Significance
Medium-sized peptides that bind tightly to a specific partner protein can be biomedically useful. However, conventional peptides, comprised exclusively of the 20 proteinogenic α-amino acid residues, are rapidly degraded in vivo by protease enzymes. We have developed a strategy that delivers protein-binding peptides that contain β-amino acid residues in addition to α-residues. The unnatural “α/β-peptide” backbone substantially diminishes susceptibility to proteolytic degradation relative to conventional peptides. Starting from a well-known family of conventional peptides that bind to diverse protein targets, we designed three sets of α/β-peptides that bind to three specific protein partners with high affinity and selectivity. These results suggest a general strategy for creating protease-resistant protein-targeting agents for diagnostic and therapeutic applications.
Keywords: α/β-peptides, foldamers, protein–protein interactions, inhibitors, molecular recognition
Abstract
Peptide-based agents derived from well-defined scaffolds offer an alternative to antibodies for selective and high-affinity recognition of large and topologically complex protein surfaces. Here, we describe a strategy for designing oligomers containing both α- and β-amino acid residues (“α/β-peptides”) that mimic several peptides derived from the three-helix bundle “Z-domain” scaffold. We show that α/β-peptides derived from a Z-domain peptide targeting vascular endothelial growth factor (VEGF) can structurally and functionally mimic the binding surface of the parent peptide while exhibiting significantly decreased susceptibility to proteolysis. The tightest VEGF-binding α/β-peptide inhibits the VEGF165-induced proliferation of human umbilical vein endothelial cells. We demonstrate the versatility of this strategy by showing how principles underlying VEGF signaling inhibitors can be rapidly extended to produce Z-domain–mimetic α/β-peptides that bind to two other protein partners, IgG and tumor necrosis factor-α. Because well-established selection techniques can identify high-affinity Z-domain derivatives from large DNA-encoded libraries, our findings should enable the design of biostable α/β-peptides that bind tightly and specifically to diverse targets of biomedical interest. Such reagents would be useful for diagnostic and therapeutic applications.
Designed molecules that bind selectively to specific sites on proteins may serve as inhibitors of medically important macromolecular interactions or diagnostic tools for biomarker detection. Small molecules often fail for these applications because of the relatively large and irregularly shaped target surfaces (1–3). In contrast, large polypeptides (e.g., antibodies) can frequently be developed to recognize a protein surface with high affinity and selectivity and represent the state of the art for engineering ligands for specific biomacromolecular targets. Large polypeptides, however, suffer several disadvantages for in vivo applications, including costly production, low storage stability, and/or low bioavailability because of rapid proteolytic degradation (4, 5).
Backbone-modified peptides, an underexplored class of molecules, are proving to be a fruitful source of tight-binding and specific protein ligands. Peptidic oligomers that contain β-amino acid residues interspersed among α-residues (“α/β-peptides”) can effectively mimic the recognition surface projected by an α-helix and thereby disrupt or augment protein–protein interactions in which one partner contributes a single helix to the interface (6, 7). The unnatural backbone diminishes α/β-peptide susceptibility to proteolytic degradation relative to conventional peptides (α-residues only, “α-peptides”). As a result, α/β-peptides can exhibit improved pharmacokinetic properties in vivo relative to analogous α-peptides (8, 9). To date, however, the α/β-peptide strategy has been restricted to mimicry of isolated α-helices, which is a significant limitation given that most protein–protein interactions are mediated by surfaces that are broader than can be covered by a single, regular helix (1–4, 10).
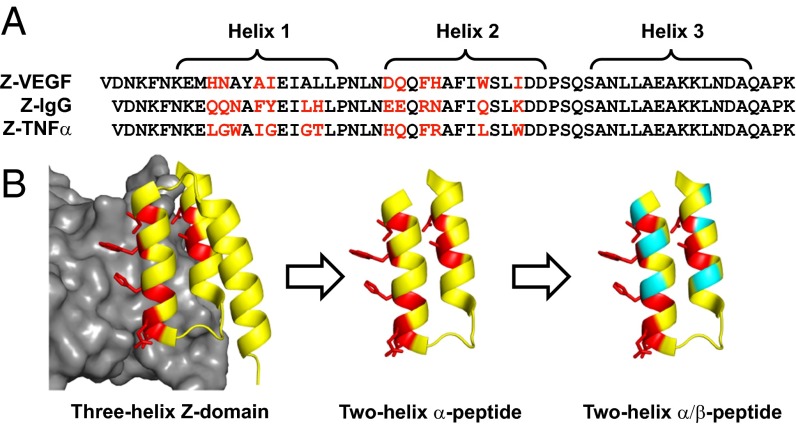
Several small proteins have been explored as scaffolds that can be adapted to interact with structurally diverse protein-binding partners (11–13). The defined tertiary structures of such scaffolds allow them to present large binding surfaces that can engage large and complementary surfaces on target proteins. The “Z-domain” or “affibody” scaffold (14) is a widely studied example that is derived from domain B of staphylococcal protein A (15). The parent Z-domain (Z-IgG) (Fig. 1A) is a 58-residue engineered analog of domain B that retains affinity for the Fc portion of IgG, the natural binding partner of protein A (16). Z-IgG adopts a three-helix bundle tertiary structure, with a large surface (>600 Å2 buried in the interface with Fc) formed by helices 1 and 2 contributing most of the Fc-contacting residues. Helix 3 stabilizes the Z-domain fold by packing against the other two helices (15, 17).
Fig. 1.
Design of α/β-peptides based on the Z-domain scaffold. (A) Sequences of peptides previously derived from the Z-domain scaffold Z-VEGF, Z-IgG, and Z-TNFα targeting VEGF (19), IgG (16), and TNFα (20), respectively. Helices 1, 2, and 3 are indicated by brackets. For Z-VEGF and Z-TNFα, residues on the protein-binding face of helices 1 and 2 that were identified via randomization and selection (including the unintentionally incorporated Ala14 in Z-VEGF) are shown in red. For Z-IgG, the parent Z-domain, red positions indicate the corresponding residues that contact IgG. Sequences are arranged based on structural alignment of helical regions. (B) Strategy for the design of α/β-peptide mimics of Z-VEGF (shown in yellow and red). Red residues indicate selected residues that contact VEGF8–109 (shown in gray) in the cocrystal structure. Sites targeted for nonnatural amino acid substitutions shown in teal. Figure is based on PDB ID code 3S1K.
