Significance
α-Synuclein (α-syn) aggregates released from neurons activate microglia, leading to chronic neuroinflammation that causes damage to neurons in brains with synucleinopathies, such as Parkinson’s disease (PD). However, little is known about the mechanism by which α-syn affects microglial activity, especially motility, and why microglia migrate toward the injured neurons and preferentially accumulate along with α-syn aggregates in the affected areas, e.g., in the substantia nigra of PD brains. Here we show that neuron-derived α-syn aggregates are chemoattractants that direct microglial migration by acting on NADPH oxidase and several specific downstream proteins. Blocking the targets involved in α-syn–mediated microglial directional migration may represent a therapeutic strategy to protect against progressive neuronal loss in PD and related synucleinopathies.
Keywords: α-Synuclein, microglial chemotaxis, neuroinflammation, Lyn, hydrogen peroxide
Abstract
Malformed α-Synuclein (α-syn) aggregates in neurons are released into the extracellular space, activating microglia to induce chronic neuroinflammation that further enhances neuronal damage in α-synucleinopathies, such as Parkinson’s disease. The mechanisms by which α-syn aggregates activate and recruit microglia remain unclear, however. Here we show that α-syn aggregates act as chemoattractants to direct microglia toward damaged neurons. In addition, we describe a mechanism underlying this directional migration of microglia. Specifically, chemotaxis occurs when α-syn binds to integrin CD11b, leading to H2O2 production by NADPH oxidase. H2O2 directly attracts microglia via a process in which extracellularly generated H2O2 diffuses into the cytoplasm and tyrosine protein kinase Lyn, phosphorylates the F-actin–associated protein cortactin after sensing changes in the microglial intracellular concentration of H2O2. Finally, phosphorylated cortactin mediates actin cytoskeleton rearrangement and facilitates directional cell migration. These findings have significant implications, given that α-syn–mediated microglial migration reaches beyond Parkinson’s disease.
Although progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) is a pathological hallmark of Parkinson’s disease (PD), the disorder also features activated microglia-mediated chronic inflammation and the accumulation of α-Synuclein (α-syn) in affected regions of the SNpc, suggesting that both activated microglia and α-syn contribute to the pathogenesis of PD.
α-Synuclein, a soluble protein that performs multiple physiological functions (1, 2), aggregates under challenging genetic or environmental conditions (3, 4). Accumulations of α-syn in neurons are toxic (5), most likely via impairment of ubiquitin-proteasomal and autophagy-lysosomal pathways, increased chronic endoplasmic reticulum stress, and mitochondrial dysfunction, among other mechanisms (6–9). In vitro and in vivo studies also have demonstrated that α-syn species, predominantly oligomers, can be released from neurons into the extracellular space (10, 11), stimulating microglial activation (10, 12) and initiating neuroinflammation. If unregulated, microglial activation may induce the release of inflammatory mediators, such as TNF-α, monocyte chemotactic protein-1 (MCP-1), and reactive oxygen species (ROS), to sites of neuroinflammation, causing progressive neuronal damage (13, 14).
Currently little is known about the mechanism by which α-syn affects microglial activity, especially motility. Several studies have reported that the extent of microglial activation in the SNpc correlates with α-syn accumulation. This result, together with the finding that microglia preferentially colocalize with aggregated α-syn in the SNpc (15, 16), led us to hypothesize that α-syn aggregates may act as chemoattractants to direct microglial migration toward neurons releasing α-syn into the interstitial tissue.
Here we show that the binding of α-syn to CD11b on microglia is sufficient and necessary for microglial directional migration. Furthermore, H2O2 originating from activated NADPH oxidase (Nox2), a key enzyme in microglia, serves as a direct signal that drives microglial migration. Finally, we identify a unique molecular event as critical to microglial directional migration: Lyn, a Src family kinase (SFK), senses changes in the intracellular concentration of H2O2 in microglia and phosphorylates the F-actin–associated protein cortactin, resulting in actin filament rearrangement and microglial directional migration.
Results
α-Syn Aggregates Act as Chemoattractants to Direct Microglial Migration.
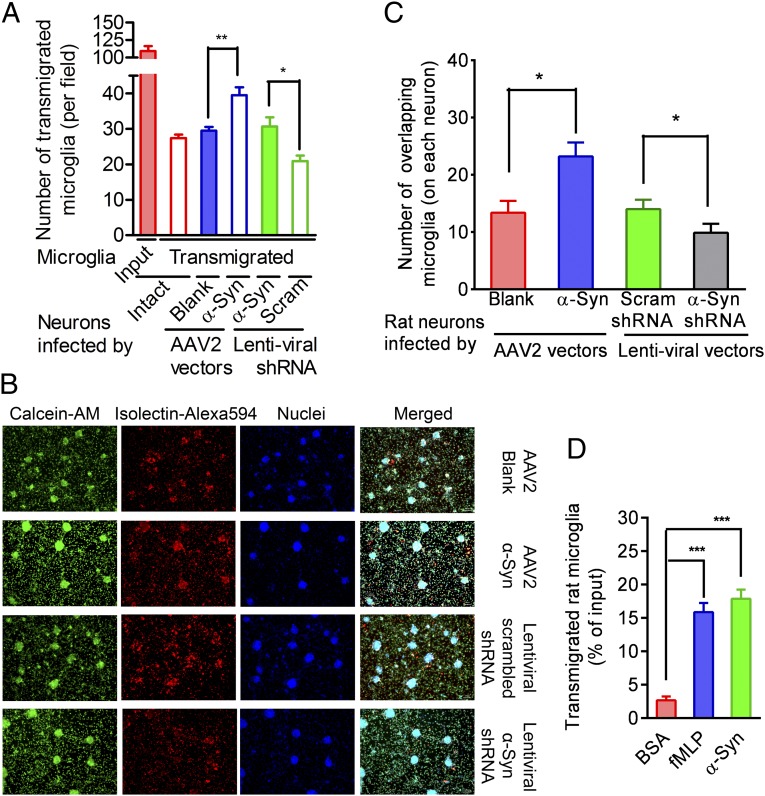
To determine whether neuron-derived α-syn acts as a microglial chemoattractant, we tested whether alteration of neuronal α-syn changed microglial migration toward affected neurons. Using viral expression systems carrying either specific α-syn cDNA or short-hairpin RNA (shRNA), α-syn was overexpressed or knocked down in rat primary neuron-enriched cultures (Fig. S1 A–E). On day 14 after seeding, the infected cells remained viable but appeared dysmorphic (Fig. S1F). As shown in Fig. 1A, neuronal α-syn overexpression accelerated microglial migration toward neurons, whereas decreasing expression of α-syn suppressed this migration.
Fig. 1.
