Significance
Sex-determining region Y-related HMG box 2 (SOX2) is a well-established marker of neural stem and progenitor cells, and its function was shown to be required for the self-renewal of these cells. However, the function of SOX2 in neuronal differentiation is poorly understood. Here we described a novel role of SOX2 in neuronal differentiation in which SOX2 binds to bivalently marked promoters of poised proneural genes in neural progenitor cells and limits the activity of polycomb repressive complex 2 and excessive levels of histone H3 Lys 27 trimethylation. We propose a novel function of SOX2 in maintaining a permissive epigenetic state thus enabling proper activation of the neuronal differentiation program under neurogenic cue.
Keywords: SOX2, neurogenesis, epigenetics
Abstract
Newborn granule neurons generated from neural progenitor cells (NPCs) in the adult hippocampus play a key role in spatial learning and pattern separation. However, the molecular mechanisms that control activation of their neurogenic program remain poorly understood. Here, we report a novel function for the pluripotency factor sex-determining region Y (SRY)-related HMG box 2 (SOX2) in regulating the epigenetic landscape of poised genes activated at the onset of neuronal differentiation. We found that SOX2 binds to bivalently marked promoters of poised proneural genes [neurogenin 2 (Ngn2) and neurogenic differentiation 1 (NeuroD1)] and a subset of neurogenic genes [e.g., SRY-box 21 (Sox21), brain-derived neurotrophic factor (Bdnf), and growth arrest and DNA-damage–inducible, beta (Gadd45b)] where it functions to maintain the bivalent chromatin state by preventing excessive polycomb repressive complex 2 activity. Conditional ablation of SOX2 in adult hippocampal NPCs impaired the activation of proneural and neurogenic genes, resulting in increased neuroblast death and functionally aberrant newborn neurons. We propose that SOX2 sets a permissive epigenetic state in NPCs, thus enabling proper activation of the neuronal differentiation program under neurogenic cue.
Cell fate and differentiation decisions of adult neural progenitor cells (NPCs) are controlled by intrinsic and extrinsic signals from the neurogenic niche (1–3). Recent genomewide analyses of epigenetic regulators in the brain have provided considerable insight into the mechanisms that regulate neural development, neurological disease, and aging (4, 5). The chromatin states of NPCs change dynamically during cell-fate determination and cell differentiation, and chromatin marks such as histone H3 trimethylated Lys 27 (H3K27me3), histone H3 trimethylated Lys 4 (H3K4me3), and histone H3 acetylated Lys 9 (H3K9ac) are essential for regulating the expression of key genes involved in these processes (6). Sex-determining region Y (SRY)-related HMG box 2 (SOX2) is a member of the SOXB1 family of transcription factors, which play important roles in maintaining neural stem/progenitor cell properties, including their capacity to proliferate and self-renew (7, 8). In humans, SOX2 mutations are associated with anophthalmia, defective hippocampal development, and seizures (9–11). Most patients with this syndrome experience intellectual disabilities (11), suggesting that loss of SOX2 function affects areas of the brain involved in cognition (e.g., hippocampus). SOX2 deficiency also causes neurodegeneration and impaired neurogenesis in the adult mouse brain (12, 13). However, the molecular mechanisms underlying the function of SOX2 in adult neurogenesis and its role in the human SOX2 anophthalmia syndrome are largely unknown.
The subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus is a germinal zone of active neurogenesis during adulthood (14). Indeed, approximately one-third of DG neurons lost during this period are replaced by adult-born neurons (15), suggesting an essential role for newborn neurons in cognitive plasticity (16, 17). Adult-born neurons originate from a population of NPCs with glial cell characteristics, having cell bodies in the SGZ and radial processes extending into the granular cell layer (GCL) (18). SOX2 is expressed in both types of neural stem/progenitors; quiescent radial NPCs (type 1) and amplifying progenitors (type 2a) (12, 19–22). The requirement for SOX2 in the establishment and maintenance of pluripotency in NPCs is well documented (12, 13); however, its role in neuronal differentiation is not well understood.
SOX2 overexpression represses neuronal differentiation (23–25), whereas SOX2 down-regulation induces exit from the cell cycle and expression of early differentiation markers (25). A recent genomewide analysis of SOX2-binding sites in NPCs revealed that SOX2 occupies the regulatory regions of hundreds of genes involved in neuronal differentiation (26), including proneural genes such as MASH1 and NGN1 (27). Previous studies have shown that activation of proneural genes requires the replacement of SOX2 by neurogenic signals at the regulatory regions of the promoters [e.g., SOX2 replaced by β-catenin to activate neurogenic differentiation 1 (NeuroD1) expression] (28). There now is evidence that epigenetic regulators are necessary for adult neural progenitors to generate neurons that will integrate into the hippocampal circuitry (29–31). Before cell differentiation, methylation of H3K27 and H3K4 by the polycomb (PcG) and trithorax (TrxG) complexes maintain genes transcriptionally silent but poised for activation once differentiation cues are received (32, 33). Taken together, these findings suggest that SOX2 is a transcriptional repressor of neuronal target genes. However, loss-of-function studies have shown that SOX2 is required for neurogenesis in the central (13, 34–36) and peripheral (27) nervous systems. Therefore, determining the mechanism by which SOX2 regulates expression of neurogenic genes that are essential for neuronal differentiation is vital to our understanding of the overall regulatory network that directs NPCs to generate neurons.
Here, we investigated SOX2 regulation of adult neuronal differentiation using an in vitro model of SOX2 deficiency in adult hippocampal NPCs (hipNPCs) and a genetic mouse model in which SOX2 is deleted in adult NPCs, bypassing the deleterious effects of SOX2 ablation during embryogenesis. We show that SOX2 deficiency in cultured adult hipNPCs reduces activation of “poised” bivalently marked (H3K4me3 and H3K27me3) transcription factors, including the neurogenic genes neurogenin 2 (Ngn2) and NeuroD1. SOX2 binds to their regulatory regions and prevents accumulation of the H3K27me3 closed chromatin mark, likely limiting the activity of the polycomb repressive complex 2 (PRC2) histone methyltransferase. We argue that SOX2 promotes a poised chromatin state at the regulatory regions of neurogenic genes, thereby enabling appropriate activation of the differentiation program upon exposure to a neurogenic stimulus.
Results
SOX2 Deficiency Leads to Epigenetic Repression at the Promoters of Bivalently Marked Proneural Genes.
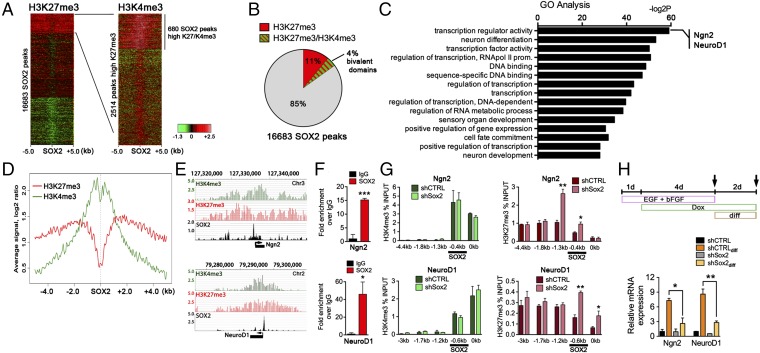
In NPCs, the chromatin regions of proneural and neurogenic genes contain high levels of both H3K27me3 and H3K4me3 (37, 38); such bivalent marks maintain the genes transcriptionally silent but poised for activation once differentiation cues are received (32). To understand the regulatory function of SOX2 in activation of the neurogenic program, we examined previously published ChIP-sequencing (ChIP-seq) data including the occurrence of H3K27me3 and H3K4me3 marks at SOX2-binding sites in mouse ES cell-derived NPCs (6, 26). Genomewide analysis revealed that of the 16,683 SOX2-binding sites, 2,514 were enriched for H3K27me3 (15.1%) (Fig. 1A). Moreover, 680 (27%) of these H3K27me3-enriched regions also contained high levels of H3K4me3 (Fig. 1 A and B). Our analysis also revealed that these SOX2-binding regions constitute 25% of all promoter regions with enriched H3K27me3 and H3K4me3 marks in NPCs (SI Appendix, Fig. S1A), suggesting that SOX2 controls a subset of genes with bivalent marks. Many of the bivalently marked SOX2-binding sites were located near the genes encoding for key transcriptional regulators of stem/progenitor cell fate and differentiation (Fig. 1C). Other genes identified are involved in neurogenesis (Sox factors, components of the PDGF and EGF signaling pathways, and proneural genes) or encode secreted molecules [such as brain-derived neurotrophic factor (Bdnf) or Vgf] and transcription factors implicated in cell-fate specification (including members of the bHLH and homeobox gene families). We also examined genes with bivalent promoters to which SOX2 does not bind (75% of promoters with bivalent marks) and which, presumably, SOX2 does not regulate directly. These genes are mostly implicated in the later stages of neurogenesis, such as neuronal maturation (e.g., synapse and channel activity), and in neuronal function (SI Appendix, Fig. S1B). Based on this analysis, we propose that SOX2 is involved in the regulation of a subset of neurogenic genes that are essential for the early steps of neuronal commitment and differentiation (here called “early neurogenic genes”). Intriguingly, further examination of the H3K27me3 and H3K4me3 profile surrounding the SOX2-binding sites (± 5 kb) at bivalent chromatin regions revealed a depression (valley) in H3K27me3 density flanking SOX2-binding sites (Fig. 1D), indicating that SOX2 may limit H2K27me3 deposition at these sites.
