Abstract
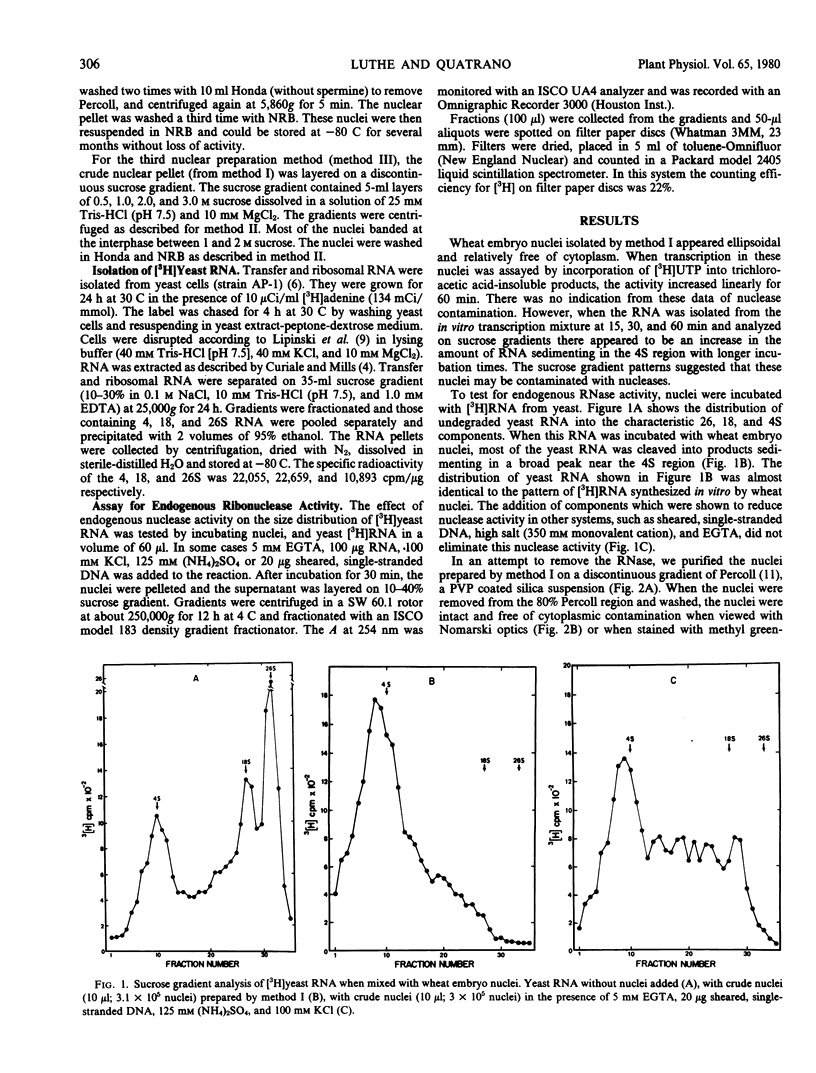
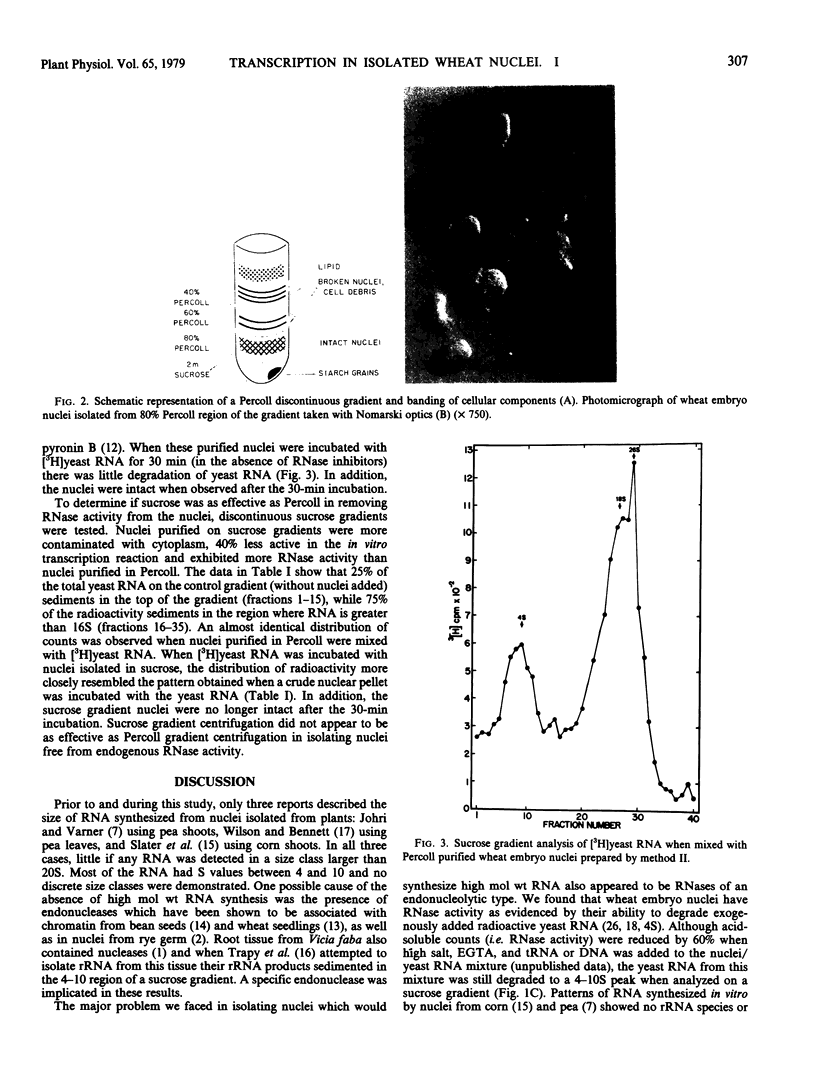
Nuclei isolated from embryos of wheat (var. Yamhill) incorporated [3H]UTP into a trichloroacetic acid-insoluble product linearly for 60 minutes. When the RNA synthesized in vitro was analyzed on a sucrose gradient, the amount of RNA in the 4S region increased with longer incubation times. These data and the absence of higher molecular weight RNA of specific size classes in our work (and previously published reports) suggested that nuclear fractions from plant tissue contained active nucleases. This was confirmed when wheat nuclei were mixed with [3H]yeast RNA (4, 18, 26S). All of the radioactive yeast RNA was degraded within 30 minutes to species sedimenting between 4 and 10S. The inclusion of high salt (125 millimolar (NH4)2SO4, 100 millimolar KCl), EGTA, and exogenous RNA or DNA reduced but did not eliminate endogenous RNase activity. Wheat embryo nuclei were further purified by centrifugation on a gradient of a polyvinylpyrrolidone-coated colloidal silica suspension (Percoll). These nuclei were ellipsoidal, free of cytoplasmic material, and lacked endogenous nuclease activity when assayed with [3H]yeast RNA. Sucrose gradients were not as effective as Percoll gradients in purifying nuclei free of RNase activity. The Percoll method of isolating nuclei and the RNase assay reported here will be useful in isolating plant nuclei that are capable of synthesizing distinct RNA species in vitro.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beopoulos N., Esnault R., Buri J. F. Study on plant RNAases. Isolation and properties of several activities from Vicia faba root cells. Biochim Biophys Acta. 1978 Jan 26;517(1):216–227. doi: 10.1016/0005-2787(78)90049-7. [DOI] [PubMed] [Google Scholar]