Evidence that α-syn mediates microglial directional migration. (A) Quantitative analysis of microglial passage across the insert filters and migration to the bottom wells of 24-well Boyden chambers. The closed red bar indicates total input (1.0 × 105). Transmigrated microglia (isolectin-positive cells) were counted. n = 4. (B) Representative images showing microglial migration toward neurons. Rat neuron-enriched cultures in the 24-well plates were infected with the AAV2-blank, AAV2-α-syn, lentiviral scrambled (Scram), or rat α-syn–specific shRNA-carrying vectors. Then 3.0 × 105 microglia were directly added to the wells and allowed to migrate toward neurons overnight before staining with Alexa Fluor 594-conjugated isolectin, calcein-AM, and Hoechst. (C) Quantitative analysis of microglial migration toward neurons. Images, as represented in B, were captured, and the microglia that overlapped neurons were counted. n = 5. (D) Chemotaxis assays of microglial migration toward rH α-syn aggregates or fMLP in the 96-well Boyden chambers. After overnight incubation, the transmigrated microglia were measured using a CytoQuant assay kit. n = 4. Group comparisons were tested by ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001. (Scale bar: 100 µm.)
Hypothesizing that neurons could serve as a concentrated source of α-syn aggregates that could diffuse throughout the culture media, establishing a concentration gradient that recruits microglia, we examined colocalization of neurons and microglia after the addition of microglia directly to virus-infected neuron cultures (Fig. 1 B and C). Colocalization of microglia with neurons was assessed after labeling with calcein-AM (which labels the cytoplasm of both cell types; green), Alexa Fluor 594-conjugated isolectin (which labels microglia; red), and Hoechst (nuclear stain; blue) and quantifying the number of microglia overlapping with neuronal cell bodies. After overnight incubation, we found a significantly higher number of microglia colocalizing with the neurons overexpressing α-syn than with neurons with reduced α-syn.
Although these data strongly suggest that α-syn released from damaged neurons induces microglial migration, they also could be explained if manipulating neuronal α-syn modulates other potential microglial chemoattractants (e.g., MCP-1). Therefore, we examined whether recombinant human (rH) α-syn oligomers could directly initiate the process. As shown in Fig. 1D, whereas only 2.67 ± 1.27% of the microglia migrated toward media containing BSA, 17.88 ± 3.06% migrated toward α-syn aggregates, comparable to the 16.94 ± 4.37% of microglia that migrated to wells containing formyl-methionyl-leucyl-phenylalanine (fMLP), a myeloid cell chemoattractant exclusively dependent on the CD11b receptor (17), confirming that α-syn aggregates are potent and sufficient microglial chemoattractants.
CD11b Is Essential for Microglia to Migrate Toward Sources of α-Syn.
Because our previous study revealed that α-syn–mediated microglial activation is strongly dependent on CD11b (12), we probed its role in inducing α-syn–mediated microglial migration. We examined the ability of antibodies (Abs) against CD11b or α-syn, along with an isotype-control IgG and the fMLP receptor antagonist cyclosporin H, to block the migration of rat and mouse microglia toward α-syn aggregates or fMLP. Cyclosporin H and Ab against CD11b, but not α-syn, partially inhibited the migration response to the CD11b-dependent chemoattractant fMLP. In contrast, both CD11b and α-syn antibodies significantly suppressed migration toward α-syn aggregates (Fig. 2 A and B). Moreover, neither the anti-CD11b Ab nor the anti–α-syn Ab further inhibited CD11b−/− (or CD11 null) mouse microglial migration after stimulation by fMLP or α-syn (Fig. 2C), indicating that CD11b is required for microglial chemotaxis toward both fMLP and α-syn, but that the mechanisms involved are distinctively different.
Fig. 2.
CD11b is involved in the regulation of α-syn–mediated microglial migration. (A) Effect of fMLP receptor antagonist cyclosporin H and Ab against either α-syn or CD11b on migration of rat microglia toward purified rH α-syn aggregates or fMLP. Here 1.0 × 105 microglia were loaded onto each insert. (B) Effect of anti–α-syn or anti-CD11b Ab on migration of WT mouse microglia toward purified rH α-syn aggregates or fMLP. (C) Migration of CD11b−/− mouse microglia toward purified rH α-syn aggregates or fMLP. In A, B, and C, the transmigrated microglia were measured in the 96-well Boyden chambers using a CytoQuant assay kit. (D) Migration of rat primary microglia toward rat neuron-enriched cultures in which α-syn expression was intact, enhanced or knocked down. “Input” control reflects addition of 1.0 × 105 microglia directly to the well. (E) Migration of mouse WT or CD11b−/− microglia toward mouse or rat neuron-enriched cultures with or without an anti-CD11b Ab. Microglial migration in D and E was measured in the 24-well Boyden chambers. In A–E, n = 4. The Student t test was performed in E, whereas ANOVA followed by the Newman–Keuls multiple-comparisons test were performed in A–D. (F) Representative images showing rat primary microglial migration toward rat neurons with or without an anti-CD11b blocking Ab. Here 3.0 × 105 microglia were loaded onto each insert. (G) Quantitative analysis of microglia that overlapped neurons, counted in images as represented in F. n = 5, Student’s t test. In A–E and G, *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the corresponding BSA or IgG controls; ##P < 0.01 and ###P < 0.001 compared as indicated. (Scale bar: 100 µm.)
To confirm this mechanism in an independent system, we evaluated rat microglial migration toward rat neuronal cultures with enhanced or reduced α-syn expression. As expected, α-syn overexpression increased the number of transmigrated microglia, whereas knockdown of α-syn decreased it (Fig. 2D). Treatment with anti-CD11b Ab dramatically suppressed microglial migration toward intact neuronal cultures or cultures overexpressing α-syn (Fig. 2D), but did not further inhibit microglial chemotaxis when neuronal α-syn production was decreased (Fig. 2D), suggesting that α-syn is a key signaling factor recognized by CD11b. Considering α-syn generated by neurons from WT or CD11b−/− mouse or rat neurons, we found that regardless of the genotype of the neurons, far fewer CD11b−/− than WT mouse microglia migrated toward neurons, and that treatment with anti-CD11b Ab decreased WT microglial migration to a level roughly equivalent to that of CD11b−/− microglia (Fig. 2E). When rat microglia were pretreated with control IgG or anti-CD11b Ab and directly incubated overnight in the wells containing rat neurons, fewer anti-CD11b Ab-pretreated microglia colocalized with the neurons compared with control IgG-treated microglia (Fig. 2 F and G). Collectively, our data suggest that CD11b detects sources of α-syn and directs microglial migration.
α-Syn Binds Directly to Microglial CD11b.
The requirement of CD11b for microglial movement toward sources of α-syn, together with our previous finding that α-syn–induced microglial activation is CD11b-dependent (18), strongly suggest that α-syn is likely a ligand of CD11b. To directly test this hypothesis, we used purified Myc-DDK–fused human CD11b to coimmunoprecipitate rH α-syn aggregates. Myc-DDK–fused human CD11b pulled down α-syn (Fig. 3A, left lane), whereas α-syn alone failed to precipitate (Fig. 3A, right lane). Reciprocally, α-syn aggregates were able to coprecipitate CD11b from lysates collected from WT microglia, but not CD11b−/− microglia (Fig. 3B, left two lanes), whereas neither CD11b nor α-syn was pulled down by IgG (Fig. 3B, right two lanes). Flow cytometry and image analysis showed that both α-syn and CD11b Ab were localized to the surface of live microglia. Finally, preincubation in α-syn significantly inhibited additional binding of APC-conjugated anti-CD11b Ab to the microglial cell surface (Fig. S2 A, C, and E), and less Dylight 488-labeled α-syn bound to WT microglia pretreated with anti-CD11b blocking Abs or to CD11b−/− microglia than to the control IgG-treated WT microglia (Fig. S2 B and D). Taken together, these data indicate that α-syn is indeed a ligand of CD11b.