Fig. 1.
SOX2-deficient NPCs show epigenetic repression at promoters of neurogenic genes. (A, Left) Heatmap showing H3K27me3 densities at all 16,683 unique SOX2-binding sites ± 5.0 kb clustered by H3K27m3 occupancy. (Right) Heatmap showing H3K4me3 densities at all 2,514 unique SOX2-binding sites with high density of H3K27me3. Signal is represented by the log2 ratio. Green color indicates a low level; red represents a high level of a mark. Data are from ChIP-seq experiments in mouse NPCs (see Experimental Procedures for details). (B) Percentage of SOX2-binding sites with bivalent chromatin marks (H3K27me3/H3K4me3) within the group with a high density of H3K27me3. (C) Gene Ontology analysis of genes with bivalent chromatin marks and SOX2 binding at their regulatory regions in mouse NPCs. (D) Density profile of H3K27me3 and H3K4me3 at SOX2-binding sites ± 5.0 kb at bivalent domains. (E) ChIP-seq plot of H3K4me3, H3K27me3, and SOX2 at Ngn2 (Upper) and NeuroD1 (Lower) loci in mouse NPCs. (F) SOX2 binding at Ngn2 and NeuroD1 loci in mouse adult hipNPCs. (G) H3K4me3 (Left) and H3K27me3 (Right) levels at regulatory regions of Ngn2 (Lower) and NeuroD1 (Upper) promoters in mouse adult hipNPCs induced with a scrambled shRNA (shCTRL) or an shRNA against Sox2 (shSox2). Highlighted is SOX2 binding at these promoters. (H, Upper) Schematic of a 7-d cell culture protocol used to differentiate SOX2-deficient hipNPCs under neurogenic signals (Wnt3a, 50 ng/mL). (Lower) qRT-PCR analysis of Ngn2 and NeuroD1 gene-expression changes in SOX2cKO and wild-type NPCs under self-renewal or exposure to neurogenic signals (Wnt3a). For all quantifications, data are plotted as the mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
Commitment of progenitor cells to a neuronal fate is a crucial step in adult neurogenesis and is accompanied by the expression of transcription factors such as Ngn2 (39) and NeuroD1 (40). Both Ngn2 and NeuroD1 contain bivalent chromatin marks around the SOX2 regulatory regions (Fig. 1E), and we confirmed, using ChIP-qualitative PCR (qPCR) assays, that SOX2 binds to Ngn2 and NeuroD1 regulatory regions in the wild-type hipNPCs generated in this study (Fig. 1F). To study the consequences of SOX2 deficiency on the epigenetic landscape of Ngn2 and NeuroD1 genes, we transduced wild-type hipNPCs with a doxycycline (dox)-inducible vector encoding shRNA against Sox2 (shSox2) or control shRNA (shCTRL) (SI Appendix, Fig. S2) and found significantly higher levels of the repressive H3K27me3 mark in genomic regions proximal to the SOX2-binding sites at Ngn2 and NeuroD1 promoters in hipNPCs transduced with shSOX2, whereas levels of H3K4me3 were unaffected (Fig. 1G). We next asked whether the increase in H3K27me3 at these bivalent promoters could affect their expression upon exposure to neurogenic cues (Wnt3a). Consistent with the epigenetic data, Wnt3a induced much higher expression of Ngn2 and Neurod1 in hipNPCs transduced with shCTRL than with shSox2 (Fig. 1H). These data suggest that the loss of SOX2 shifts the poised state of Ngn2 and NeuroD1 chromatin toward a more repressed configuration.
Loss of SOX2 Causes Impaired Transcriptional Activation of Proneural Genes in Response to Neurogenic Cues.
The finding that bivalent proneural genes in SOX2-deficient hipNPCs show reduced activation raised the possibility that increased H3K27me3 deposition renders these genes less active. To test this idea further, we conditionally ablated Sox2 in vivo in adult hipNPCs postnatally using the previously described Sox2flox mice (13) crossed with mGFAP-Cre::Nestin-CFPnuc mice (SI Appendix, Fig. S3) (17, 21, 41). The resulting mouse strain is designated Sox2flox::mGFAP-Cre::nestin-CFPnuc (SOX2cKO). Notably, although wild-type hipNPCs responded to Wnt/β-catenin signaling by up-regulating Ngn2 and NeuroD1 transcription, SOX2cKO cells were unaffected by Wnt treatment (SI Appendix, Fig. S4A). Because there was little difference in the expression of other Wnt/β-catenin target genes (e.g., Lef1, cJun, cMyc, Btrc, and Axin2) in hipNPCs from SOX2cKO and from wild-type mice (SI Appendix, Fig. S4B), these results suggest that Wnt/β-catenin signaling in stem/progenitor cells is generally unaffected by the loss of SOX2, except for the failure to activate the expression of proneural genes.
Treatment with the histone deacetylase (HDAC) inhibitor valproic acid (VPA) previously has been shown to increase acetylation of the NeuroD1 promoter and activate its expression in NPCs, resulting in neuronal differentiation (42). Therefore, we asked whether VPA could rescue the expression of Ngn2 and NeuroD1 in SOX2-deficient cells. We found that treatment with HDAC inhibitor VPA had no effect on Ngn2 and NeuroD1 expression in SOX2cKO hipNPCs (SI Appendix, Fig. S4C). These observations suggest a profound chromatin change at the NeuroD1 regulatory regions in SOX2-deficient NPCs that renders NeuroD1 expression insensitive to global epigenetic modifiers.
SOX2 Limits the Activity of the Polycomb Complex PRC2 at the Regulatory Regions of Poised Neurogenic Genes.