The composite surface displayed by helices 1 and 2 of the Z-domain scaffold can be crafted for specific binding to diverse protein partners because the three-helix bundle tertiary structure tolerates substitutions at solvent-exposed positions (18). Combinatorial randomization of as many as 13 solvent-exposed positions on helices 1 and 2, followed by affinity-based selection, has identified Z-domain derivatives that bind to a variety of targets (12, 14), including vascular endothelial growth factor (VEGF) (peptide Z-VEGF; Fig. 1 A and B) (19), tumor necrosis factor-α (TNFα) (peptide Z-TNFα; Fig. 1A) (20), and human epidermal growth factor receptor 2 (HER2) (21). Such Z-domain analogs might represent alternatives to antibodies for selective detection of disease marker proteins or for blocking deleterious signal transduction (11–14). In many cases, selection from a phage library has identified Z-domain derivatives that exhibit dissociation constants (KD) in the nanomolar range for a chosen protein target. Affinity maturation can enhance binding to KD values in the picomolar range (21). Recent clinical evaluations of radiolabeled Z-domain derivatives targeting HER2 revealed that these peptides could be safely used to image HER2-overexpressing lesions in breast cancer patients (22), a result that highlights the medical promise of the Z-domain scaffold.
The high α-helix content of the Z-domain scaffold led us to envision that α/β-peptide analogs could be developed as binding partners for target proteins (23). We hypothesized that α→β replacements focused at sites distinct from the positions within helices 1 and 2 that mediate target recognition could reduce susceptibility to proteolytic degradation while maintaining high affinity for the partner. This design hypothesis is encouraged by two reports of Z-domain derivatives lacking helix 3 that retained affinity for their designated targets (24–26). Here, we describe the development of α/β-peptides that structurally and functionally mimic Z-VEGF. We demonstrate the versatility of this α/β-peptide strategy by showing how principles revealed in the VEGF-based effort can be extended to achieve functional mimicry of Z-domain peptides (Z-IgG and Z-TNFα) that bind to two other protein partners, IgG and TNFα.
Results and Discussion
Truncation of Z-VEGF to a Two-Helix Analog.
VEGF is a homodimeric protein that serves as a critical mediator of angiogenesis (27). Inappropriate VEGF signaling is associated with several diseases, and engineered proteins that bind to VEGF and block interactions with cell surface receptors have been approved as drugs to treat cancer or macular degeneration (28–30). Z-VEGF is a 59-mer that was developed by Fedorova et al. (19) via phage display. Nine residues on the protein-binding face of helices 1 and 2 of the Z-domain scaffold were randomized, and favorable variants were identified by selecting for binding to the VEGF8–109 homodimer (Fig. 1A) (19). A cocrystal structure shows that Z-VEGF occupies the receptor-binding region of VEGF8–109 [Protein Data Bank (PDB) ID code 3S1K; Fig. 1B] (19, 31). Z-VEGF adopts the expected three-helix bundle tertiary structure, and the surface defined by helices 1 and 2 makes contact with VEGF8–109, as predicted. During the phage display/selection process, an alanine residue (Ala14) was unexpectedly incorporated, shifting the preceding N-terminal residues by one position in helix 1 relative to the parent Z-IgG.
Because the VEGF-binding residues of Z-VEGF are restricted to helices 1 and 2, we sought to remove helix 3 while retaining high affinity for VEGF (Fig. 1B, left arrow). This truncation strategy, pioneered by Wells and coworkers (24, 25), should be beneficial because shorter oligomers are more amenable to chemical synthesis, which is required for access to α/β-peptides. In addition, the removal of helix 3 opens sites in helices 1 and 2 that are distal to the VEGF-binding face and therefore attractive for α→β replacement (Fig. 1B, right arrow). Two-helix analogs of Z-IgG and a Z-domain derivative targeting HER2 have been reported; in both cases, the intended helix–loop–helix structure is encouraged with α-amino acid substitutions on the face opposite the target-binding face and an interhelical disulfide (24–26).
In our competition fluorescence polarization (FP) assay for binding to the receptor contact region of the VEGF165 homodimer (32), Z-VEGF exhibited an apparent dissociation constant (Ki) of 0.41 μM (Table 1 and Fig. S1). This measurement serves as a benchmark for FP evaluation of new α- and α/β-peptide Z-VEGF analogs. Removal of helix 3 from Z-VEGF, to give Z-VEGF(1–38), abolished binding to VEGF165 (Fig. 2A and Table 1), which is consistent with the loss of target affinity seen for other Z-domain–derived peptides upon removal of helix 3 (24, 26). In the development of the two-helix Z-IgG analog (24), phage display identified several mutations that restored affinity for IgG after helix 3 removal, presumably by stabilizing the desired two-helix conformation. Subsequent incorporation of five of these substitutions (those thought not to be involved in the binding interface) into a two-helix HER2-binding Z-domain analog substantially improved affinity for HER2 after removal of helix 3 (26). However, we found that incorporation of these five substitutions into Z-VEGF(1–38), along with Met9→Gln to avoid adventitious oxidation, did not restore binding to VEGF165 (α-VEGF-1; Fig. 2A and Table 1). The far-UV CD spectrum of α-VEGF-1 suggests that this peptide is relatively unstructured in solution (Fig. S2). This observation and the lack of affinity of α-VEGF-1 for VEGF165 suggest that helix 3 of Z-VEGF plays a critical role in stabilizing the tertiary structure required for binding.
Table 1.
Binding of oligomers to VEGF165 and proteolysis data
| Oligomer | Ki, μM* | t1/2, min† |
| Z-VEGF | 0.41 | 1.6 |
| Z-VEGF(1–38) | >100 | — |
| α-VEGF-1 | >100 | — |
| α-VEGF-2 | 0.40 | 0.20 |
| α/β-VEGF-1 | 0.11 | 59 |
| α/β-VEGF-2 | 0.39 | 670 |
| α/β-IgG-1 | >50 | — |
| α/β-IgG-2 | >50 | — |
| α/β-TNFα-1 | >50 | — |
Ki values for binding to VEGF165 as determined by competition FP assay.