Fig. 3.
α-Syn aggregates directly bind to CD11b, which activates Nox2 to induce a migratory conformation of microglia. (A) In vitro binding assays. Myc-DDK–fused human CD11b was incubated with rH α-syn aggregates, and binding was detected via immunoblot for DDK and α-syn. Incubation of CD11b or α-syn alone served as controls. n = 3. (B) In vivo binding assays. Purified rH α-syn aggregates were mixed with WT or CD11b−/− microglial lysates to allow α-syn to react with CD11b, and the mixtures were further incubated in the IgG- or Ab-conjugated magnetic beads. Binding was detected via immunoblot for CD11b and α-syn. n = 3. (C) O2− production in mouse microglial cultures on stimulation using PMA (positive control) or α-syn. (D) Representative immunoblots showing plasma membrane (PM) translocation of p47phox from cytosol (Cyt) in PMA (positive controls)- or α-syn–stimulated HAPI cells. (E) Quantitative analysis of p47phox membrane translocation based on the immunoblot data. ANOVA was performed. n = 3. ***P < 0.001 compared with the BSA control. (F) Membrane translocation of p47phox and polarization of the cellular morphology in BSA- or α-syn–stimulated HAPI cells. (G) Rearrangement of the actin cytoskeleton in α-syn–stimulated HAPI cells in the presence or absence of an anti–α-syn or anti-CD11b Ab. The samples were immunostained for p47phox, F-actin (by phalloidin), and nuclei (by Hoechst). The arrows indicate the polarized distribution of p47phox in the lamellipodia. (Scale bar: 10 μm.)
We also tested α-syn–mediated adhesion of microglia by pretreating calcein-AM–labeled WT or CD11b−/− mouse microglia with control IgG or anti-CD11b blocking Abs and then adding them to plates coated with BSA or rH α-syn aggregates. We found that more WT microglia adhered to the α-syn–coated wells than to the BSA-coated wells (Fig. S2F). The CD11b−/− cells were less capable of adhering to the α-syn–coated wells, and pretreatment with the anti-CD11b Abs decreased the adhesion of WT microglia to approximately the same level as that of the CD11b−/− microglia (Fig. S2F). These findings suggest that α-syn–mediated microglial adhesion is specific to and directly dependent on the binding of α-syn to CD11b.
Nox2 Activation Is Required for Microglial Directional Migration.
We next explored the downstream events occurring after the binding of α-syn to CD11b. Consistent with our previous study (12), exposing WT microglia to α-syn aggregates increased Nox2 activity, as evidenced by increased production of the superoxide anion (O2−) (Fig. 3C). We compared the ability of α-syn aggregates to cause translocation of p47phox to the cell membrane, a surrogate marker of Nox2 activation (19), with that of phorbol 12-myristate 13-acetate (PMA), a compound that increases Nox2-generated O2− levels via a CD11b-independent pathway requiring the activation of protein kinase C (19). Using this measure, both PMA and α-syn aggregates increased Nox2 activation in rat microglia-derived HAPI cells (Fig. 3 D–G). Serendipitously, we noticed that, simultaneous with the translocation of p47phox to the plasma membrane, α-syn disrupted the peripheral actin rings in HAPI cells, resulting in a polarized microglial morphology in which p47phox accumulated along the leading edge of growing lamellipodia. Remarkably, lamellipodia growth was blocked by pretreating the microglia with an anti-CD11b or α-syn Ab (Fig. 3G).
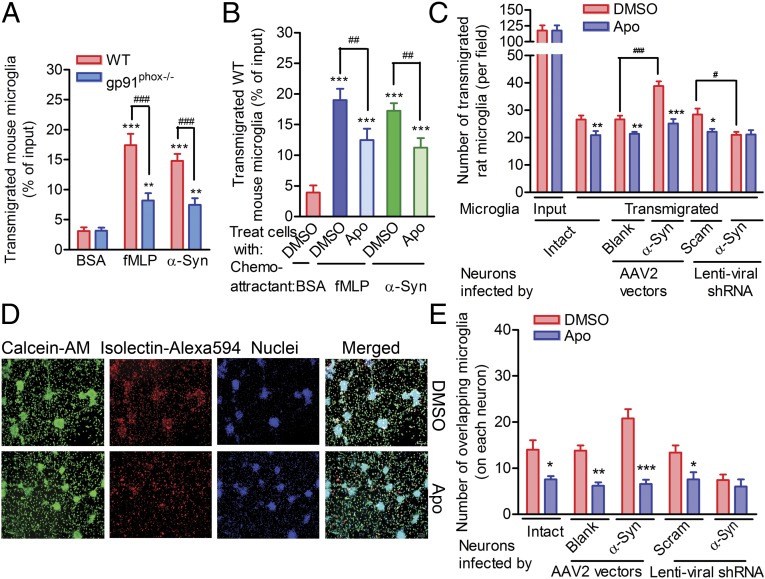
In addition, we inhibited mouse microglial chemotaxis toward α-syn aggregates or fMLP by knocking out gp91phox (gp91phox−/−), the catalytic subunit of Nox2 (Fig. 4A). In fact, simply inhibiting Nox2 using apocynin (Apo) (20) markedly suppressed microglial migration toward rH α-syn aggregates or fMLP (Fig. 4B). We found that Apo treatment decreased microglial migration toward either intact or α-syn–overexpressing neurons (Fig. 4C), but not toward neurons treated with α-syn shRNA, further demonstrating that α-syn–induced microglial migration depends on Nox2 activity (Fig. 4C). Moreover, when microglia were added directly to rat neuron cultures, treatment with Apo reduced the number of microglia that colocalized with rat neurons in which α-syn expression was intact (Fig. 4 D and E) or enhanced (Fig. 4E). Interestingly, Apo treatment did not further decrease the number of microglia colocalizing with neurons in rat primary neuronal cultures with decreased α-syn (Fig. 4E). Taken together, these data indicate that Nox2 activity is obligatory for directional microglial migration, although the mechanism by which the binding of α-syn to CD11b induces Nox2 activation remains to be investigated.
Fig. 4.
Activation of Nox2 is essential to α-syn–induced microglia directional migration. (A) Migration of WT or gp91phox−/− mouse microglia toward fMLP or rH α-syn aggregates. n = 5. The Student t test was performed. (B) Effect of the Nox2 inhibitor Apo on mouse microglial migration toward fMLP or α-syn. One-way ANOVA followed by the Newman–Keuls multiple-comparisons test were performed. n = 5. The transmigrated cells in A and B were detected in the 96-well Boyden chambers using a CytoQuant kit; 1.0 × 105 microglia were loaded. (C) Effect of the Nox2 inhibitor Apo on rat microglial migration toward neuron-enriched cultures. ANOVA and the Newman–Keuls multiple-comparisons test were applied. n = 5. The transmigration assays were performed in the 24-well Boyden chambers. (D) Representative images showing rat microglial migration toward neurons with or without Apo. Here 3.0 × 105 microglia were loaded. (E) Quantitative analysis of the microglia that overlapped neurons after the direct addition to neuron-enriched cultures overnight. Images, as represented in D, were captured, and the number of microglia that overlapped neurons was compared between DMSO treatment and the corresponding Apo treatment. The Student t test was performed. n = 5. *P < 0.05; **P < 0.01; ***P < 0.001. (Scale bar: 100 µm.)