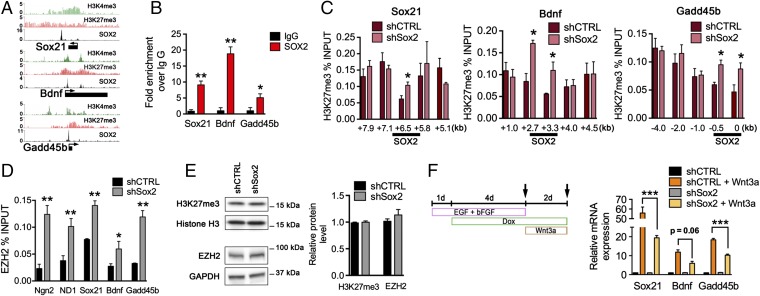
To determine whether SOX2 is necessary to ensure a balance of bivalent chromatin marks at the regulatory regions of poised neurogenic genes, we examined several genes known to be associated with the differentiation of hippocampal adult-born neurons, including the transcription factor Sox21 (43), the neurotrophic factor Bdnf (44), and the activity-inducible DNA demethylation factor growth arrest and DNA-damage–inducible, beta (Gadd45b). Consistent with the genome-wide ChIP-seq data from mouse ES cell-derived NPCs (Fig. 2A) (26), hipNPCs also showed SOX2 binding at Sox21, Bdnf, and Gadd45b genes (Fig. 2B). Analysis of the SOX2-bound regulatory regions revealed an increased level of H3K27me3 at these sites in SOX2-deficient as compared with control hipNPCs (Fig. 2C). Moreover, binding of EZH2, the catalytic subunit of the PRC2 histone methyltransferase complex, was increased at SOX2-regulatory regions of Ngn2, NeuroD1, Sox21, Bdnf, and Gadd45b in SOX2-deficient hipNPCs (Fig. 2D). Notably, shSox2- and shCTRL-expressing hipNPCs contained comparable total levels of H3K27me3 and EZH2 (Fig. 2E), indicating that H3K27me3 was increased selectively only at the SOX2-binding sites. Similar to our observations with Ngn2 and NeuroD1, we found that expression of Sox21, Bdnf, and Gadd45b was reduced in SOX2-deficient hipNPCs exposed to the Wnt3a differentiation cue (Fig. 2F).
Fig. 2.
Loss of SOX2 Increases polycomb complex PRC2 activity at bivalent genes required for neuronal differentiation. (A) ChIP-seq plot of H3K4me3, H3K27me3, and SOX2 at Sox21, Bdnf, and Gadd45b loci in mouse NPCs. (B) SOX2 binding at Sox21, Bdnf, and Gadd45b loci in mouse adult hipNPCs. (C) H3K27me3 level at regulatory regions of Sox21 (Left), Bdnf (Center), and Gadd45b (Right) loci in mouse adult hipNPCs induced with shCTRL or shSox2. Highlighted is SOX2 binding at these promoters. (D) EZH2 binding in SOX2-binding sites of Ngn2, NeuroD1, Sox21, Bdnf, and Gadd45b loci in mouse hipNPCs. (E) Western blot showing H3K27me3 and EZH2 levels in SOX2-deficient and wild-type hipNPCs. (F, Left) Schematic of a 7-d cell-culture protocol used to differentiate SOX2-deficient hipNPCs under neurogenic signals (Wnt3a). (Right) Quantification of Sox21, Bdnf, and Gadd45b mRNA levels in adult hipNPCs under self-renewal or neurogenic signals (Wnt3a). For all quantifications, data are plotted as the mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
We next investigated the levels of H3K27me3 in SOX2-deficient hipNPCs under differentiation conditions. Analysis of epigenetic marks after neurogenic activation revealed a pronounced increase in H3K27me3 levels at proneural (Ngn2 and NeuroD1) and neurogenic (Sox21, Bdnf, and Gadd45b) genes in cells lacking SOX2 (SI Appendix, Fig. S5 A and B). H3K9ac is found at actively transcribed promoters and is increased during neuronal differentiation (45). Consistent with the repressive epigenetic signature in SOX2-deficient hipNPCs, we found that H3K9ac levels were higher within the regulatory regions of poised proneural/neurogenic genes in shCTRL- than in shSOX2-expressing hipNPCs following exposure to Wnt3a (SI Appendix, Fig. S5C). Because H3K9 acetylation is catalyzed primarily by the histone acetyltransferase GCN5 (46), we monitored the dynamics of GCN5 binding at proneural/neurogenic genes during neuronal differentiation of control and SOX2-knockdown hipNPCs. Indeed, Wnt3a treatment increased the binding of GCN5 to the regulatory regions of these genes in both cell types but did so to a much lower extent in SOX2-deficient hipNPCs than in control cells (SI Appendix, Fig. S5D).
Taken together, these data suggest that SOX2 functions to maintain a bivalent chromatin state at the poised genes, likely by limiting EZH2 binding and PRC2 activity (H3K27me3 levels). The absence of SOX2 thus would result in the increased H3K27me3, leading to a more compact chromatin structure and impaired gene expression during differentiation.
Loss of SOX2 in hipNPCs Reduces the Expression of NGN2 and NEUROD1 in Vivo.
To evaluate whether SOX2 deficiency influences the expression of these pivotal neurogenic genes in vivo, we examined their expression in DG sections from SOX2cKO and wild-type mice. NGN2 is transiently expressed by late-amplifying progenitors, and NGN2 deficiency impairs neurogenesis by reducing the proliferation of these cells (Fig. 3A) (39). Immunohistochemical staining of NGN2 revealed that expression in hippocampal progenitor cells was significantly lower in SOX2cKO mice than in wild-type mice (Fig. 3B). NEUROD1 was not detected in the majority of neural stem/progenitor cells but was present in only a small fraction of cells transitioning from the progenitor to neuroblast stage (Fig. 3A) (28, 40). Similar to our findings with NGN2, NEUROD1 expression was reduced in SGZ neuroblasts of SOX2cKO mice compared with wild-type mice (Fig. 3C). To determine whether NEUROD1 expression is reduced at a particular stage of differentiation, we examined expression in neuroblasts (DCX+) identified morphologically as early- (nondendritic processes), mid- (short dendritic processes), or late- (large dendritic processes) stage neuroblasts. Intriguingly, NEUROD1 expression was similar in early, mid, and late neuroblasts of SOX2cKO mice, whereas expression in wild-type mice peaked at the early neuroblast stage and did so at significantly higher levels (Fig. 3D). We also analyzed the expression of PROX1, a transcription factor up-regulated in neuroblasts and required for granule cell maturation (Fig. 3A) (47), but we found no differences in expression between SOX2cKO and wild-type neuroblasts (SI Appendix, Fig. S6A). Interestingly, the PROX1 regulatory region bound by SOX2 is not bivalently marked, suggesting that the epigenetic mechanism described here specifically affects poised genes. To substantiate our in vivo results, we examined the expression of Prox1 in adult hipNPCs grown from 2-mo-old SOX2cKO mice. In agreement with the in vivo data, the expression of Prox1 mRNA (SI Appendix, Fig. S6B) was similar in SOX2cKO and wild-type hipNPCs. Together, these data indicate that SOX2 deletion in adult stem/progenitor cells decreases the expression of proneural genes, compromising the differentiation of newborn neurons.
Fig. 3.
Loss of SOX2 in adult hipNPCs reduces the expression of NGN2 and NEUROD1 in early neuroblasts. (A) Graphical representation of the expression of NGN2, NEUROD1, and PROX1 in adult hippocampal neuronal maturation. (B, Left) Sections from 2-mo-old mice were immunostained for NGN2. (Scale bar: 20 µm.) (Right) Quantification results are expressed as the average intensity of NGN2 staining in stem/progenitor (CFPnuc+) cells relative to mature granule neurons. (C, Left) Sections from 2-mo-old mice were immunostained for NEUROD1. (Scale bar: 50 µm.) (Right) Quantification results are expressed as the average intensity of NEUROD1 staining in neuroblasts (DCX+) cells relative to mature granule neurons. (D, Left) Confocal imaging showing colocalization of NEUROD1 in neuroblasts (DCX+) of wild-type and SOX2cKO mice. (Scale bar: 20 µm.) (Right) Quantification results are expressed as the average intensity of NEUROD1 staining in NPCs, early, mid, and late neuroblasts (NB) and neurons (NeuN). For all quantifications, data are plotted as the mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001).
Loss of SOX2 Reduces Adult Hippocampal Neurogenesis.