Half-life of 45 μM α- or α/β-peptide in the presence of 10 μg/mL proteinase K in TBS, pH 7.5.
Fig. 2.
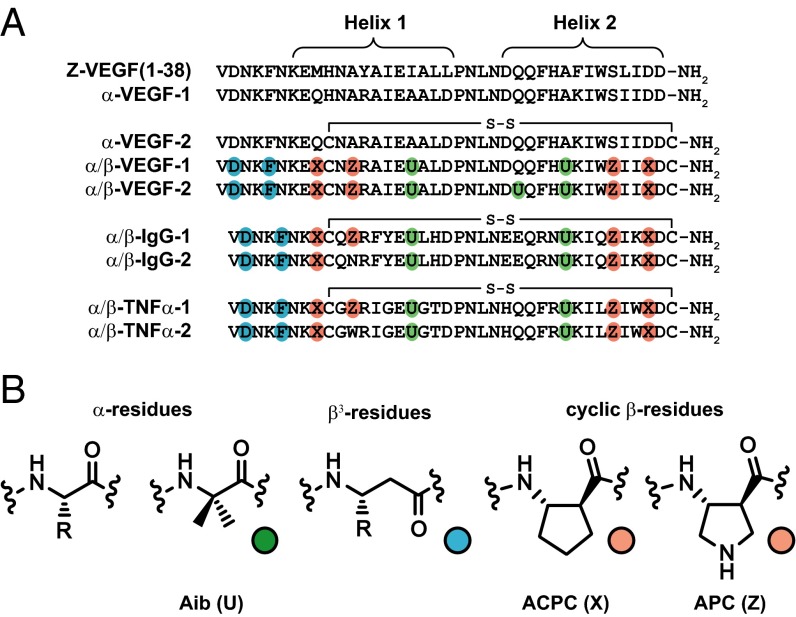
Sequences of two-helix α- and α/β-peptides used in this study, all of which were derived from three-helix Z-domain analogs shown in Fig. 1A. (A) Primary sequences of α- and α/β-peptides. Nonnatural residues are indicated by colored circles. Each cysteine is engaged in an intramolecular disulfide bond. (B) Structures of a generic α-residue, the Aib residue (green), a generic β3-residue (teal), and the cyclic β-residues ACPC and APC (orange).
We next incorporated the previously explored disulfide cross-link to stabilize the helix–loop–helix conformation (25, 26). We overlaid helices 1 and 2 of Z-VEGF in the cocrystal structure with VEGF8–109 with the NMR structure of the two-helix Z-IgG analog (PDB ID code 1ZDD) (25); this comparison suggested two sites in α-VEGF-1 for cysteine substitution (Fig. S3): His10, which occurs near the N-terminal side of helix 1, and Pro39, which lies at the C terminus of helix 2. α-VEGF-2 contains the His10→Cys and Pro39→Cys substitutions; the disulfide form bound to the VEGF165 dimer with Ki = 0.40 μM, which is indistinguishable from the Ki for 59-mer Z-VEGF binding to VEGF165.
Development of α/β-Peptide Mimics of Z-VEGF.
α-VEGF-2, which is expected to approximate the helix 1–helix 2 segment of Z-VEGF, represents an excellent starting point for implementing α→β replacements in pursuit of a ligand that binds tightly to VEGF but resists proteolysis. We have previously shown that β-amino acid residues containing a five-membered ring constraint (e.g., ACPC and APC; Fig. 2B) can enhance the affinity of helical α/β-peptides for their protein targets, presumably by increasing their folding propensity through constraint of the Cα-Cβ backbone torsion angle (7). We therefore sought to replace α-residues of α-VEGF-2 with cyclic β-residues. Using an iterative design strategy, we explored multiple sites within the helical segments and found that several tolerated cyclic β-substitutions, with the resulting oligomers retaining significant affinity for VEGF165. An initial attempt at α→β substitution near the central loop (Pro21 to Asn24) led to loss of affinity for VEGF165 (Fig. S1), and we therefore used the helix-promoting α-residue Aib (Fig. 2B) at positions 17 and 30 (33, 34). Aib residues can protect peptides from proteolysis, but we have observed that cyclic β-residues provide superior protection relative to Aib residues (9), and so we used Aib residues only in regions of the scaffold peptide that did not seem to tolerate cyclic β-residues (i.e., only near the central loop). Residues within the loop itself form intramolecular hydrophobic contacts and hydrogen bonds to residues in both helix 1 and helix 2, and these interactions may be critical to the helix–loop–helix conformation; therefore, we did not make substitutions in the loop. Flexible β3-residues (Fig. 2B) were used for replacements in the N-terminal segment (residues 1–6, which are not resolved in the Z-VEGF+VEGF8–109 cocrystal structure). These efforts led to α/β-VEGF-1 (Fig. 2A), which contains nonnatural residues throughout the N-terminal segment and both helices and exhibits Ki = 0.11 μM in the competition FP assay for binding to VEGF165 (Table 1).
The central segment of α/β-VEGF-1, from Ala18 to His29, contains only proteinogenic α-residues, and we feared that this segment would be susceptible to proteolytic degradation. We therefore introduced the Gln26→Aib substitution, to create α/β-VEGF-2. α/β-VEGF-2 exhibited Ki = 0.39 μM in the competition FP assay for binding to VEGF165, which is experimentally indistinguishable from the Ki value measured for the full-length α-peptide Z-VEGF, although slightly higher than the Ki for α/β-VEGF-1.
α/β-VEGF-1 and α/β-VEGF-2 represent the first examples in which functional mimicry of an α-peptide prototype has been achieved with α/β-peptides that are significantly shorter than the prototype (39 residues vs. 59 residues for Z-VEGF). Moreover, α/β-VEGF-1 and α/β-VEGF-2 highlight the successful extension of the principles for α/β-peptide mimicry of single helices (6, 7) to a multihelical tertiary structure. Most positions for α→β residue substitution were rationally chosen based on available structural data. In the case of α/β-VEGF-1, the affinity for VEGF165 was modestly improved relative to Z-VEGF, presumably because the nonnatural residues promote the helical secondary structure required for binding. The slightly diminished affinity of α/β-VEGF-2 relative to α/β-VEGF-1 may reflect loss of a modestly stabilizing contact resulting from Gln26→Aib substitution.