H2O2, a Downstream Product of α-Syn–Activated Nox2, Serves as a Direct Signal to Regulate Microglial Directional Migration.
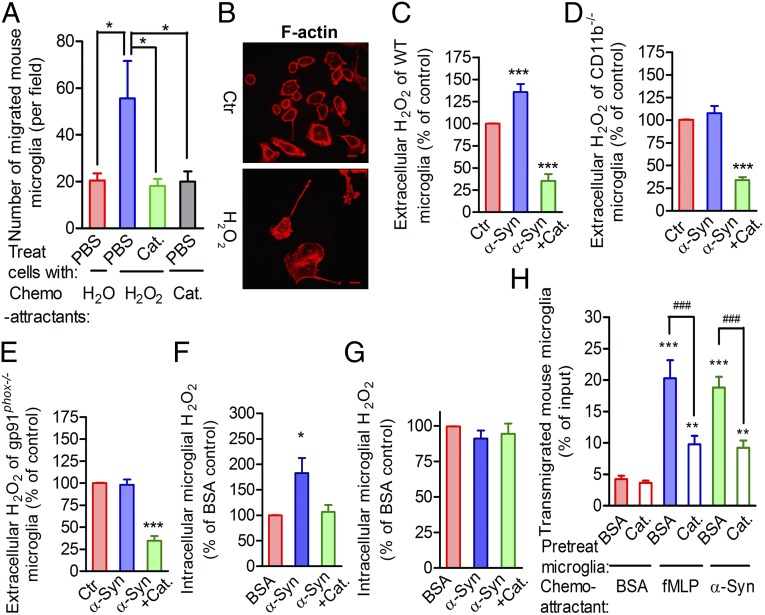
Nox2 activation increases extracellular O2− levels (21), and O2− is spontaneously converted to H2O2. This fact, combined with other reports that redox reactions play a role in cell migration (22), prompted us to examine whether ROS contribute to microglial migration toward sources of α-syn. Unlike O2−, H2O2 is membrane-permeable. Therefore, we hypothesized that H2O2 might be the principal signal that regulates microglial migration toward sources of α-syn. Using under-agarose chemotaxis assays, we showed that mouse microglia moved preferentially toward the source of H2O2 (Fig. 5A and Fig. S3 A–C). This response was attenuated by the presence of catalase in the microglial media (Fig. 5A). Remarkably, when directly exposed to H2O2, the microglia broke down their cortical actin rings and extended lamellipodia at the leading edges of the cells, exhibiting a migratory morphology (Fig. 5B) similar to that of α-syn–stimulated microglia.
Fig. 5.
H2O2, a product of α-syn–activated Nox2, serves as a direct signal to regulate microglial directional migration on the interaction between α-syn and CD11b. (A) Microglial chemotaxis toward H2O2 with or without catalase (Cat) based on an under-agarose gel migration assay. n = 4. ANOVA, followed by the Newman–Keuls multiple-comparisons test. (B) Polarized microglial morphology and F-actin distribution after direct exposure of cells to 10 µM H2O2 for 30 min. (Scale bar: 10 μm.) (C) Effect of α-syn stimulation on extracellular H2O2 in WT mouse microglia with or without catalase, as measured by an Abcam kit. BSA served as a control (Ctr). (D) Extracellular H2O2 in stimulated CD11b−/− mouse microglial cultures. (E) Extracellular H2O2 in stimulated gp91phox−/− mouse microglial cultures. (F) Quantitative analysis of the changes in intracellular H2O2 concentration in WT mouse microglia. (G) Quantitative analysis of the changes in intracellular H2O2 concentration in WT mouse microglia pretreated with catalase overnight. In C, D, E, F, and G, ANOVA was performed comparing with the BSA-treated control. n = 4. (H) BSA- or catalase-preincubated microglia migrated toward BSA, fMLP, or α-syn. Assays were performed in the 96-well Boyden chambers using a CytoQuant kit, with 1.0 × 105 microglia loaded onto each insert. n = 4. ANOVA followed by the Newman–Keuls multiple-comparisons test were performed. *P < 0.05; **P < 0.01; ***P < 0.001 compared with the corresponding BSA-treated control. ###P < 0.001 as indicated.
To further confirm the role of H2O2 in microglial migration, we monitored the changes in concentration of both extracellular (i.e., supernatant) and intracellular H2O2. We found that α-syn increased the extracellular H2O2 levels in WT mouse microglial cultures, and that the generation of H2O2 was suppressed by exogenous catalase (Fig. 5C). Moreover, α-syn failed to generate extracellular H2O2 from either CD11b−/− or gp91phox−/− microglia (Fig. 5 D and E). The intracellular accumulation of H2O2 in WT mouse microglia mirrored the extracellular accumulation of H2O2 in response to α-syn, and, as before, this accumulation was suppressed when α-syn was applied together with catalase (Fig. 5F and Fig. S3D). In addition, after overnight preincubation of the cells in catalase, the intracellular H2O2 levels remained relatively low, and no difference in intracellular H2O2 was detected between BSA- and α-syn–stimulated microglia (Fig. 5G and Fig. S3E). However, preincubating the microglia in catalase did not affect the α-syn–induced increase in the extracellular H2O2 levels (Fig. S3F). These results suggest that in α-syn–stimulated microglia, most of the intracellular H2O2 originates from the extracellular space and diffuses down the concentration gradient. Furthermore, the presence of catalase significantly inhibited microglial migration to the source of α-syn or fMLP (Fig. 5H). Taken together, these data indicate that α-syn–activated Nox2 produces extracellular O2− that is converted to H2O2, either spontaneously or with the assistance of extracellular SOD (23), leading to an extracellular-to-intracellular H2O2 gradient that guides microglial migration toward to the source of α-syn.
Lyn Acts as a Sensor of H2O2 Levels to Regulate the Phosphorylation of Cortactin and to Facilitate Microglial Migration Toward α-Syn.
