Significance
In this study, we demonstrate that guanine nucleotide-binding protein G(i) subunit alpha-1 and alpha-3 (Gαi1/3) regulate the downstream signaling pathways of Toll-like receptor 4 (TLR4). We show that Gαi1/3 form complexes containing the pattern recognition receptor (PRR) CD14 and growth factor receptor binding 2 (Grb2)-associated binding protein (Gab1), which are required for activation of PI3K-Akt signaling and NF-κB activation. Besides, Gαi1/3 act at both the plasma membrane and the endosome levels and are involved in TLR4 endocytosis. Furthermore, Gαi1/3 participated in the induction of M1 polarization of macrophages, and their decreased expression contributed to LPS tolerance. Thus, Gαi1/3 are important in controlling inflammation.
Keywords: Gαi1, Gαi3, macrophage polarization, Toll-like receptor 4, endosome
Abstract
Heterotrimeric G proteins have been implicated in Toll-like receptor 4 (TLR4) signaling in macrophages and endothelial cells. However, whether guanine nucleotide-binding protein G(i) subunit alpha-1 and alpha-3 (Gαi1/3) are required for LPS responses remains unclear, and if so, the underlying mechanisms need to be studied. In this study, we demonstrated that, in response to LPS, Gαi1/3 form complexes containing the pattern recognition receptor (PRR) CD14 and growth factor receptor binding 2 (Grb2)-associated binding protein (Gab1), which are required for activation of PI3K-Akt signaling. Gαi1/3 deficiency decreased LPS-induced TLR4 endocytosis, which was associated with decreased phosphorylation of IFN regulatory factor 3 (IRF3). Gαi1/3 knockdown in bone marrow-derived macrophage cells (Gαi1/3 KD BMDMs) exhibited an M2-like phenotype with significantly suppressed production of TNF-α, IL-6, IL-12, and NO in response to LPS. The altered polarization coincided with decreased Akt activation. Further, Gαi1/3 deficiency caused LPS tolerance in mice. In vitro studies revealed that, in LPS-tolerant macrophages, Gαi1/3 were down-regulated partially by the proteasome pathway. Collectively, the present findings demonstrated that Gαi1/3 can interact with CD14/Gab1, which modulates macrophage polarization in vitro and in vivo.
The innate immune recognition of bacterial lipopolysaccharide (LPS) is mediated by Toll-like receptor 4 (TLR4) with activation of proinflammatory signaling pathways including activation of nuclear factor (NF)-κB, mitogen-activated protein kinases (MAPKs) and transcription factor IFN regulatory factor 3 (IRF3) (1). Activation of these pathways is essential to protect the host from infection, but this activation must be tightly regulated, because uncontrolled inflammation may have detrimental effects on hosts, resulting in inflammatory diseases including diabetes, hypertension, cardiovascular disorders, and septic shock (2). Macrophages are an essential component of innate immunity and play a central role in inflammation and host defense. Depending on the environmental cues, macrophages can assume a spectrum of activation states ranging from classically activated M1 inflammatory macrophages to various alternatively activated M2 macrophages, the latter being involved in immune regulation and tissue repair (3). The M1 phenotype is characterized by the expression of high levels of proinflammatory cytokines [i.e., tumor necrosis factor α (TNF-α), interleukin (IL)-6, and IL-1], high production of reactive nitrogen and oxygen intermediates, promotion of T helper 1 response, and strong microbicidal and tumoricidal activity. M2 macrophages, however, mainly exert immunoregulatory functions, and are involved in parasite containment, tissue remodeling, and tumor development (4). They are characterized by efficient phagocytic activity, high expression of scavenging molecules, and an F4/80hiCD11bhi phenotype (5). Endotoxin tolerance is a transient state of LPS refractoriness after an initial and nonlethal exposure to LPS. Endotoxin-tolerant macrophages have been found to express a set of molecules that are similar to those expressed by M2-polarized macrophages.
G proteins are heterotrimers composed of α, β, and γ subunits. The α subunit is a GTPase. When a receptor activates a G protein, the α subunit releases GDP and binds GTP and changes conformation, acquiring its activated state. In the case of Gαi proteins, the activated Gαi subunits, which inhibit adenylyl cyclases, are likely candidates to regulate leukocyte differentiation, thus shaping inflammatory reactions. The Gαi proteins, including Gαi1, Gαi2, and Gαi3, are highly similar, sharing 87–93% of amino acid sequence identity and showing overlapping expression patterns (6). Although Gαi1 is primarily found in the brain, both Gαi1 and Gαi3, here collectively referred to as Gαi1/3, are abundantly expressed in the immune system, being involved in many receptors signaling processes, including responses to both GPCRs and non-GPCRs [i.e.FcγRs (refs. 7 and 8) and EGFR (ref. 9)]. Recent studies also have implicated a role of heterotrimeric Gαi proteins in lipopolysaccharide (LPS)-induced inflammatory responses (10–12). However, the molecular mechanisms how Gαi1/3 regulate septic shock are poorly understood. In addition, the role of Gαi proteins in macrophage polarization and LPS tolerance remains unknown.
Here, we demonstrated that Gαi1/3 regulate the downstream signaling pathways of TLR4, namely those leading to activation of NF-κB, MAPKs, and IRF3, and participate in the induction of M1 polarization of macrophages.
Results
Gαi1/3 Promote LPS-Induced Inflammatory Response.