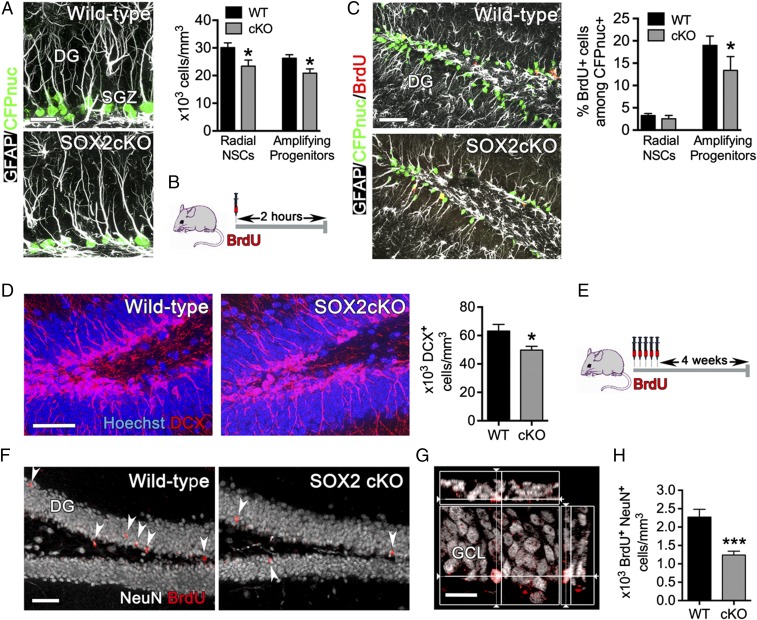
The conditional loss of SOX2 in adult hipNPCs of SOX2cKO mice provides us with an opportunity to follow the stem/progenitor cell population with the Nestin-CFPnuc transgene (41). The total number of neural stem/progenitor (CFPnuc+) cells in the DG of SOX2cKO mice was 71.2%, 78.4%, and 64.8% of wild-type levels at 1, 2, and 6 mo of age, respectively (SI Appendix, Fig. S7A). The number of radial NPCs and amplifying progenitors in the SGZ was examined by comparing the expression of GFAP, an intermediate filament protein organized in granule layer-traversing processes of radial NPCs, with the expression of CFPnuc, which is localized in the nuclei of all Nestin-expressing cells (both radial NPCs and amplifying progenitors) (Fig. 4A). Two-month-old SOX2cKO mice contained fewer radial NPCs (CFPnuc+ cells with radial GFAP+ processes) and amplifying progenitors (CFPnuc+ GFAP− cells) than did wild-type mice, suggesting that SOX2 deficiency may affect the self-renewal and/or survival of both cell populations.
Fig. 4.
Reduced number of adult hipNPCs and adult newborn neurons in SOX2cKO mice. (A, Left) Sections of the SGZ of the DG from 2-mo-old mice (SOX2cKO and littermate controls) were stained for GFAP and CFPnuc. (Scale bar: 20 µm.) (Right) Quantification of the number of radial NPCs and amplifying progenitors in the SGZ of SOX2cKO and wild-type mice. (B) Protocol for BrdU (100 mg/kg body weight) injection used in 2-mo-old SOX2cKO and wild-type mice. Cells were examined 2 h after injection. (C, Left) Sections from BrdU-injected mice (as in B) were immunostained for BrdU, GFAP, and CFPnuc. (Scale bar: 50 µm.) (Right) The percentage of BrdU+ cells that are radial NPCs or amplifying progenitors was quantified among CFPnuc+ cells. (D, Left) Hippocampal DG sections from 2-mo-old mice (SOX2cKO and the littermate control) were stained for the neuroblast marker DCX. (Scale bar: 50 µm.) (Right) The number of neuroblasts (DCX+ cells) in the DG was analyzed in SOX2cKO and wild-type mice. (E) Protocol for BrdU injection (5 d of twice-daily injections given 12 h apart, at 50 mg/kg body weight) used in 2-mo-old SOX2cKO and wild-type mice. BrdU-labeled cells were examined 4 wk after the last injection. (F) Sections from BrdU-injected mice (as in E) were immunostained for BrdU and NeuN. (Scale bar: 50 µm.) (G) Confocal imaging showing colocalization of a BrdU+ NeuN+ neuron in the GCL. (Scale bar: 20 µm.) (H) Quantification of BrdU+ NeuN+ cells showing reduced neurogenesis in the GCL of the DG in SOX2cKO mice. For all quantifications, data are plotted as the mean ± SEM (*P < 0.05, ***P < 0.001).
To determine whether SOX2 affects the proliferation of adult stem/progenitor cells, we analyzed the expression of the nuclear proliferative cell marker proliferating cell nuclear antigen (PCNA) and the incorporation of the thymidine analog BrdU into DNA. We found that the number of PCNA+ stem/progenitor cells in the SGZ was lower in SOX2cKO than in wild-type mice at each age examined (76.0%, 61.4%, and 45.6% of wild-type levels at 1, 2, and 6 mo, respectively) (SI Appendix, Fig. S7B). Analysis of BrdU incorporation 2 h after injection (Fig. 4B) revealed that SOX2cKO and wild-type mice contained approximately the same percentage of proliferating radial NPCs in the DG, but the percentage of proliferating amplifying progenitors was reduced significantly in SOX2cKO mice (Fig. 4C). To analyze the effect of SOX2 loss on cell-cycle progression, mice were injected sequentially with 5-chloro-2-deoxyuridine (CldU) and 5-iodo-2-deoxyuridine (IdU), which allows monitoring of cells reentering the cell cycle (CldU+ IdU+) (SI Appendix, Fig. S8A) (48, 49). Very few (∼4%) radial NPCs were detected in mice of either genotype (SI Appendix, Fig. S8B). In contrast, the number of amplifying progenitors that reenter the cell cycle was reduced significantly in SOX2cKO mice (19%) compared with wild-type mice (31%) (SI Appendix, Fig. S8B). We then analyzed the number of DCX+ neuroblasts and found reduced numbers in SOX2cKO mice (73.2%, 78.8%, and 49.7% of wild-type levels at 1, 2, and 6 mo, respectively) (Fig. 4D and SI Appendix, Fig. S7C). These results indicate that loss of SOX2 causes an age-associated progressive reduction in the total number of neural stem/progenitor cells and immature neuroblasts in the DG.
To analyze neurogenesis in the DG of SOX2cKO mice, 2-mo-old mice were injected with BrdU for 5 d, and cells were analyzed 4 wk later (Fig. 4E). Mature newborn neurons can be identified microscopically by colocalization of BrdU with the mature neuron marker NeuN (Fig. 4 F and G). Quantitative analysis of double-stained cells revealed a 45.5% decrease in the density of newborn neurons in the DG of SOX2cKO mice compared with wild-type mice (Fig. 4H). To confirm these results, we grew adult hipNPCs from 2-mo-old mice (SI Appendix, Fig. S9A), cultured them in the presence of Wnt3a to promote neural differentiation, and then examined the expression of the neuroblast marker TUJ1. In agreement with our in vivo data, the frequency of TUJ1+ cells was reduced dramatically in cultures of SOX2cKO NPCs (∼2.5% TUJ1+) compared with wild-type NPCs (∼12.5% TUJ1+) (SI Appendix, Fig. S9B). Together, these results indicate that neurogenesis is decreased substantially in SOX2cKO mice.
SOX2 Deficiency Reduces Adult Neurogenesis by Increasing Apoptosis of Neuroblasts.
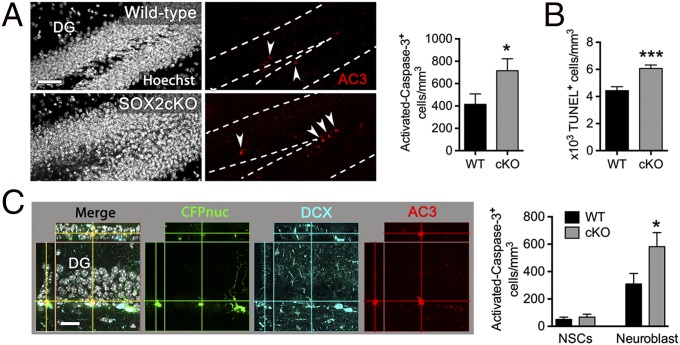
The finding that the DG of SOX2cKO mice shows a mild reduction in the number and proliferation of neuroblasts but a dramatic decrease in the number of new integrating neurons raised the possibility that a large proportion of neuroblasts undergo apoptosis during differentiation. In fact, loss of NeuroD1 in the hippocampal neurogenic lineage results in impaired maturation and survival of new neurons (40). Therefore we analyzed the density of apoptotic cells within the hippocampal neurogenic niche of 2-mo-old mice by detecting activated caspase 3 (AC3) or fragmented DNA (TUNEL). We found that the density of AC3+ and TUNEL+ cells in the SGZ was ∼1.5- to twofold higher in SOX2cKO than in wild-type mice (Fig. 5 A and B). To identify the cell subpopulation(s) undergoing apoptosis, we immunostained for AC3, CFPnuc, and DCX (Fig. 5C). Apoptotic neuroblasts (AC3+ DCX+) were more abundant in SOX2cKO mice than in wild-type mice, but the number of apoptotic adult neural stem/progenitor cells (AC3+ CFPnuc+) was approximately the same in both strains. These results are in agreement with a previous study (50) showing that the majority of apoptotic cells in the adult SGZ are neuroblasts (PSA-NCAM+ cells). Because SOX2 is not expressed in neuroblasts, our results suggest that the loss of SOX2 affects the activation of the genetic program controlling neuronal differentiation, and as a consequence, new neurons in SOX2cKO mice undergo apoptosis before maturation.