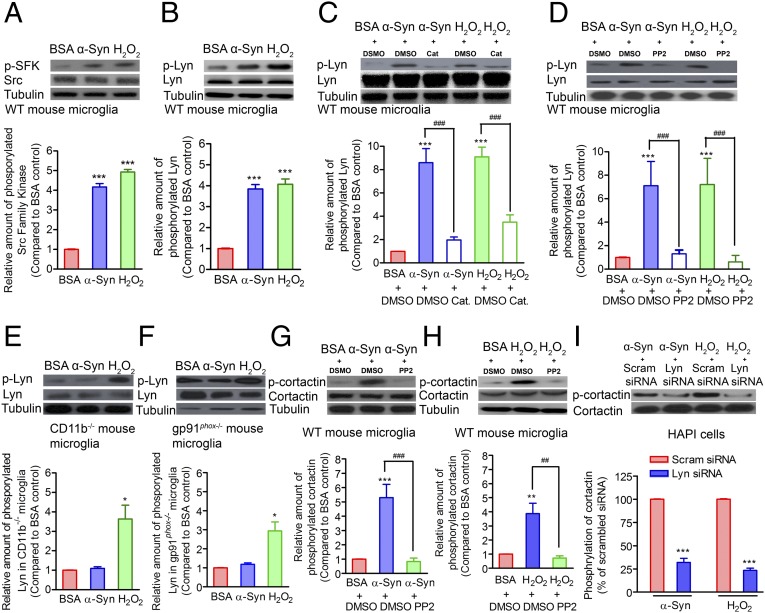
We next examined the mechanism by which α-syn–induced H2O2 guides the directional movement of microglia. H2O2 is known to regulate the activity of numerous tyrosine kinases by oxidizing cysteine residues (24, 25). Specifically, a recent study showed that SFKs are activated by H2O2 to direct neutrophil migration (26). Therefore, we hypothesized that H2O2 activates SFKs and induces the rearrangement of actin filaments, which provides the basis for the microglial detection of and migration toward sources of extracellular α-syn. To test this hypothesis and the signaling mechanisms involved, we examined the effect of α-syn and H2O2 on SFK phosphorylation, and found that it was increased in microglia by either treatment (Fig. 6A). By isolating proteins containing phosphorylated tyrosine from the lysates of α-syn– or H2O2-treated mouse microglia, we found that phosphorylated Lyn, a member of the SFK family, was substantially enriched in these cell lysates (Fig. 6B). Further demonstrating that H2O2 induces Lyn phosphorylation, the simultaneous addition of catalase markedly attenuated Lyn phosphorylation induced by either α-syn or H2O2 (Fig. 6C). Similarly, the Src inhibitor PP2 suppressed Lyn phosphorylation (Fig. 6D).
Fig. 6.
Lyn acts as an H2O2 sensor to regulate the phosphorylation of cortactin. (A) Exposure to either α-syn or H2O2 induced the phosphorylation of SFKs (pSFK) in WT primary mouse microglia. Tubulin (Tub) blots served as protein loading controls. (B) Lyn, an SFK family member, is phosphorylated in WT mouse microglia stimulated using either α-syn or H2O2. (C) Effect of DMSO (DM) or catalase (Cat) on α-syn– or H2O2-induced Lyn phosphorylation in WT mouse microglia. (D) Effect of DMSO (DM) or PP2 on α-syn– or H2O2-induced Lyn phosphorylation in WT mouse microglia. (E) Lyn phosphorylation (pLyn) in CD11b−/− mouse microglia stimulated by α-syn or H2O2. (F) Lyn phosphorylation (pLyn) in gp91phox−/− microglia stimulated by α-syn or H2O2. (G) Effect of DMSO (DM) or PP2 on α-syn–induced cortactin (Cort) phosphorylation (pCort) in WT mouse primary microglia. (H) Effect of DMSO (DM) or PP2 on H2O2-induced cortactin phosphorylation (pCort) in WT mouse primary microglia. (I) Effect of scrambled (Scram) or Lyn siRNA treatment on α-syn– or H2O2-induced cortactin phosphorylation in rat microglia-derived HAPI cells. In A–H, ANOVA and the Newman–Keuls multiple-comparisons test were performed. In I, the Student t test was performed. ***P < 0.001 comparing Lyn shRNA- and its corresponding scrambled shRNA-treated HAPI cells, stimulated by either α-syn or H2O2. In A–I, representative Western blots are shown on the top, and a corresponding summary of three to five independent experiments is shown on the bottom.
Because both CD11b and gp91phox are required for the α-syn–induced activation of microglial Nox2, we examined whether Lyn was phosphorylated in CD11b−/− and gp91phox−/− mouse microglia stimulated by α-syn or H2O2. As expected, Lyn phosphorylation was not induced by α-syn in the CD11b−/− or gp91phox−/− microglia, but could be induced by directly exposing the knockout microglia to H2O2 (Fig. 6 E and F), confirming that H2O2 is the signaling molecule that acts downstream of both CD11b and Nox2.
To identify the downstream targets of phosphorylated SFK that regulate the rearrangement of cytoskeletal actin filaments, we stimulated WT mouse primary microglia and rat microglia-derived HAPI cells with either α-syn or H2O2. We detected increased phosphorylation of cortactin, an F-actin–associated protein that promotes actin cytoskeleton polymerization and rearrangement (27), in both cell types. However, both PP2 (Fig. 6 G and H and Fig. S4 A and B) and catalase (Fig. S4 A and B) attenuated this response, indicating that Src serves as the second messenger between α-syn– or H2O2-mediated microglial stimulation and cortactin phosphorylation.
To further clarify the role of Lyn in cortactin phosphorylation using a more specific approach, we knocked down Lyn in HAPI cells using siRNA (Fig. S5 A and B), and found that decreased Lyn dramatically reduced cortactin phosphorylation in both α-syn– and H2O2-stimulated HAPI cells (Fig. 6I). As before, α-syn treatment of WT primary microglia and HAPI cells induced actin filament reorganization and long lamellipodia extension, as well as relocalization of phosphorylated cortactin to the leading edge of the cells and colocalization of phosphorylated cortactin with polarized actin filaments, whereas PP2 or Lyn siRNA treatment prevented this effect (Fig. 7 A and B). Chemotaxis assays further showed that treatment with PP2 inhibited the migration of rat primary microglia and HAPI cells toward the source of H2O2 (Fig. 7C, Left and Fig. S3C), and that Lyn knockdown decreased the number of migrating HAPI cells (Fig. 7C, Right and Fig. S5B). In migration assays, treatment with PP2 (rat primary microglia and HAPI cells) or Lyn siRNA (HAPI cells) decreased migration toward α-syn aggregates or fMLP (Fig. 7D), confirming the role of Lyn in microglial migration toward α-syn or fMLP. Moreover, overnight coincubation of HAPI cells and dysmorphic rat neurons showed that treatment with either PP2 or Lyn siRNA reduced the number of HAPI cells that colocalized with the dysmorphic rat neurons (Fig. S5 C and D).
Fig. 7.
Activation of Lyn and cortactin facilitates microglial migration toward the source of H2O2, fMLP or α-syn. (A) Effect of the Src-inhibitor PP2 on the α-syn–induced distribution of F-actin and phosphorylated cortactin (pCortactin) in WT mouse primary microglia. (B) Effect of the Lyn siRNA on the α-syn–induced distribution of F-actin and phosphorylated cortactin in HAPI cells. Arrows are indicating the polarized localization of F-actin and pCortactin. (Scale bar: 10 μm.) (C) Effect of PP2 or Lyn siRNA on rat primary microglial or HAPI cell chemotaxis toward the source of H2O2 by under-agarose migration assays. (D) Effect of PP2 or Lyn siRNA on rat primary microglial or HAPI cell chemotaxis toward the source of fMLP or α-syn in the 96-well Boyden chambers. In C and D, 1.0 × 105 microglia were loaded onto each well or insert. Cell migration with DMSO (DM) or PP2 is shown on the left, and migration of cells transfected with scrambled (Scram) or Lyn siRNA for 3 or 10 d is shown on the right. n = 4–5. The Student t test was performed. *P < 0.05 and **P < 0.01 compared with the corresponding DMSO- or scrambled siRNA-treated controls.