Crystal Structure of α/β-VEGF-1 in Complex with VEGF8–109.
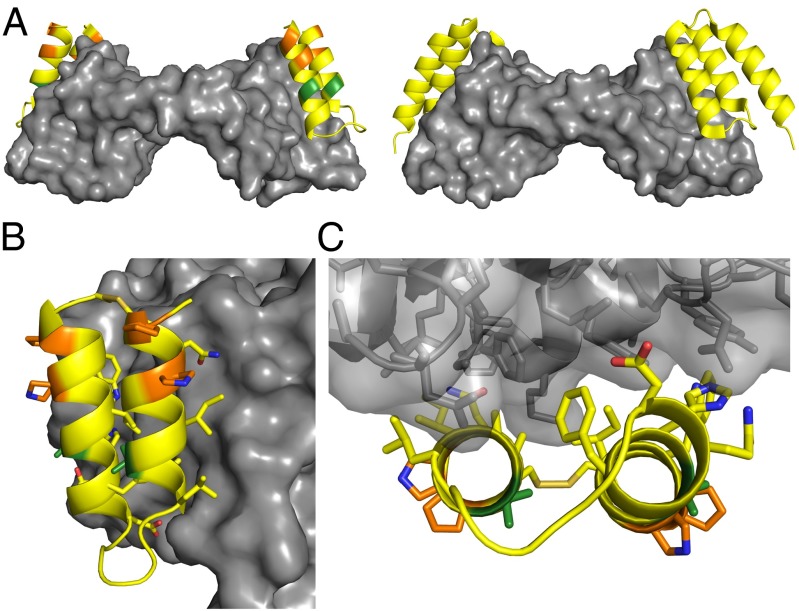
We crystallized α/β-VEGF-1 in complex with the VEGF8–109 homodimer and refined the structure to 3.1-Å resolution, R = 24.5% (Fig. 3, Fig. S4, and Table S1). The crystal structure shows two molecules of α/β-VEGF-1 bound to the equivalent receptor-binding sites of the VEGF8–109 dimer; the same sites on VEGF8–109 are occupied by Z-VEGF in its cocrystal structure (Fig. 3A) (19). Both α/β-VEGF-1 molecules adopt the anticipated helix–loop–helix conformation. As expected, the positions of the four cyclic β-amino acid residues and two Aib residues of α/β-VEGF-1 project away from VEGF8–109 and toward the medium (Fig. 3 B and C). Radiation damage is evident at the disulfide bonds, and a total of four side chains were disordered on the solvent-exposed face and the helix 2 terminus, as well as the N terminus (residues 1–7), for each of the α/β-peptides. In contrast, most side chains from the α/β-peptides that contact VEGF8–109 were well ordered (Fig. 3 B and C). Overall, our cocrystal structure indicates that α/β-VEGF-1 accurately mimics the binding mode of helices 1 and 2 of Z-VEGF with VEGF8–109, confirming that the removal of helix 3 and incorporation of multiple α- and β-amino acid residue replacements distal to the VEGF-binding face does not alter recognition geometry.
Fig. 3.
Crystal structure of α/β-VEGF-1 in complex with VEGF8–109. Residues in α/β-VEGF-1 are colored by type of residue (yellow for α, green for Aib, and orange for cyclic β). VEGF8–109 is depicted as a gray surface. (A, Left) Cartoon representation of α/β-VEGF-1 bound to VEGF8–109. (Right) Cartoon representation of α-peptide Z-VEGF bound to VEGF8–109 (PDB ID code 3S1K) (19), for comparison. The α/β-peptide binds to the same surface of VEGF8–109 as Z-VEGF. (B) Side view of α/β-VEGF-1+VEGF8–109 highlighting the interface between these molecules. The side chains are displayed as sticks for residues of α/β-VEGF-1 appearing to make contact with VEGF8–109, for Aib and cyclic β-residues, and for the disulfide bond. Carbon atoms are colored by residue type, blue atoms are nitrogen, red atoms are oxygen, and tan atoms are sulfur. (C) View of the α/β-VEGF-1+VEGF8–109 interface looking along the α/β-peptide helical axes. VEGF8–109 shown as transparent surface with cartoon backbone and side chains represented as sticks.
Proteolytic Susceptibility.
We examined the impact of nonnatural residue incorporation on susceptibility to degradation by proteinase K (7), a promiscuous and aggressive enzyme (Table 1 and Fig. S5). The 59-mer Z-VEGF was rapidly degraded, displaying a half-life of 1.6 min under the assay conditions. α-VEGF-2, the disulfide-constrained 39-mer α-peptide that matched the VEGF165 affinity of Z-VEGF, was degraded even more rapidly (half-life, 0.20 min). The susceptibility trend for this pair may reflect decreased conformational stability of the two-helix scaffold relative to the three-helix scaffold. This trend is consistent with a previous report that the two-helix Z-IgG analog manifests reduced conformational stability relative to full-length Z-IgG despite nearly identical affinities for IgG (25). In contrast, 39-mer α/β-VEGF-1, which contains six β-amino acid residues and two Aib residues, exhibited a half-life of 59 min, which represents a >290-fold increase relative to the analogous α-peptide, α-VEGF-2. Indeed, α/β-VEGF-1 showed substantially greater resistance to proteinase K than the 59-mer α-peptide Z-VEGF. These observations are consistent with previous findings, involving other α- vs. α/β-peptide comparisons, that have shown that α→β replacements at multiple sites can substantially enhance polypeptide resistance to protease action (6–9, 35). Introduction of one additional Aib residue, to convert α/β-VEGF-1 into α/β-VEGF-2, conferred additional resistance to proteinase K (half-life, 670 min; >400-fold increase relative to Z-VEGF and >3,300-fold increase relative to α-VEGF-2). Thus, despite having considerably fewer residues than Z-VEGF, the α/β-peptides developed here match or exceed the target affinity of the initial 59-mer α-peptide while far surpassing this prototype in terms of resistance to proteolytic destruction.
Antagonism of VEGF-Induced Human Umbilical Vein Endothelial Cell Proliferation.