Fig. 5.
Increase in programmed cell death in SOX2cKO mice. (A, Left) Sections from 2-mo-old mice were immunostained for the apoptotic cell marker AC3. (Scale bar: 50 µm.) The number of apoptotic cells was increased in the SGZ of SOX2cKO mice. (Right) Results are expressed as the number of AC3+ cells within the total volume of the DG. (B) Quantification of TUNEL+ cells in the DG of 2-mo-old mice revealed an increase in the number of apoptotic cells in SOX2cKO mice. (C, Left) Confocal imaging showing an apoptotic neuroblast (colocalization of AC3 and DCX staining). (Scale bar: 25 µm.) (Right) The number of apoptotic neuroblasts is higher in SOX2cKO mice than in wild-type mice. Results are expressed as the number of AC3+ cells within the total volume of the DG. For all quantifications, data are plotted as the mean ± SEM (*P < 0.05, ***P < 0.001).
NeuroD1 Expression Recovers Adult Neurogenesis in SOX2cKO Mice.
Next, we asked whether exogenous expression of proneural genes could recover the neuroblast defect resulting from SOX2 deficiency. Because NeuroD1 is essential for the survival of adult-born neuroblasts (40) and has a strong prodifferentiation activity, we transduced hipNPC cultures from SOX2cKO or wild-type mice (SI Appendix, Fig. S10A) with lentiviral vectors encoding NeuroD1 or GFP (control) and examined neuronal differentiation in vitro. We found that transduction of SOX2cKO hipNPCs with the NeuroD1 lentivirus at the initiation of differentiation increased the number of cells expressing the neuronal marker MAP2ab relative to the number observed in wild-type hipNPC cultures (SI Appendix, Fig. S10B), demonstrating that NEUROD1 expression recovered neurogenesis in SOX2-deficient cultures.
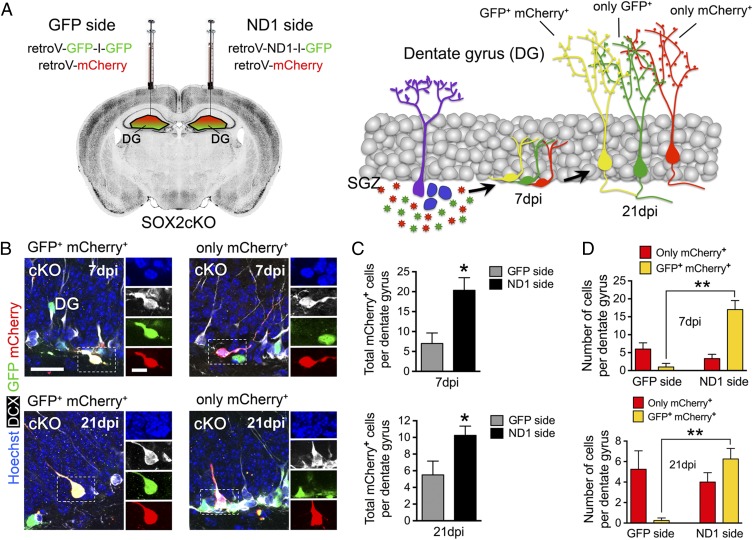
To examine this recovery of neuroblasts in vivo, we delivered NEUROD1 or GFP specifically to hippocampal neural stem/progenitor cells by using retroviral vectors, which infect only proliferating cells and are passed on to their progeny. For these experiments, SOX2cKO mice were injected into the right and left DG with either ND1-IRES-GFP or GFP-IRES-GFP retroviruses. Both sides received the same control retrovirus encoding mCherry (Fig. 6 A and B). In this paradigm, mCherry serves as an internal control, which ensures the equal infection of the progenitor cells in the left and the right DG. Therefore, the ability of NEUROD1 expression to recover neurogenesis is reflected in the total number of mCherry+ cells in each side and the number of cells expressing both GFP+ and mCherry+ (yellow) compared with the number of cells expressing only mCherry (red) on each side. These quantifications are largely independent of slight variations in viral titer and injection site (51). We found that DG of SOX2cKO mice injected with NEUROD1-encoding retrovirus (the ND1 side) contained a higher number of total mCherry+ (red + yellow) cells than did the DG injected with GFP-encoding retrovirus (the GFP side) at both 7 and 21 d postinjection (dpi) (Fig. 6C). The morphology of mCherry+ cells at both sites was consistent with that of neuroblasts at 7 dpi and DG granule neurons at 21 dpi. Because equal numbers of progenitors on both sides were infected with mCherry (an equal volume of the same virus was injected) the increase in total mCherry+ cells at the NeuroD1 side indicates a specific effect of NEUROD1 expression. The numbers of mCherry-only (red) cells were similar in both sides (Fig. 6D) at both 7 and 21 dpi, suggesting similar numbers of uninfected (and, therefore, originally infected) cells in both NEUROD1- and GFP-injected sides. However, much larger numbers of yellow cells were seen on the NEUROD1-injected side than on the GFP-injected side, further demonstrating a specific effect of NEUROD1 expression (Fig. 6D) on cell survival at both 7 and 21 dpi. Together, these results indicate that expression of NeuroD1 in differentiating stem/progenitor cells recovers the newly generated neuroblasts in SOX2-deficient hipNPCs both in vitro and in vivo.
Fig. 6.
NeuroD1 transduction recovers neurogenesis in SOX2cKO mice. (A, Left) Retroviral injections were performed in hippocampal DG of 2-mo-old SOX2cKO mice. One side was injected with a mixture of GFP-IRES-GFP–encoding retrovirus and mCherry-encoding control retrovirus. The other side was injected with a mixture of ND1-IRES-GFP–expressing retrovirus and mCherry-encoding control retrovirus. The same amount of control virus was injected into both sides. (Right) The resulting infected newborn neurons at 7 and 21 dpi. (B) Confocal imaging showing transduced neuroblasts (DCX+) with GFP and mCherry retroviruses (Left) or with only mCherry retrovirus (Right) at 7 and 21 dpi. (Scale bars: 25 µm for merged images and 10 µm for single channels.) (C) Quantification of total mCherry+ (GFP+ mCherry+) and only mCherry+ cells per DG at 7 (Upper) and 21 (Lower) dpi. (D) Quantification of GFP+ mCherry+ and only mCherry+ cells in the GFP and ND1 sides at 7 (Upper) and 21 (Lower) dpi. For all quantifications, data are plotted as the mean ± SEM (*P < 0.05, **P < 0.01).
SOX2-Deficient Adult NPC Progeny Show Aberrant Dendritic Maturation and Electrophysiology.