To further demonstrate that the actions of Lyn are both necessary and sufficient to mediate the effects of α-syn, Lyn protein was transiently silenced by transfection of Lyn-specific or scrambled siRNA into HAPI cells. Western blot analysis demonstrated that Lyn protein was markedly reduced by day 3 after transfection, but normal levels were restored by day 10 (Fig. S5B). In parallel, we tested the ability of transfected cells to migrate toward the source of H2O2, fMLP or α-syn. As shown on the right side of Fig. 7 C and D, chemotaxis was reduced by Lyn-specific siRNA on day 3 after transfection regardless of which chemoattractant was used; however, this reaction was restored to the control level on day 10 after transfection, further suggesting that Lyn is required for microglial directional migration. Taken together, these data clearly suggest that H2O2, which is generated by microglia on stimulation by α-syn, enables phosphorylated Lyn to activate cortactin, which in turn induces actin filament rearrangement, thereby facilitating microglial migration.
Because α-syn is also abundant in the periphery, and recent work has demonstrated dysregulation of monocytes in the blood in PD (28), we examined the effects of α-syn on migration of primary monocytes. We found that fMLP enhanced chemotaxis of monocytes isolated from peripheral rat blood, a response that was partially inhibited by Src kinase inhibitor PP2; however, unlike microglia, rat primary monocytes failed to directionally move toward a source of α-syn (Fig. S6A). Exposure of monocytes to fMLP increased the phosphorylation of both the Src member Lyn and the F-actin–associated protein cortactin (Fig. S6B), indicating that Lyn-dependent cortactin phosphorylation is involved in the response of monocytes to fMLP. Therefore, these data support the idea that the microglial response is quite different from that of other myeloid-derived cells such as monocyte-macrophages, despite the fact that they share the same origin, and that the directional migration of monocytes, at least toward fMLP, is Src-dependent.
Discussion
It is well known that α-syn aggregates indirectly damage neurons by eliciting microglia-mediated neuroinflammation (12, 29, 30); however, the mechanism by which α-syn aggregates interact with microglia remains incompletely understood. Previous studies found that activated microglia preferentially accumulate with α-syn aggregates in affected areas of the PD brain. This finding prompted us to speculate that α-syn aggregates act as chemoattractants to recruit microglia toward the sources of α-syn, thus inadvertently damaging neurons, e.g., in SNpc. Although a recent study suggested that neuronal α-syn could potentially induce microglial migration in a β1 integrin-dependent manner (14), it did not examine the directional movement of microglia or the potential mechanisms involved in this process. Our investigation has made several advances in understanding how α-syn affects microglial motility. We have shown that α-syn induces microglial directional migration in vitro by binding to CD11b, which activates Nox2 to produce H2O2, leading to the phosphorylation of the SFK member Lyn and the F-actin–associated protein cortactin. Activated cortactin ultimately promotes the reorganization of cytoskeletal actin scaffolds into lamellipodia that extend toward the source of α-syn (Fig. S7).
The first of these key findings is that extracellular α-syn aggregates are sufficient to directionally mobilize microglia via direct binding to the receptor CD11b. It is known that neurons can release several mediators, such as gastrin-releasing peptide (31), that are capable of attracting leukocytes. Our findings that overexpression of α-syn accelerated microglial migration, whereas decreasing α-syn expression suppressed this migration, demonstrate that neuron-derived α-syn can function in a similar capacity, serving as a microglial chemoattractant. Moreover, although we found that purified rH α-syn aggregates directly recruit microglia, we did not eliminate monomeric forms as contributors to this process. However, it has been shown the majority of α-syn released from neurons is in oligomeric forms (10), making these the most likely candidates. Collectively, α-syn aggregates released from neurons appear to be sufficient to initiate directional microglial migration, although we cannot exclude the possible involvement of other chemoattractants in our experimental paradigm.
We further investigated the mechanism by which aggregated α-syn affects microglial behavior and morphology. It was previously shown to bind to several cellular receptors or adapters, such as TLR2 (10), whereas our data indicate that CD11b functions as a chemotactic sensor of α-syn to direct microglial migration via direct interaction. However, neither genetic ablation of CD11b nor treatment with a CD11b-neutralizing Ab completely abolished microglial directional migration, implying that other microglial receptors, e.g., TLR2 (10) or β1 integrin (14), also may contribute to this cellular event. Whether β1 integrin binds directly to α-syn remains to be investigated.
The second major discovery of the present investigation is that Nox2 activity is an absolute requirement for microglial directional migration, regardless of which chemoattractant to which the microglia are exposed. Our previous studies demonstrated that extracellular α-syn aggregates increase the production of extracellular O2− by Nox2 (12), and others have reported that redox reactions are involved in cell migration (22). In the present study, we further found that activation of Nox2 and relocalization of the Nox2 p47phox subunit to the plasma membrane (particularly within extended lamellipodia) are vital steps in initiating α-syn–induced microglial directional migration.
The critical issue raised by these findings is the identity of the signaling molecule(s) downstream of Nox2 activation that mediate(s) microglial migration. Several lines of evidence led us to focus on H2O2 in particular: (i) it can be spontaneously generated by O2− even in the absence of SOD, at a rate of 8 × 104 M-1s−1 (32); (ii) it readily diffuses across the cell membrane (33); and (iii) although this activity has not been reported for microglia, it can effectively recruit leukocytes to wounded tissues (26). In this investigation, exogenous H2O2 clearly stimulated microglial migration, which was abolished by coincubation of the microglia with catalase. It must be stressed that many investigators, including us, have established that O2− generated by activated Nox2 is secreted primarily extracellularly (21). Thus, our data, along with those of others, support the argument that intracellular H2O2 transduces the α-syn–elicited extracellular signals to the microglial cytosol.
Finally, in this study we examined the molecular events leading to microglial directional migration. In cytosol, H2O2 oxidizes cysteine residues in many kinases and phosphatases, regulating their activity (34). We found that exposure to either α-syn or H2O2 led to increased phosphorylation of SFK Lyn, provided that the upstream requirements (i.e., CD11b and gp91phox) were intact. We also identified Lyn phosphorylation as essential for microglial directional migration toward sources of either α-syn or H2O2, further clarifying the signaling pathway for this important event.