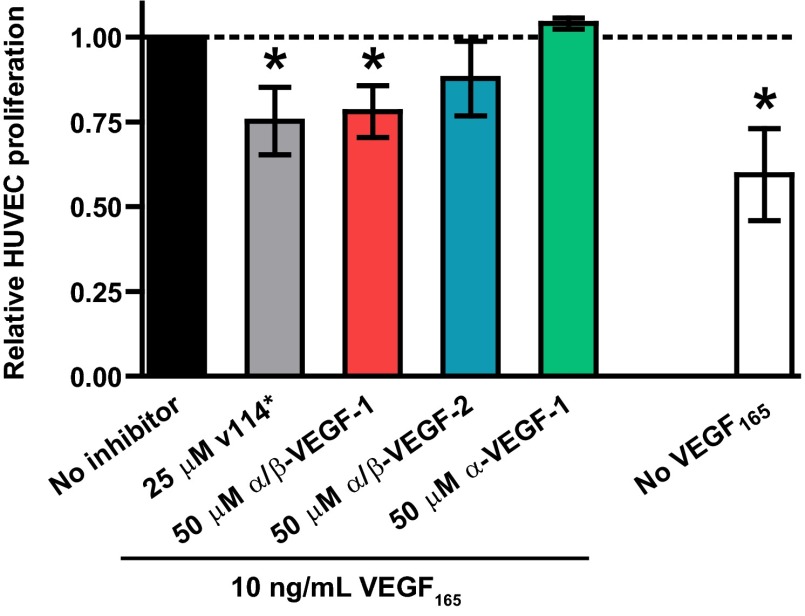
Human umbilical vein endothelial cells (HUVECs) proliferate when exposed to VEGF in cell culture (27). Because α/β-VEGF-1 and α/β-VEGF-2 bind VEGF on the receptor-recognition surface, we hypothesized that these α/β-peptides would antagonize VEGF165-mediated HUVEC proliferation (Fig. 4 and Fig. S6). α/β-VEGF-1 at 50 μM attenuated VEGF165-induced HUVEC proliferation. A positive control, α-peptide v114* at 25 μM, attenuated proliferation to about the same extent. v114* is the Met10→norleucine variant of phage-derived peptide v114 (36) and displays Ki = 0.060 μM in the competition FP assay for binding to VEGF165; α-peptide v114* has previously been shown to antagonize VEGF165-stimulated HUVEC proliferation (35), as has v114 itself (36). Although we qualitatively observed that α/β-VEGF-2 seemed to attenuate VEGF165-induced proliferation of HUVECs, the response was not statistically different from the “No-inhibitor” (VEGF165 only) control condition. The apparently reduced activity of α/β-VEGF-2 relative to α/β-VEGF-1 is consistent with the lower affinity for VEGF165 of α/β-VEGF-2 relative to α/β-VEGF-1. A negative control, α-VEGF-1, which did not bind to VEGF165 according to the FP assay, showed no antagonism of proliferation at 50 μM. Neither α/β-VEGF-1 nor α/β-VEGF-2 reduced HUVEC viability at 50 μM under the assay conditions, which suggests that the decline in VEGF165-stimulated proliferation was not due to nonspecific cytotoxicity (Fig. S6A). We probed the mechanism of action of α/β-VEGF-1 by attempting to inhibit HUVEC proliferation that was induced by 10 ng/mL FGF-2 rather than VEGF165 (36, 37); however, no inhibition was observed under these conditions (Fig. S6B). Thus, the antagonistic properties of α/β-VEGF-1 are specific for proliferation induced by VEGF165. Overall, these data strongly suggest that α/β-peptides derived from Z-VEGF can function as specific inhibitors of VEGF165-mediated signal transduction in the complex environment provided by cell culture medium.
Fig. 4.
Proliferation of HUVECs after treatment with 10 ng/mL VEGF165 and the indicated concentration of inhibitor: v114* (positive control; n = 6), α/β-VEGF-1 (n = 7), α/β-VEGF-2 (n = 6), or α-VEGF-1 (n = 3). Each bar represents the mean ± SD collected from eight independent experiments. Data from each experiment were normalized to the respective No-inhibitor control condition treated with VEGF165 only (black bar, dotted line). White bar indicates relative levels of proliferation when no exogenous VEGF165 is added (n = 8). Statistical differences are denoted for a value of P ≤ 0.01 (*) relative to No-inhibitor (VEGF165-only) control using a two-tailed ratio t test.
α/β-Peptide Mimicry of Z-IgG.
Peptides derived from the Z-domain scaffold are presumed to adopt very similar three-helix bundle tertiary structures even if multiple changes are made among residues that define the partner-binding surface (18, 19, 38); therefore, we anticipated that the strategy leading to α/β-peptides that functionally mimic Z-VEGF could be extended to Z-domain derivatives that bind to other target proteins. To test this hypothesis, we sought to design an α/β-peptide that mimics the first two helices of Z-IgG (Fig. 1A), which binds to the Fc portion of IgG (15–17). Following the path that led from Z-VEGF to α/β-VEGF-1, we removed helix 3 of Z-IgG, introduced a disulfide, and implemented α→β and Aib replacements to generate α/β-IgG-1 (Fig. 2A). α/β-IgG-1 differs from α/β-VEGF-1 at 11 residues in helices 1 and 2, positions that differ between the corresponding Z-domains, Z-VEGF and Z-IgG. These 11 differences account for the divergent protein-binding specificities of α-peptides Z-VEGF and Z-IgG. Because Z-IgG contains one fewer residue than Z-VEGF, α/β-IgG-1 correspondingly has one fewer residue than α/β-VEGF-1. In the development of Z-VEGF (19), Ala12 was not a residue randomized to optimize VEGF affinity, and Ala12 does not appear to make contact with VEGF in the Z-VEGF+VEGF8–109 cocrystal structure. Therefore, we placed the β-residue APC at position 12 in the design of α/β-VEGF-1 and α/β-VEGF-2, and α/β-IgG-1 retains this APC residue (now at position 11). However, the corresponding residue in the native domain B (Asn11) appears to make contact with the Fc portion of human IgG in the cocrystal structure (15), and so α/β-IgG-2 contains Asn at this position. Because our design strategy for the truncation of the Z-domain scaffold was inspired by efforts of Wells and coworkers (24, 25) involving truncation of Z-IgG, α/β-IgG-1 and α/β-IgG-2 contain many of the same α-residue substitutions and the same disulfide bond as these previously reported analogs. Thus, α/β-IgG-1 and α/β-IgG-2 are also α/β-peptide analogs of these previously described two-helix IgG-binding α-peptides.