The failure of SOX2-deficient hipNPCs to activate proneural and neurogenic gene expression efficiently suggests that neuronal maturation and function may be impaired in SOX2cKO mice. To examine adult-born neuroblasts, we stereotactically injected a GFP-encoding retrovirus into the DG of 2-mo-old SOX2cKO and wild-type mice, allowing us to visualize and quantify the dendritic outgrowth of individual newborn neurons (Fig. 7A) (52). Analysis of sections of DG at 28 dpi revealed a significant reduction in both total dendritic length and branch number of newborn neurons in SOX2cKO compared with wild-type mice (Fig. 7B). We also analyzed spine density and morphology in mice injected with a retrovirus encoding mCherry (Fig. 7C). Interestingly, SOX2 deficiency significantly decreased the spine density but increased the mushroom dendritic spine density of adult-born neurons (Fig. 7D). To determine whether SOX2 ablation affected neuronal physiology and connectivity, whole-cell recordings were performed in GFP-labeled newborn neurons in acute slices. Cells from SOX2cKO mice showed slightly lower resting membrane potentials than cells from wild-type mice (SI Appendix, Fig. S11A); however, the cells showed similar levels of excitability (action-potential spikes generated in response to positive current injections in current-clamp conditions) (SI Appendix, Fig. S11B). The average amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) was increased in SOX2cKO cells (Fig. 7E); given that sEPSC amplitude correlates with spine-head size (53), this finding is consistent with the observed increase in mushroom spines in newborn neurons from SOX2cKO mice. In contrast, SOX2 ablation had no effect on the frequency of sEPSC events (Fig. 7E). Although the reduction in dendritic spines would be expected to reduce the frequency of sEPSCs in SOX2-deficient cells, the observed increase in the density of mushroom spines may compensate.
Fig. 7.
SOX2 deficiency leads to abnormal newborn granule neuron maturation. (A) Sample projected confocal images of newborn neurons in the DG at 28 dpi of a retrovirus encoding GFP. (Scale bar: 50 µm.) (B) Quantification of total dendritic length and branching points of GFP+ newborn neurons at 28 dpi. (C) Sample-projected confocal images of dendritic spines of newborn neurons at 28 dpi of a retrovirus expressing mCherry. (Scale bar: 2 µm.) (D) Quantification of the number of spines and the number of mushroom spines of mCherry+ newborn neurons at 28 dpi. (E, Left) Plot of spontaneous excitatory postsynaptic currents (sEPSC) in GFP+ newborn neurons at 42 dpi. (Scale bars: 20 pA and 5 s.) (Right) Quantification results. For all quantifications, data are plotted as the mean ± SEM (*P < 0.05, ***P < 0.001).
Thus, SOX2 deletion leads to aberrant neuronal maturation and integration during adult hippocampal neurogenesis, as illustrated by the defects in dendritic outgrowth, dendritic spine formation, and neuronal activity. Our findings suggest that, in addition to its function in maintaining the survival and proliferative capacity of neural progenitors, SOX2 also plays a role in the maturation of newborn granule neurons long after its expression has ceased. This “delayed” function of SOX2 is consistent with our finding that it regulates the epigenetic landscape of many neurogenic genes, enabling their robust activation at the onset of neurogenic program (SI Appendix, Fig. S12).
Discussion
Although SOX2 is arguably a cornerstone of neural stem cell biology (54, 55) and plays an essential role in neurogenesis in the developing and adult brain (13), the mechanisms by which SOX2 regulates neuronal differentiation have remained unclear. In our previous study focusing on the LIN28 gene, which is fully active in NPCs, we found that SOX2 promotes an open chromatin state at the promoter (H3K9ac mark) through recruitment of the TRRAP/GCN5 complex (56). Here, we uncovered a distinct epigenetic mechanism of SOX2 function on poised proneural and early neurogenic genes in NPCs. We discovered that SOX2 binds within bivalently marked regulatory regions of such poised genes where it limits the activity of the PRC2 complex. Our data suggest that SOX2 promotes the functional bivalent chromatin state and thus enables proper activation of differentiation program upon exposure to neurogenic cues. Finally, we demonstrate that the reduction in proneural gene expression in differentiating SOX2-deficient hipNPCs increases cell death during neuronal differentiation and impairs dendritic development and electrophysiological properties of the surviving new neurons.
SOX2 Limits PRC2 Complex Activity at Bivalent Genes.
Previous ChIP-seq studies have found that SOX2 is bound to hundreds of known or presumably poised loci that are transcriptionally inactive but will be expressed during differentiation (26, 38); however, its biological role at these genes was unknown. It is widely accepted that bivalent domains are central to maintaining genes in a poised state, ensuring proper and robust responses to differentiation cues (57). During development, TrxG and PcG complexes at bivalent domains play a key role in fine-tuning the expression of genes encoding crucial factors and in defending against unscheduled gene activation (57). Differentiation of neural progenitors is accompanied by an epigenetic switch characterized by a decrease of H3K27me3 and a gain of H3K4me3 at the promoters of proneural genes (6). Exogenous SOX2 expression previously was shown to be sufficient to reprogram fibroblasts into multipotent neural stem cells (NSCs) (55) or, in combination with Mash1, to reprogram human brain pericytes into neuronal cells (58). These findings suggested that SOX2 plays a key role in the epigenetic control of the neurogenic program, cooperating with the differentiation capacities of neurogenic factors. Consistent with this SOX2 cooperative function in neuronal differentiation, we found that loss of SOX2 increased the repressive H3K27me3 mark at proneural (Ngn2 and NeuroD1) and neurogenic (Sox21, Bdnf, and Gadd45b) genes normally bound by SOX2. Furthermore, H3K27me3 was increased only at SOX2-binding sites, whereas EZH2 binding was increased in broader regulatory regions of these genes. This result is consistent with the recent observation that EZH2 binding does not equate with PRC2 complex activity (i.e., H3K27me3 levels) (59). At the same time, global H3K27me3 and EZH2 levels were not altered in SOX2-deficient cells, suggesting that SOX2 acts locally (directly or through cofactors) to modulate PRC2 complex binding in the vicinity of SOX2-binding sites. Moreover, our whole-genome analysis suggests that SOX2 regulates the bivalent marks on a subset of early neurogenic genes involved in the onset of neuronal differentiation (25% of bivalently marked promoters), whereas the remaining 75% (genes involved in neuronal maturation and function) are mainly devoid of SOX2, implicating other factors in their regulation.
Based on our results, we propose that in neural precursors SOX2 functions at bivalent domains as a lineage-specific transcriptional regulator to maintain the bivalent chromatin/poised state by limiting the activity of the PRC2 complex. SOX2 could compete directly with PRC2 for chromatin binding at bivalent domains and/or act indirectly, e.g., through its control of Utf1 expression (60). To our knowledge, this is the first example of a lineage-specific transcription factor that maintains the bivalent state at the promoters of poised genes, thus coordinating the onset of a developmental (neurogenic) program and consequently ensuring a robust and appropriate terminal (neuronal) differentiation process. It is worth pointing out potential limitations of our whole-genome analysis, which has been performed using the ChIP-seq datasets from mouse ES cell-derived NPCs. In the future, it will be important to generate the whole-genome maps of SOX2 binding and the epigenetic landscape of hipNPCs and to investigate the exact mechanisms of SOX2 interference with PRC2, which could involve steric hindrance of the same locus, recruitment of other transcription factors, DNA methylation, or changes in chromatin structure in hipNPCs.
SOX2 Regulates the Transcriptional Response to Neurogenic Cues.
Neural progenitors in the adult hippocampus decrease SOX2 levels before exiting the cell cycle and initiating the expression of markers associated with neural differentiation. For example, NeuroD1 transcriptional activation by Wnt/β-catenin requires the removal of SOX2 binding at the NeuroD1 promoter (28). Previously, however, it was unclear whether SOX2 binding at the promoters of essential neurogenic genes is simply repressive or might contribute to their activation. We found that SOX2-deficient hipNPCs showed a significantly impaired neurogenic response to Wnt3a, a physiological ligand expressed in the adult hippocampus (61), suggesting that SOX2 presence at the poised promoters indeed is required for full transcriptional activation.
We correlated the reduced neurogenic response in SOX2-deficient NPCs with the increased abundance of repressive H3K27me3 marks at the poised proneural/neurogenic genes. These early changes in NPCs also affected epigenetic regulation at later stages of the neurogenic program, including the GCN5-dependent deposition of H3K9ac activating marks. Notably, attempts to maintain high levels of acetylation in SOX2-knockdown NPCs by treatment with VPA did not rescue the expression of proneural genes, suggesting that the early SOX2-dependent imbalance in H3K4me3 and H3K27me3 marks has a profound impact throughout the entire differentiation process. Further work is needed to understand fully this previously unknown function of SOX2 and to identify downstream factors involved in the epigenetic regulation of poised proneural/neurogenic genes.