Because cell migration relies on remodeling of the actin cytoskeleton (35), we also explored the molecular targets downstream of Lyn and found that cortactin, an F-actin–associated protein, was phosphorylated after exposure to either α-syn or H2O2. It has been suggested that phosphorylated cortactin relocalizes to specific cellular regions, particularly lamellipodia, and propels cell migration toward its targets (27, 36). Consistent with these findings, our data show that exposure to either α-syn or H2O2 increased cortactin phosphorylation and shifted cortactin expression from the cytoplasm to the cellular leading edge, which occurred in parallel with the rearrangement of actin filaments. This result suggests that Lyn-dependent cortactin phosphorylation is essential for microglial migration. In contrast, although stimulation of peripheral monocytes with fMLP also resulted in increased phosphorylation of both Lyn and cortactin, suggesting that their migration toward fMLP follows a process similar to that of microglia, monocyte migration was not stimulated by α-syn. This finding suggests that, despite the common origin of microglia and monocytes, this process is specific to the brain. These results are particularly interesting in light of the finding of inflammatory dysregulation of PD blood monocytes (28). Changes in the functions of peripheral blood leukocytes, which are frequently “primed” by an initial insult and become more sensitive to a second stimulation, or “hit,” have been reported in many chronic inflammatory conditions, e.g., chronic kidney disease (37). Although we did not observe normal monocytes responding to the challenge of α-syn, this does not necessarily exclude the possibility that monocytes in PD obtain the ability to directionally migrate toward the source of α-syn. Owing to a lack of fresh samples obtained from either PD patients or Parkinsonian animal models, we cannot test this possibility at present. The difference between cells from these sources also suggests an additional caveat of the study: the microglia that we tested are derived from newborn animals and might have a different molecular signature than adult microglia (38). Therefore, further investigation of whether neuron-derived α-syn also influences the mobility of adult microglia is needed.
In summary, we have confirmed that neuron-derived α-syn acts as a chemoattractant to direct microglial migration and revealed the signaling pathway that mediates this cellular function. The directional recruitment of microglia may benefit the central nervous system by removing unhealthy neurons or tissue debris (39, 40). However, exposure to such undesirable materials may further activate microglia, sustaining chronic neuroinflammation in PD and related synucleinopathies and causing progressive neuronal damage. In this study we did not address whether α-syn–induced microglial migration is beneficial or harmful to the central nervous system; however, previous studies have suggested that inappropriate microglial activation harms neurons (29). Based on this scenario, blocking of α-syn–mediated microglial migration may provide a therapeutic strategy to protect neurons against progressive loss in PD and other synucleinopathies.
Materials and Methods
Animals.
Mice deficient in gp91phox (gp91phox−/−) or CD11b (CD11b−/−) and their WT C57BL/6J controls were housed in a pathogen-free facility at the National Institute of Environmental Health Sciences (NIEHS). Animal housing, breeding, and experiments were approved by NIEHS and performed in strict accordance with National Institutes of Health guidelines.
Cultures of Mixed Glia and Harvesting of Microglia.
The brains of mouse or rat pups were dissected on postnatal day 1 as described previously (12), and the brain cells were seeded on T175 flasks. Two weeks later, the microglia were harvested by shaking the flasks.
Primary Neuron-Enriched Cultures.
The cultures were established as described previously (12) by seeding mouse or rat embryonic day 14 mesencephalic cells on 6- or 24-well plates or MakTek dishes. Two days later, 10 µM arabinofuranosyl cytidine (Ara-C; Sigma-Aldrich) was added to the cultures to remove the glia and enrich the neurons. On day 14, these neuron-enriched cultures, either intact or infected by AAV2 or lentiviral vectors (see below), were used for various experimental purposes.
Isolation of Rat Peripheral Blood Monocytes.
Rat monocytes were isolated from the peripheral blood using Ficoll-Paque PREMIUM (GE Healthcare Life Sciences) as described previously (41) and detailed SI Materials and Methods.
AAV2 Vectors and Infection of Rat Primary Neurons.
AAV2 vectors harbor the cDNA encoding green fluorescent protein (GFP) or human α-syn. To infect rat primary neuron-enriched cultures, 1.0 × 1013 Vg/mL of vectors was added overnight on day 6 after cell seeding, and the cultures were maintained for 7 d in fresh media for different experimental purposes.
Retroviral Packing of Lentiviral Vectors and Infection of Rat Primary Neurons.
As described previously (42), retroviral packing was performed using a combination of human 293FT cells, the packing plasmids, and the pGFP-C-shLenti-vector carrying scrambled or rat α-syn–specific shRNA (Origene Technologies). Infection of rat neuron-enriched cultures was performed overnight by adding the media collected from the transfected 293FT cell cultures to each neuron-enriched culture, along with 6 µg/mL polybrene.
Knockdown of Lyn in HAPI Cells Using SiRNA.
HAPI cells (a rat microglia-derived cell line) were seeded on six-well plates and transfected by adding Opti-MEM reduced serum media containing a mixture of Lipofectamine RNAiMAX transfection reagent (7.5 µL/well; Life Technologies) and ON-TARGETplus SMARTpool rat Lyn-specific siRNA (40–80 pmol/well; GE Dharmacon).
Preparation of rH α-Syn.
Dissolved in water, rH α-syn (endotoxin level, <0.024 EU/μg) was incubated with agitation at 37 °C for 7 d. This process allowed α-syn to form oligomers (12), which were used in this study.
Microglial Cell Migration Assays Using 96-well Boyden Chambers.
In a 96-well Boyden chamber, each bottom well was filled with 200 µL of serum-free media containing 0.1% BSA, 1.0 × 10−7 M fMLP or 1.0 μM rH oligomeric α-syn with or without the control IgG, or an anti–α-syn Ab (Abcam). Before the insert frame was placed back on the plate and incubated at 37 °C overnight, each insert, with 5-µm pores in its filter membrane, was loaded with 1.0 × 105 microglia, HAPI cells, or monocytes pretreated with the control IgG or an anti-CD11b Ab (Biolegend) or a compound such as Apo (0.25 mM), cyclosporin H (1.0 μM; Santa Cruz Biotechnology), PP2 (10 μM), or catalase (100 U/well; EMD Chemicals). The number of transmigrated cells was measured using a CytoQuant Kit (Life Technologies) according to the manufacturer’s protocol.
Microglial Cell Migration Assays Using 24-well Boyden Chambers.
Enriched mouse or rat primary neurons were cultured in the bottom wells of 24-well Boyden chambers. A total of 1.0 × 105 microglia pretreated with the control IgG or an anti-CD11b Ab or a compound such as DMSO, Apo, PP2, or catalase were loaded on each insert, which contained 5-µm pores in its filter membrane. Then the inserts were placed back on the bottom chamber. In some experiments, the neuron-enriched cultures in the bottom wells were treated with the control IgG or an anti–α-syn Ab when the chemotaxis assays were initiated. After overnight incubation, the cultures were stained with calcein-AM and Alexa Fluor 594-conjugated isolectin. The isolectin-positive cells in 15 random fields per well were counted as microglia (43).
Chemotaxis Assays Using Live Cell Imaging.
Intact or viral vector-infected enriched rat primary neurons were cultured in 24-well plates. Then 1.0 or 3.0 × 105 of microglia treated by anti-CD11b Ab or compounds (e.g., PP2), or HAPI cells transfected with scrambled or Lyn siRNA or treated with DMSO or PP2, were directly applied to the bottom wells containing the enriched rat neurons. On the next day, the cells were labeled with calcein-AM (cytoplasm of both neurons and microglia), Alexa Fluor 594-conjugated isolectin (microglia only), and Hoechst (cell nuclei). Fifteen fields in each well for each experimental condition were selected at random for imaging with an Axio Observer epifluorescent microscope equipped with a 10× objective (Carl Zeiss Microscopy). The amount of microglia overlapping neurons was quantified with Imaris 7.7 (Bitplane).
