the manuscript. M. M. performed the experiments and analysed the data.
Conflict of interest
The authors have no conflict of interest to declare.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatta M, Mühlberger A, Yarali A, Gerber B, Pauli P. A rift between implicit and explicit conditioned valence in human pain relief learning. Proc Biol Sci. 2010;277:2411–2416. doi: 10.1098/rspb.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatta M, Fendt M, Mühlberger A, Wieser MJ, Imobersteg S, Yarali A, et al. Onset and offset of aversive events establish distinct memories requiring fear- and reward networks. Learn Mem. 2012;19:518–526. doi: 10.1101/lm.026864.112. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PF. The diversity of synaptic plasticity. Nat Neurosci. 2001;4:556–558. doi: 10.1038/88367. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, Sangha S. Inhibition of fear by learned safety signals: a mini-symposium review. J Neurosci. 2012;32:14118–14124. doi: 10.1523/JNEUROSCI.3340-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Theobald DEH, Armstrong HC, Corlett PR, Chudasama Y, et al. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Astrachan DI. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M. Translational value of startle modulations. Cell Tissue Res. 2013;354:287–295. doi: 10.1007/s00441-013-1599-5. [DOI] [PubMed] [Google Scholar]
- Gerber B, Yarali A, Diegelmann S, Wotjak CT, Pauli P, Fendt M. Pain-relief learning in flies, rats, and man: basic research and applied perspectives. Learn Mem. 2014;21:232–252. doi: 10.1101/lm.032995.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol Learn Mem. 2008;89:504–512. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Falls WA, Gewirtz JC, Pistell P, Davis M. The nucleus accumbens is not critically involved in mediating the effects of a safety signal on behavior. Neuropsychopharmacology. 2005;30:17–26. doi: 10.1038/sj.npp.1300530. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, SmithRoe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-n-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci U S A. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 2000;23:345–346. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Predicting danger: the nature, consequences, and neural mechanisms of predictive fear learning. Learn Mem. 2006;13:245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Bergado-Acosta JR, Fendt M. Relief learning is distinguished from safety learning by the requirement of the nucleus accumbens. Behav Brain Res. 2014;272:40–45. doi: 10.1016/j.bbr.2014.06.053. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Schauz C, Koch M. Blockade of NMDA receptors in the amygdala prevents latent inhibition of fear-conditioning. Learn Mem. 2000;7:393–399. doi: 10.1101/lm.33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Koch M, Schnitzler H-U. Conditioned pleasure attenuates the startle response in rats. Neurobiol Learn Mem. 1995;64:1–3. doi: 10.1006/nlme.1995.1037. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Long-term potentiation in the nucleus accumbens requires both NR2A- and NR2B-containing N-methyl-d-aspartate receptors. Eur J Neurosci. 2008;27:1957–1964. doi: 10.1111/j.1460-9568.2008.06173.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto H, Heisenberg M, Gerber B. Event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Interactions of MK-801 and GYKI 52466 with morphine and amphetamine an place preference conditioning and behavioural sensitization. Behav Brain Res. 1997;84:99–107. doi: 10.1016/s0166-4328(97)83329-3. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Yarali A, Niewalda T, Chen YC, Tanimoto H, Duerrnagel S, Gerber B. Pain relief' learning in fruit flies. Anim Behav. 2008;76:1173–1185. [Google Scholar]
- Zahm DS, Brog JS. On the significance of subterritories in the ‘accumbens’ part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]