We evaluated binding of α/β-IgG-1 and α/β-IgG-2 to human IgG1-κ by indirect enzyme-linked immunosorbent assay (ELISA). α/β-IgG-1 and α/β-IgG-2 were each conjugated to BSA, and each conjugate was immobilized. Both conjugates bound human IgG1-κ, as detected using goat anti-human IgG-alkaline phosphatase (Fig. S7). When BSA alone was immobilized, no increase in ELISA signal over background was observed. In addition, no increase in signal was observed in the absence of human IgG1-κ, which indicates that neither α/β-IgG-1 nor α/β-IgG-2 bound to the goat antibody used for detection (Fig. S7 A and B). These data indicate that α/β-IgG-1 and α/β-IgG-2 bind to human IgG1-κ, as designed.
A competition ELISA format was used to compare the affinities of α/β-IgG-1 and α/β-IgG-2 for IgG1-κ (Table 2 and Fig. S7 C and D). Soluble forms of both α/β-IgG-1 and α/β-IgG-2 were able to compete with immobilized α/β-IgG-2 for binding to human IgG1-κ, with IC50 values in this assay of 3.3 and 1.4 μM, respectively. The approximately twofold weaker affinity of α/β-IgG-1 compared with α/β-IgG-2 is consistent with the hypothesis that Asn11 makes favorable contacts with IgG, as observed in the domain B+IgG Fc cocrystal structure (15), although the effect is very small. α/β-VEGF-1, which has many residues in common with α/β-IgG-1 and binds to VEGF165 with high affinity, did not bind to human IgG1-κ, according to the competition ELISA. In complementary experiments, α/β-IgG-1 and α/β-IgG-2 did not bind detectably to VEGF165 in our competition FP assay (Table 1 and Fig. S1). These comparisons highlight the selectivity of these Z-domain–derived α/β-peptides for their respective protein targets.
Table 2.
Binding of oligomers to IgG1-κ and TNFα
| IgG1-κ binding ELISA | TNFα–TNFR1 inhibition ELISA | ||
| Oligomer | IC50, μM* | Oligomer | IC50, μM† |
| α/β-IgG-1 | 3.3 | Z-TNFα | 0.0048 |
| α/β-IgG-2 | 1.4 | α/β-TNFα-1 | 0.030 |
| α/β-VEGF-1 | >60 | α/β-TNFα-2 | 0.0066 |
| α/β-TNFα-1 | >60 | α/β-VEGF-1 | >10 |
| α/β-IgG-1 | >10 | ||
IC50 values are based on the ability of each oligomer to compete with immobilized α/β-IgG-2 for binding to 10 μg/mL human IgG1-κ.
IC50 values are based on the ability of each oligomer to compete with immobilized TNFR1 for binding to 0.081 μg/mL TNFα.
α/β-Peptide Mimicry of Z-TNFα.
We further tested the generality of our design strategy by endeavoring to mimic a Z-domain that binds to tumor necrosis factor-α (TNFα). TNFα, a cytokine with a variety of functions, plays a key role in multiple inflammatory disorders (39). Engineered proteins that bind to TNFα and inhibit association with its receptors (TNFRs) are currently used to treat rheumatoid arthritis, inflammatory bowel disease, and other illnesses (40). Using phage display, Jonsson et al. (20) previously developed the 58-mer Z-TNFα (Fig. 1A), based on the Z-domain scaffold. No crystallographic or other high-resolution characterization is available for this complex, in contrast to the two systems discussed above. Z-TNFα presumably adopts the characteristic three-helix bundle tertiary structure and contacts TNFα via the surface defined by helices 1 and 2. Z-TNFα has been reported to bind TNFα with an apparent dissociation constant (KD) of 0.1–0.5 nM, as measured by surface plasmon resonance, and can block binding of TNFα to the receptor TNFR2 in vitro, as indicated by experiments with etanercept (a TNFR2+IgG1 Fc fusion protein) (20). We designed α/β-TNFα-1 and α/β-TNFα-2 by removing helix 3 of Z-TNFα, adding a disulfide, and introducing α→β and Aib replacements at positions corresponding to those in the VEGF- and IgG-targeted α/β-peptides described above (Fig. 2A). As in the α/β-IgG series, α/β-TNFα-1 contains the cyclic β-residue APC at position 11, whereas α/β-TNFα-2 contains the native residue (Trp) at this position.
We used a competition ELISA experiment to determine whether α/β-TNFα-1 and α/β-TNFα-2 inhibit TNFα–TNFR1 complexation. The TNFR1 extracellular domain was immobilized onto an ELISA plate and allowed to interact with soluble TNFα in the presence of varying concentrations of a potential competitor. The relative amount of TNFα bound to the immobilized TNFR1 was then detected using a biotinylated anti-TNFα antibody and streptavidin-alkaline phosphatase. The three-helix parent α-peptide Z-TNFα had an IC50 value of 0.0048 μM in our assay (Table 2 and Fig. S8), indicating that this peptide was able to disrupt the interaction between TNFα and the TNFR1 receptor fragment. Both α/β-peptides disrupted the TNFα–TNFR1 interaction as well, with IC50 values of 0.030 μM for α/β-TNFα-1 and 0.0066 μM for α/β-TNFα-2. These results suggest that both α/β-peptides bound to TNFα with affinities comparable to that of the parent, Z-TNFα. The modestly lower IC50 value for α/β-TNFα-2 relative to α/β-TNFα-1 suggests that the residue at position 11 in Z-TNFα (Trp11) makes favorable, but not essential, contacts with TNFα. This small advantage for the native residue at position 11 parallels our previous observations with α/β-IgG-1 vs. α/β-IgG-2. Neither α/β-VEGF-1 nor α/β-IgG-1 blocks TNFα–TNFR1 complexation, and α/β-TNFα-1 does not bind to VEGF165 (Table 1) or to IgG1-κ (Table 2). These last observations underscore the selectivity of each designed α/β-peptide for its intended protein target.