Chemotaxis Assessment Using Under-Agarose Migration Assays.
These assays were performed as described previously (26, 44) to examine the ability of microglia or HAPI cells to migrate toward the source of H2O2.
Co-IP.
To determine whether rH α-syn binds to Myc/DDK-tagged CD11b (OriGene Technologies), an equimolar mixture of these two proteins was incubated and further reacted with anti-Myc–tagged magnetic beads (MBL International). After multiple washes, the beads were boiled in Laemmli sample buffer, and the samples were examined by Western blot analysis for α-syn or DDK peptide (OriGene Technologies). To evaluate the ability of rH α-syn to bind to microglia-derived CD11b, α-syn was mixed with WT or CD11b−/− microglial lysates containing IP lysis buffer (Thermo Scientific), and anti–α-syn Ab or control IgG was conjugated to the protein G magnetic beads (Life Technologies). Next, the mixtures of α-syn and microglial lysates were incubated with the Ab- or control IgG-conjugated protein G magnetic beads. After washing, the beads were eluted and the samples were boiled in Laemmli sample buffer and Western-blotted for CD11b or α-syn.
Immunofluorescence.
To demonstrate the membrane translocation of p47phox and cell spreading, mouse primary microglia or HAPI cells, either intact or pretreated with scrambled or Lyn-specific siRNA (OriGene Technologies), were stimulated and then fixed. After blocking with PBS containing 5 ml/100 ml goat serum and 0.3% Triton-X, the cells were immunostained for p47phox or phosphorylated cortactin (phospho-Y466 Ab; Abcam), followed by staining for F-actin and nuclei. The samples were analyzed using a Zeiss LSM 510 confocal microscope.
Detection of Membrane Translocation of p47phox via Western Blot Analysis.
HAPI cells were stimulated using 0.1% BSA or 250 nM α-syn and then lysed in a hypotonic lysis buffer. The membrane fraction and cytosol were collected from the cell lysates using centrifugation as described previously (45). The proteins in the membrane fraction and cytosol were examined by Western blot analysis for p47phox and gp91phox.
O2− Production Assays.
O2− released from mouse microglia was measured according to the SOD-inhibitable reduction of the tetrazolium salt WST-1 (46).
Extracellular and Intracellular H2O2 Assays.
The level of extracellular H2O2 released from microglia into the culture supernatant was examined using a hydrogen peroxide assay kit (Abcam) according to the manufacturer’s instructions. To measure the intracellular H2O2 level, WT microglia were seeded on each well of 96-well plates with or without 1 mg/mL catalase overnight, to allow delivery of catalase to the cells (47). Next, the microglia were stimulated by 0.1% BSA, 250 nM α-syn, or a mixture of α-syn and 100 U/mL catalase at 37 °C for 1 h. After washing, 50 µL of media and 50 µL of AbGreen Indicator working solution (Abcam) were added to each well, and the plate was measured using a fluorescence reader every 5 min for 1 h.
Statistical Analysis.
Data are presented as mean ± SEM and compared using the Student t test or ANOVA plus the Newman–Keuls multiple-comparisons test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank the Michael J. Fox Foundation for providing the AAV2 vectors. We appreciate the technical support from Dr. Negin Martin at the National Institute of Environmental Health Science (NIEHS) Laboratory of Neurobiology Viral Vector Core Facility and Mr. Jeff Tucker at the NIEHS Imaging Center. We are grateful to Drs. Keith Burridge and Zachary Hoffer for their critical reading of this manuscript. This study was supported by intramural funding from the NIEHS (to J.-S.H.), National Institutes of Health Grants R01 ES019277 and R01 ES016873 (to J.Z.), and Grant 81129018 from the National Natural Science Foundation of China (to J.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417883112/-/DCSupplemental.
References
- 1.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477(7362):107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burré J, et al. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4(11):1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 4.Di Monte DA. The environment and Parkinson’s disease: Is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2(9):531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 5.Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14(9):626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AA, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313(5785):324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzulli JR, et al. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146(1):37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrucelli L, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: Proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36(6):1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 9.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 10.Kim C, et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Bae EJ, Lee SJ. Extracellular α-synuclein: A novel and crucial factor in Lewy body diseases. Nat Rev Neurol. 2014;10(2):92–98. doi: 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson's disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 13.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim C, et al. β1-integrin–dependent migration of microglia in response to neuron-released α-synuclein. Exp Mol Med. 2014;46:e91. doi: 10.1038/emm.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson's disease. J Neuroimm Pharmacol. 2009;4(4):419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J Cell Sci. 2005;118(Pt 22):5205–5220. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55(11):1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- 19.Raad H, et al. Regulation of the phagocyte NADPH oxidase activity: Phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2009;23(4):1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11(1):95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 21.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 22.Sakai J, et al. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity. 2012;37(6):1037–1049. doi: 10.1016/j.immuni.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35(3):236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 24.Burgoyne JR, et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317(5843):1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 25.Kemble DJ, Sun G. Direct and specific inactivation of protein tyrosine kinases in the Src and FGFR families by reversible cysteine oxidation. Proc Natl Acad Sci USA. 2009;106(13):5070–5075. doi: 10.1073/pnas.0806117106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480(7375):109–112. doi: 10.1038/nature10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uruno T, et al. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3(3):259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 28.Grozdanov V, et al. Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol. 2014;128(5):651–663. doi: 10.1007/s00401-014-1345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 30.Harms AS, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33(23):9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czepielewski RS, et al. Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Proc Natl Acad Sci USA. 2012;109(2):547–552. doi: 10.1073/pnas.1110996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson PL, Phillis JW. Novel Therapies for CNS Injuries: Rationales and Results. CRC; Boca Raton, FL: 1995. pp 416. [Google Scholar]
- 33.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758(8):994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Rhee SG. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacGrath SM, Koleske AJ. Cortactin in cell migration and cancer at a glance. J Cell Sci. 2012;125(Pt 7):1621–1626. doi: 10.1242/jcs.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sela S, et al. Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol. 2005;16(8):2431–2438. doi: 10.1681/ASN.2004110929. [DOI] [PubMed] [Google Scholar]
- 38.Butovsky O, et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain. 2009;132(Pt 2):288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201(4):647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richters CD, Mayen I, Havenith CE, Beelen RH, Kamperdijk EW. Rat monocyte-derived dendritic cells function and migrate in the same way as isolated tissue dendritic cells. J Leukoc Biol. 2002;71(4):582–587. [PubMed] [Google Scholar]
- 42.Wang S, et al. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS ONE. 2012;7(9):e45499. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon BK, et al. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci USA. 2002;99(5):3246–3251. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heit B, Kubes P. Measuring chemotaxis and chemokinesis: The under-agarose cell migration assay. Sci STKE. 2003;2003(170):PL5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- 45.Qian L, et al. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol. 2008;181(1):660–668. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan AS, Berridge MV. Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: A simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods. 2000;238(1-2):59–68. doi: 10.1016/s0022-1759(00)00156-3. [DOI] [PubMed] [Google Scholar]
- 47.Chinta SJ, Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: Implications for Parkinson’s disease. Free Radic Biol Med. 2006;41(9):1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.