- Chen D., Schultz G., Katchalski E. Early ribosomal RNA transcription and appearance of cytoplasmic ribosomes during germination of the wheat embryo. Nat New Biol. 1971 May 19;231(20):69–72. doi: 10.1038/newbio231069a0. [DOI] [PubMed] [Google Scholar]
- Johri M. M., Varner J. E. Enhancement of RNA synthesis in isolated pea nuclei by gibberellic acid. Proc Natl Acad Sci U S A. 1968 Jan;59(1):269–276. doi: 10.1073/pnas.59.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski C., Ferro A. J., Mills D. Macromolecule synthesis in a mutant of Saccharomyces cerevisiae inhibited by S-adenosyimethionine. Mol Gen Genet. 1976 Mar 30;144(3):301–306. doi: 10.1007/BF00341728. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: II. CHARACTERIZATION OF RNA SYNTHESIZED IN VITRO. Plant Physiol. 1980 Feb;65(2):309–313. doi: 10.1104/pp.65.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertoft H., Laurent T. C., Lås T., Kågedal L. Density gradients prepared from colloidal silica particles coated by polyvinylpyrrolidone (Percoll). Anal Biochem. 1978 Jul 15;88(1):271–282. doi: 10.1016/0003-2697(78)90419-0. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Sasaki S. Low-temperature-dependent ribonuclease in chromatin of winter wheat seedlings. Biochem Biophys Res Commun. 1976 Oct 4;72(3):850–858. doi: 10.1016/s0006-291x(76)80210-0. [DOI] [PubMed] [Google Scholar]
- Wilson P. S., Bennett J. Transcription in nuclei isolated from a higher plant. Biochem Soc Trans. 1976;4(4):812–813. doi: 10.1042/bst0040812. [DOI] [PubMed] [Google Scholar]