Conclusions
We have described a strategy for creating peptidic oligomers with unnatural backbones that bind selectively to large surfaces on specific target proteins. This approach appears to be versatile: after optimizing the Z-domain–derived α/β-scaffold for one protein partner, we found that this unnatural backbone could be readily adapted, via modification of selected α-residue side chains, to other protein targets for which Z-domain ligands were already known. The unnatural backbone hinders proteolytic degradation of these α/β-peptides and therefore represents a substantial advantage relative to the conventional α-peptide backbone.
Our approach combines elements of two long-term polypeptide engineering initiatives: (i) efforts from multiple laboratories to optimize the Z-domain (or affibody) scaffold for selective binding to diverse partner proteins; and (ii) efforts from our group to mimic information-bearing α-helices with oligomers that contain both α- and β-amino acid residues. Prior Z-domain scaffold efforts have identified a protein-binding face formed by two adjacent α-helical segments (helices 1 and 2 of the three-helix bundle tertiary structure). The recognition properties of this surface can be adapted to many different protein targets via randomization-selection protocols focused on a specific set of residues from helices 1 and 2 (12, 14, 18). Moreover, polypeptide length can be minimized by eliminating helix 3 and incorporating compensatory features (including a disulfide) to stabilize the helix–loop–helix folding pattern (24–26). Principles that have emerged from mimicry of individual α-helices with α/β-peptides (6, 7) enable α→β replacement at Z-domain sites that do not contact the target protein or interfere with tertiary packing. This integrated design strategy allows one to combine the benefits of large biosynthetically generated libraries, which offer profound advantages in terms of surveying vast regions of sequence space but are limited to the 20 proteinogenic α-amino acids, with the benefits in physiological stability provided by an unnatural backbone.
The availability of two-helix α/β-peptide analogs of Z-domains tailored to bind to three distinct protein partners (VEGF, IgG, and TNFα) allowed us to demonstrate that high target specificity is maintained despite the unnatural backbones. The data for analogs of Z-VEGF and Z-TNFα suggest that these α/β-peptides match the affinity of the prototype Z-domains for the target proteins. Therefore, we predict that the target affinity of two-helix α/β-peptide Z-domain analogs might be improved if phage display or some comparable technique were used to select a new two- or three-helix Z-domain with higher affinity for that target. Such an effort should, in principle, identify favorable changes to the set of target-contacting α-residues, and these changes could be transferred directly to the two-helix α/β-peptide scaffold.
The three-helix Z-domain α-peptide scaffold has proven to be very versatile, with customized variants reported for over 20 protein targets of biomedical interest (12, 14, 19–21). The resulting miniproteins are attractive in terms of development of therapeutic antagonists of deleterious protein–protein associations and as starting points for highly specific imaging agents (11–14, 19–23). Selection of new protein-binding molecules from Z-domain libraries usually generates polypeptides that maintain the characteristic Z-domain three-helix bundle tertiary structure and the two-helix interaction surface. The results reported here provide a strategy that should enable rapid creation of biostable two-helix α/β-peptide analogs of many Z-domain agents.
Methods
Full details for all experiments can be found in SI Methods.
Peptide Synthesis.
All α- and α/β-peptides were synthesized by Fmoc solid-phase peptide synthesis, purified by reverse-phase HPLC, and identity was confirmed by MALDI-TOF mass spectrometry.
Crystallization.
For crystallization experiments, solutions of 110 μM VEGF8–109 and 340 μM α/β-VEGF-1 were mixed and equilibrated at room temperature. Crystals of the α/β-VEGF-1+VEGF8–109 complex were grown by hanging-drop vapor diffusion with 1 μL of the premixed protein solution and 1 μL of precipitate solution containing 0.1 M magnesium formate dihydrate, 15% (wt/vol) PEG3350, and 30% (vol/vol) glycerol. Full details on crystallization, data collection, structure solution, model building, and refinement can be found in SI Methods.
HUVEC Proliferation Assays.
HUVEC proliferation assays were performed using the Click-iT EdU cell proliferation assay (Life Technologies), as previously described (35) with minor modifications. The sequence of v114*, used as a positive control, is VEPNCDIHV(Nle)WEWECFERL-NH2, where Cys residues are engaged in an intramolecular disulfide bond and “(Nle)” is norleucine.
IgG1-κ Indirect and Competition ELISA.
α/β-Peptides were conjugated to BSA by glutaraldehyde cross-linking. For competition ELISA experiments, α/β-IgG-2 conjugated to BSA was immobilized onto 96-well Nunc Maxisorp ELISA plates overnight at 4 °C. Human IgG1-κ at 10 μg/mL in PBS plus 0.05% Tween 20 was mixed with varying concentrations of α/β-peptides, and then added to the ELISA plate. The presence of IgG1-κ was then detected by goat anti-human IgG (Fab specific)-alkaline phosphatase antibody and p-nitrophenyl phosphate (pNPP) as the enzyme substrate, monitoring absorbance at 405 nm. Full details on the conjugation reaction, indirect ELISA, and competition ELISA can be found in SI Methods.
TNFα–TNFR1 Competition ELISA.
Soluble recombinant human TNFR1 was immobilized onto ELISA plates by incubating 50 μL of a 1 μg/mL TNFR1 solution in PBS at 4 °C overnight. Serial dilutions of α- or α/β-peptides were incubated with 0.081 μg/mL of recombinant human TNFα in PBS plus 0.05% Tween 20 and then added to the ELISA plate. Bound TNFα was detected by sequential addition of biotinylated anti-TNFα antibody, streptavidin-alkaline phosphatase, and pNPP, monitoring absorbance at 405 nm.
Supplementary Material
Acknowledgments
This research was supported by NIH Grant GM056414 (to S.H.G.) and Grant HL093282 (to W.L.M.). In addition, W.L.M. acknowledges support from the University of Wisconsin–Madison Nanoscale Science and Engineering Center (DMR-0832760). J.W.C. and D.F.K. were supported in part by a Biotechnology Training Grant (NIH Grant T32 GM008349). N.J.R. was supported in part by a Hilldale Undergraduate Research Fellowship from University of Wisconsin–Madison. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract DE-AC02-06CH11357. Use of the Life Sciences Collaborative Access Team Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
Footnotes
Conflict of interest statement: J.W.C. and S.H.G. are coinventors on a patent application covering the α/β-peptides described in this paper. S.H.G. is a cofounder of Longevity Biotech, Inc., which is pursuing biomedical applications of α/β-peptides.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4WPB).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420380112/-/DCSupplemental.