Our analysis of SOX2cKO mice revealed that the expression of both poised proneural genes, Ngn2 (amplifying progenitors) and NeuroD1 (neuroblasts), was reduced significantly in the adult hippocampus. In particular, the dynamics of NeuroD1 expression were perturbed in SOX2cKO mice, and the typical peak in expression in early neuroblasts was absent (40). In contrast, there was no effect on PROX1, which is required for granule cell maturation and is bound by SOX2. However, the Prox1 promoter is not bivalently marked, reinforcing the notion that SOX2-dependent epigenetic regulation specifically affects poised genes during neuronal differentiation.
Loss of SOX2 Impairs Neuronal Survival and Differentiation.
Deletion of SOX2 in adult neural progenitors was shown previously to reduce drastically the number and proliferation of radial neural progenitors and, consequently, the number of neuroblasts in the adult hippocampus (13). However, we observed only a slight reduction in radial glia cells in SOX2cKO mice between 1 and 6 mo of age. One possible explanation for this discrepancy is the use of tamoxifen to induce Sox2 deletion in the former study (13); tamoxifen might have had a deleterious effect on the SOX2-deficient hipNPCs.
We found that SOX2 regulates the abundance of transiently amplifying progenitors by opposing premature exit from the cell cycle. The cell-cycle deficits observed in these proliferating cells likely result from the reduced expression of Lin28. Our previous work showed that SOX2 directly controls Lin28 expression in several types of NPCs, and Lin28 rescues the proliferation defect in SOX2-deficient NPCs through effects on cell-cycle regulators such as CyclinD1, TLX, and CDC25A (56). On the other hand, the absence of a cell-cycle deficit in SOX2-deficient radial glia stem cells is consistent with the limited self-renewal of this stem cell population (48).
The role of SOX2 in regulating neuronal differentiation and functional integration in the adult hippocampus had not been investigated previously. The defects in hippocampal neurogenesis observed in SOX2cKO mice are of particular importance, because patients with SOX2 anophthalmia syndrome are known to have learning and memory deficits, and most have anomalies of the hippocampus (62, 63). We found that apoptosis of immature neuroblasts was increased in SOX2cKO mice, leading to dramatically reduced integration of newborn neurons in the hippocampal DG. Given that the proneural genes Ngn2 (39) and NeuroD1 (40) are required for the survival of hippocampal newborn neurons, we predicted that exogenous overexpression of one or both of these genes would recover cell survival in SOX2cKO mice. Indeed, the recovery of neuroblasts in vivo was shown by retroviral delivery of NeuroD1 in the adult hippocampus of these mice, demonstrating that appropriate NeuroD1 expression is sufficient for neuroblast survival in the absence of SOX2.
We reasoned that deficient activation of proneural and neurogenic genes would lead to impaired neuronal development and function. Indeed, we found that newborn neurons in the DG of SOX2cKO mice had prominent morphological alterations and functional abnormalities compared with the newborn neurons in the DG of wild-type mice. Consistent with its role in setting up the epigenetic landscape, we argue that SOX2 affects neuroblast survival and neuronal maturation long after its expression has ceased. These results indicate that SOX2 plays a much broader role than previously anticipated. Further experiments will be required to understand the impact of newborn neurons in SOX2-deficient animals on hippocampal functions such as contextual learning and pattern separation. We expect that these hippocampal functions will be affected in SOX2cKO mice. However, because the number of newborn neurons decreased, and the function of the remaining neurons was altered, a behavioral paradigm capable of separating these phenomena will be required.
Finally, the neuronal differentiation defect we observed in SOX2cKO mice provides insight into the human SOX2 anophthalmia syndrome. The phenotype of SOX2cKO mice observed here suggests one possible epigenetic mechanism for this clinical syndrome. The defects observed in neuronal differentiation, dendritic development, and functional integration of newborn neurons in the hippocampus of adult SOX2cKO mice may contribute to the learning and intellectual disabilities experienced by patients with SOX2 anophthalmia syndrome (63). Given the reversibility of epigenetic changes, our results suggest that pharmacological therapies directed at restoring the levels of neurogenic genes down-regulated in SOX2-deficient hipNPCs might be beneficial for anophthalmia syndrome patients, particularly in ameliorating hippocampal-related learning disability.
Experimental Procedures
Mouse Models.
The generation of conditional Sox2 mutant mice has been described (13). Briefly, Sox2flox mice were established by inserting loxP sites into a 6.5-kbp region containing the Sox2 locus. Mice were crossed with transgenic mice expressing Cre recombinase under the control of the mouse glial fibrillary acidic protein promoter (mGFAP-Cre) (21), resulting in Sox2flox::mGFAP-Cre mice. These mice were crossed with Nestin-CFPnuc transgenic mice (41), resulting in Sox2flox::mGFAP-Cre::nestin-CFPnuc mice (SOX2cKO mice). All mice were backcrossed onto a C57BL/6 background. Mutant mice were compared directly with nontransgenic littermates. Experiments were conducted according to guidelines and protocols approved by the Institutional Animal Care and Use Committee of Sanford-Burnham Medical Research Institute.
BrdU, CldU, and IdU Experiments.
For proliferation studies, 8-wk-old mice were injected once with BrdU (i.p. 100 mg/kg body weight) (51-7581KZ; BD Pharmingen) and were perfused with 4% paraformaldehyde 2 h later. To study adult neurogenesis, 8-wk-old mice were injected twice daily, 12 h apart, for five consecutive days with BrdU (i.p. 50 mg/kg body weight) and were perfused with 4% paraformaldehyde 4 wk after the last injection. To determine the mitotic potential of stem and progenitor cells, 8-wk-old mice were injected i.p. with equimolar concentrations of CldU (85 mg/kg in saline; Sigma) and IdU [115 mg/kg in saline containing 2% (vol/vol) of 0.2N NaOH; Sigma].
Cell Culture and in Vitro Quantification.
Neurosphere cultures from the hippocampal DG of 6- to 8-wk-old mice were established as described, with some modifications (64). Briefly, the DG was dissected, disaggregated in papain, and maintained in culture in base medium (1:1 ratio of DMEM/F12 Glutamax-neurobasal medium [Gibco], 2% B27 supplement without vitamin A [Gibco], 10% BIT 9500 [StemCell Technologies], and 1 mM glutamine [Gibco]) supplemented with 20 ng/mL EGF (Chemicon), 20 ng/mL bFGF, 1 µM purmorphamine (Calbiochem), 5 µg/mL insulin (Sigma), and 5 mM nicotinamide (Sigma). After the second passage, neurospheres were propagated as monolayer cultures on Matrigel-coated plates. hipNPCs were subcultured enzymatically with Accutase (Chemicon) at a 1:3 ratio approximately once a week. For neuronal differentiation experiments, hipNPCs were transferred to base medium supplemented with 5 µg/mL insulin (Sigma), 5 mM nicotinamide (Sigma), and 50 ng/mL Wnt3a ligand (R&D Systems) for the indicated times. To inhibit histone deacetylase activity, 1 mM VPA was added to hipNPC cultures at the time of withdrawal from EGF and bFGF.
qPCR.
Total RNA was extracted using an RNeasy kit (Qiagen), and 1 µg was reverse transcribed using the QuantiTect kit (Qiagen) according to the manufacturer’s instructions. Real-time PCR analysis was performed with 3.125 ng of cDNA per sample using a LightCycler 480 II (Roche) and SYBR Green master mix (Invitrogen) according to the manufacturers’ recommendations. Primer sequences are listed in SI Appendix, Table S1. Gene expression was normalized against the ubiquitously expressed HPRT gene. Data were analyzed using the Δ(ΔCT) method.
ChIP.
ChIP was performed using the EZ-ChIP kit (Millipore) according to the manufacturer’s recommendations with the following modifications: 1 × 106 cell equivalents were used for each immunoprecipitation, cells were sonicated to obtain chromatin fragments of 200–500 bp, and 5–10 µg of antibody was used in each ChIP. To evaluate factor-specific enrichment at different promoter sites, qPCR was performed using the purified chromatin as a template. Amplification was performed with site-specific primers designed to flank the genomic region of interest (SI Appendix, Table S1). qPCR data were normalized to the values obtained with normal rabbit IgG. To compare factor-specific enrichment at particular sites across different cell lines (i.e., shCTRL vs. shSox2), qPCR data were normalized to 1% of the purified input DNA, which was used as a measure of the total amount of chromatin present in the sample.
Stereotactic Injections and Analysis of Coinjection Assays.
All experiments were performed with 5- to 6-wk-old wild-type or SOX2cKO mice (n = 4 per group). Mice were anesthetized with a mixture of ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight). Mice were stereotactically injected with 1.5 µL of a 1:1 mixture of CAG-NeuroD1-IRES-GFP/CAG-mCherry and CAG-GFP-IRES-GFP/CAG-mCherry into the left and right DG, respectively. Coordinates from the bregma were (in mm): −2.1 anterior/posterior, ±1.6 medial/lateral, and −1.95 dorsal/ventral from the dura. Viral titers were adjusted to ensure equal amounts were injected. At the appropriate times after injection, animals were transcardially perfused as previously described.
To phenotype the transduced cells, equidistant sections throughout the entire DG were selected and stained. Sections were immunostained for DCX, counterstained with Hoechst dye, and analyzed by confocal fluorescence microscopy (n = 20–40 cells per animal and marker). To analyze the ratio of colabeled cells after injection of viral mixtures, the fraction of double-transduced cells (GFP+ mCherry+) to all transduced cells (all mCherry+) was determined. Statistical analysis was performed with data from at least 10 sections from each hemisphere per mouse.
Morphological Analysis.
Dendritic lengths, branch points, spines, and mushroom spines were analyzed in DG neurons of mice that had been transduced 4 wk earlier with a GFP-encoding retrovirus. 3D reconstruction of GFP-transduced neurons was made from Z-series stacks of confocal images. The projection images were traced with ImageJ (Nationals Institutes of Health) (imagej.nih.gov/ij/) using the NeuronJ plug-in (www.imagescience.org/meijering/software/neuronj/). Spine density was calculated by dividing the spine number by the length of the dendritic segment. A spine was classified as mushroom if its diameter was more than twice the length of the spine neck.
Statistical Analysis.
Data were analyzed by unpaired two-tailed t test or ANOVA followed by post hoc tests for multiple comparisons when appropriate. A P value of ≤0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank S. K. Nikolis of the University of Milano-Bicocca, M. V. Sofroniew of the University of California, Los Angeles, and G. Enikolopov of Cold Spring Harbor Laboratory for Sox2flox, mGFAP-Cre, and Nestin-CFPnuc mice, respectively. This work was supported by transient research from the Sanford-Burnham Medical Research Institute and Grant RO1 NS066278 (to A.V.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421480112/-/DCSupplemental.
References
- 1.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42(4):535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 2.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6(1):21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 4.Jakovcevski M, Akbarian S. Epigenetic mechanisms in neurological disease. Nat Med. 2012;18(8):1194–1204. doi: 10.1038/nm.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Numata S, et al. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90(2):260–272. doi: 10.1016/j.ajhg.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn MA, et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Reports. 2013;3(2):291–300. doi: 10.1016/j.celrep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archer TC, Jin J, Casey ES. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Dev Biol. 2011;350(2):429–440. doi: 10.1016/j.ydbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyagi S, Kato H, Okuda A. Role of SoxB1 transcription factors in development. Cell Mol Life Sci. 2009;66(23):3675–3684. doi: 10.1007/s00018-009-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fantes J, et al. Mutations in SOX2 cause anophthalmia. Nat Genet. 2003;33(4):461–463. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- 10.Kelberman D, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116(9):2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisodiya SM, et al. Role of SOX2 mutations in human hippocampal malformations and epilepsy. Epilepsia. 2006;47(3):534–542. doi: 10.1111/j.1528-1167.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferri AL, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131(15):3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 13.Favaro R, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12(10):1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- 14.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 15.Spalding KL, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153(6):1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amador-Arjona A, et al. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: Implications for learning and memory. J Neurosci. 2011;31(27):9933–9944. doi: 10.1523/JNEUROSCI.1062-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis P, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26(2-4):148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 20.Suh H, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 22.Seri B, García-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478(4):359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 23.Bani-Yaghoub M, et al. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295(1):52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6(11):1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 25.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39(5):749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 26.Lodato MA, et al. SOX2 co-occupies distal enhancer elements with distinct POU factors in ESCs and NPCs to specify cell state. PLoS Genet. 2013;9(2):e1003288. doi: 10.1371/journal.pgen.1003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cimadamore F, et al. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell. 2011;8(5):538–551. doi: 10.1016/j.stem.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuwabara T, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh J, Gage FH. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev. 2004;14(5):461–469. doi: 10.1016/j.gde.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Ma DK, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323(5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z, et al. The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J Neurosci. 2011;31(26):9772–9786. doi: 10.1523/JNEUROSCI.1604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein BE, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125(2):315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 33.Boyer LA, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091):349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 34.Cavallaro M, et al. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135(3):541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- 35.Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30(2):714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taranova OV, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20(9):1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim DA, et al. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458(7237):529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergsland M, et al. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25(23):2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roybon L, et al. Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS ONE. 2009;4(3):e4779. doi: 10.1371/journal.pone.0004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao Z, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469(3):311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101(47):16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda S, et al. Sox21 promotes hippocampal adult neurogenesis via the transcriptional repression of the Hes5 gene. J Neurosci. 2012;32(36):12543–12557. doi: 10.1523/JNEUROSCI.5803-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39(3):372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hezroni H, et al. H3K9 histone acetylation predicts pluripotency and reprogramming capacity of ES cells. Nucleus. 2011;2(4):300–309. doi: 10.4161/nucl.2.4.16767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suka N, Suka Y, Carmen AA, Wu J, Grunstein M. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol Cell. 2001;8(2):473–479. doi: 10.1016/s1097-2765(01)00301-x. [DOI] [PubMed] [Google Scholar]
- 47.Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8(8):e000460. doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Encinas JM, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8(5):566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vega CJ, Peterson DA. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat Methods. 2005;2(3):167–169. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]
- 50.Sierra A, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 52.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nusser Z, et al. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21(3):545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 54.Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42(3):421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 55.Ring KL, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11(1):100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cimadamore F, Amador-Arjona A, Chen C, Huang CT, Terskikh AV. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci USA. 2013;110(32):E3017–E3026. doi: 10.1073/pnas.1220176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voigt P, Tee WW, Reinberg D. A double take on bivalent promoters. Genes Dev. 2013;27(12):1318–1338. doi: 10.1101/gad.219626.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heinrich C, et al. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rev. 2014;3(6):1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davidovich C, Zheng L, Goodrich KJ, Cech TR. Promiscuous RNA binding by Polycomb repressive complex 2. Nat Struct Mol Biol. 2013;20(11):1250–1257. doi: 10.1038/nsmb.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jia J, et al. Regulation of pluripotency and self- renewal of ESCs through epigenetic-threshold modulation and mRNA pruning. Cell. 2012;151(3):576–589. doi: 10.1016/j.cell.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 62.Ragge NK, et al. SOX2 anophthalmia syndrome. Am J Med Genet A. 2005;135(1):1–7, discussion 8. doi: 10.1002/ajmg.a.30642. [DOI] [PubMed] [Google Scholar]
- 63.Ragge NK, Quaghebeur G, Stewart H. SOX2 anophthalmia syndrome in adulthood - a neurodegenerative picture? Clin Genet. 2013;83(5):482–484. doi: 10.1111/j.1399-0004.2012.01922.x. [DOI] [PubMed] [Google Scholar]
- 64.Ferrari D, Binda E, De Filippis L, Vescovi AL. 2010. Isolation of neural stem cells from neural tissues using the neurosphere technique. Curr Protoc Stem Cell Biol Chapter 2:Unit2D.6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.