References
- 1.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450(7172):1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 2.Arkin MR, Tang Y, Wells JA. Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem Biol. 2014;21(9):1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson AJ. Inhibition of protein-protein interactions using designed molecules. Chem Soc Rev. 2009;38(12):3289–3300. doi: 10.1039/b807197g. [DOI] [PubMed] [Google Scholar]
- 4.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: Science and market. Drug Discov Today. 2010;15(1-2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Kariolis MS, Kapur S, Cochran JR. Beyond antibodies: Using biological principles to guide the development of next-generation protein therapeutics. Curr Opin Biotechnol. 2013;24(6):1072–1077. doi: 10.1016/j.copbio.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Johnson LM, Gellman SH. α-Helix mimicry with α/β-peptides. Methods Enzymol. 2013;523:407–429. doi: 10.1016/B978-0-12-394292-0.00019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horne WS, et al. Structural and biological mimicry of protein surface recognition by α/β-peptide foldamers. Proc Natl Acad Sci USA. 2009;106(35):14751–14756. doi: 10.1073/pnas.0902663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheloha RW, Maeda A, Dean T, Gardella TJ, Gellman SH. Backbone modification of a polypeptide drug alters duration of action in vivo. Nat Biotechnol. 2014;32(7):653–655. doi: 10.1038/nbt.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson LM, et al. A potent α/β-peptide analogue of GLP-1 with prolonged action in vivo. J Am Chem Soc. 2014;136(37):12848–12851. doi: 10.1021/ja507168t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo Conte L, Chothia C, Janin J. The atomic structure of protein-protein recognition sites. J Mol Biol. 1999;285(5):2177–2198. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- 11.Binz HK, Amstutz P, Plückthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol. 2005;23(10):1257–1268. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- 12.Grönwall C, Ståhl S. Engineered affinity proteins—generation and applications. J Biotechnol. 2009;140(3-4):254–269. doi: 10.1016/j.jbiotec.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Moore SJ, et al. Engineered knottin peptide enables noninvasive optical imaging of intracranial medulloblastoma. Proc Natl Acad Sci USA. 2013;110(36):14598–14603. doi: 10.1073/pnas.1311333110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Löfblom J, et al. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010;584(12):2670–2680. doi: 10.1016/j.febslet.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-Å resolution. Biochemistry. 1981;20(9):2361–2370. [PubMed] [Google Scholar]
- 16.Nilsson B, et al. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;1(2):107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- 17.Tashiro M, et al. High-resolution solution NMR structure of the Z domain of staphylococcal protein A. J Mol Biol. 1997;272(4):573–590. doi: 10.1006/jmbi.1997.1265. [DOI] [PubMed] [Google Scholar]
- 18.Nord K, et al. Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nat Biotechnol. 1997;15(8):772–777. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- 19.Fedorova A, et al. The development of peptide-based tools for the analysis of angiogenesis. Chem Biol. 2011;18(7):839–845. doi: 10.1016/j.chembiol.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson A, Wållberg H, Herne N, Ståhl S, Frejd FY. Generation of tumour-necrosis-factor-α-specific affibody molecules capable of blocking receptor binding in vitro. Biotechnol Appl Biochem. 2009;54(2):93–103. doi: 10.1042/BA20090085. [DOI] [PubMed] [Google Scholar]
- 21.Orlova A, et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006;66(8):4339–4348. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 22.Baum RP, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med. 2010;51(6):892–897. doi: 10.2967/jnumed.109.073239. [DOI] [PubMed] [Google Scholar]
- 23.Henchey LK, Jochim AL, Arora PS. Contemporary strategies for the stabilization of peptides in the α-helical conformation. Curr Opin Chem Biol. 2008;12(6):692–697. doi: 10.1016/j.cbpa.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braisted AC, Wells JA. Minimizing a binding domain from protein A. Proc Natl Acad Sci USA. 1996;93(12):5688–5692. doi: 10.1073/pnas.93.12.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starovasnik MA, Braisted AC, Wells JA. Structural mimicry of a native protein by a minimized binding domain. Proc Natl Acad Sci USA. 1997;94(19):10080–10085. doi: 10.1073/pnas.94.19.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webster JM, Zhang R, Gambhir SS, Cheng Z, Syud FA. Engineered two-helix small proteins for molecular recognition. ChemBioChem. 2009;10(8):1293–1296. doi: 10.1002/cbic.200900062. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 29.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grothey A, Galanis E. Targeting angiogenesis: Progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6(9):507–518. doi: 10.1038/nrclinonc.2009.110. [DOI] [PubMed] [Google Scholar]
- 31.Wiesmann C, et al. Crystal structure at 1.7 Å resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell. 1997;91(5):695–704. doi: 10.1016/s0092-8674(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 32.Peterson KJ, et al. A fluorescence polarization assay for identifying ligands that bind to vascular endothelial growth factor. Anal Biochem. 2008;378(1):8–14. doi: 10.1016/j.ab.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karle IL, Balaram P. Structural characteristics of α-helical peptide molecules containing Aib residues. Biochemistry. 1990;29(29):6747–6756. doi: 10.1021/bi00481a001. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi H, et al. Effect of α,α-dialkyl amino acids on the protease resistance of peptides. Biosci Biotechnol Biochem. 2003;67(10):2269–2272. doi: 10.1271/bbb.67.2269. [DOI] [PubMed] [Google Scholar]
- 35.Haase HS, et al. Extending foldamer design beyond α-helix mimicry: α/β-Peptide inhibitors of vascular endothelial growth factor signaling. J Am Chem Soc. 2012;134(18):7652–7655. doi: 10.1021/ja302469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairbrother WJ, et al. Novel peptides selected to bind vascular endothelial growth factor target the receptor-binding site. Biochemistry. 1998;37(51):17754–17764. doi: 10.1021/bi981931e. [DOI] [PubMed] [Google Scholar]
- 37.Beenken A, Mohammadi M. The FGF family: Biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eigenbrot C, Ultsch M, Dubnovitsky A, Abrahmsén L, Härd T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc Natl Acad Sci USA. 2010;107(34):15039–15044. doi: 10.1073/pnas.1005025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 40.Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-α therapies: The next generation. Nat Rev Drug Discov. 2003;2(9):736–746. doi: 10.1038/nrd1175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.