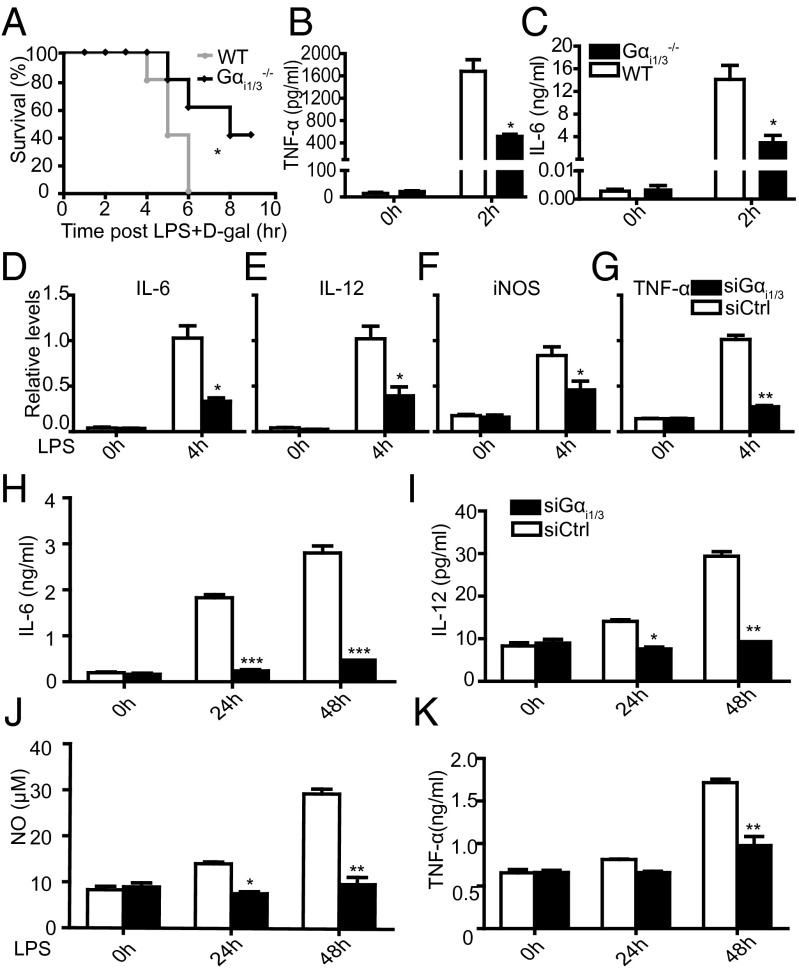
To explore the influence of Gαi1/3 in endotoxic shock, we injected a lethal dose of LPS plus d-galactosamine i.p. into WT (129SvEv) and Gαi1/3 double knockout (DKO) mice. All WT mice died within 8 h after administration while 40% of Gαi1/3 DKO mice survived (Fig. 1A). WT mice showed significantly higher serum levels of TNF-α and IL-6 2 h after LPS administration, compared with Gαi1/3 DKO mice (Fig. 1 B and C). These results indicate that Gαi1/3 may play an important role in regulation of LPS-induced TLR4 activation and the inflammatory response. Because macrophages play a major role in inflammation, we used siRNA strategies in bone marrow-derived macrophage cells (BMDMs) as the cell model to study the participation of Gαi1/3 in LPS-initiated proinflammatory cytokine production. The results showed that Gαi1/3 deficiency significantly impaired LPS-induced IL-6, TNF-α, iNOs, and IL-12 mRNA expression (Fig. 1 D–G). Accordingly, the secretion of IL-6, TNF-α, NO, and IL-12 was also significantly reduced in Gαi1/3 DKO BMDMs after LPS stimulation (Fig. 1 H–K). Collectively, these data indicate that Gαi1/3 may play important role in regulating LPS responses.
Fig. 1.
Survival of WT and Gαi1/3−/− mice after LPS and d-galactosamine challenge. (A) WT and Gαi1/3 DKO mice were injected i.p. with 4 mg/kg LPS plus 500 mg/kg d-galactosamine. Survival was monitored over 10 h. Keplan–Meler plots were shown after LPS challenge. Statistical significance was determined by log rank test. Data were representative of at least three independent experiments (five mice per group). *P < 0.05. (B and C) Serum assayed for TNF-α (B) and IL-6 (C) by ELISA 2 h after 4 mg/kg LPS plus 500 mg/kg d-galactosamine treatment. *P < 0.05, **P < 0.01. (D–G) BMDMs from WT mice were transfected with control small interference RNA (siCtrl) or Gαi1 and Gαi3-specific siRNA. After 48 h, cells were stimulated with LPS (100 ng/mL) for 4 h. IL-6 (D), IL-12 (E), iNOs (F), and TNF-α (G) mRNA expressions were measured by quantitative real-time RT-PCR (QRT-PCR). *P < 0.05, **P < 0.01. (H–K) BMDMs from WT mice were transfected with control small interfering RNA (siCtrl) or Gαi1 and Gαi3-specific siRNA. After 48 h, cells were stimulated with LPS (100 ng/mL) for 24 and 48 h, and IL-6 (H), IL-12 (I), NO (J), and TNF-α (K) were then measured by ELISA in the supernatants. The results shown are representative of three independent experiments. Data are shown as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Gαi1/3 Deficiency Impairs the TLR4 Triggered NF-κB, MAPKs, and TIR-Domain-Containing Adapter-Inducing Interferon-β Signaling Pathways.
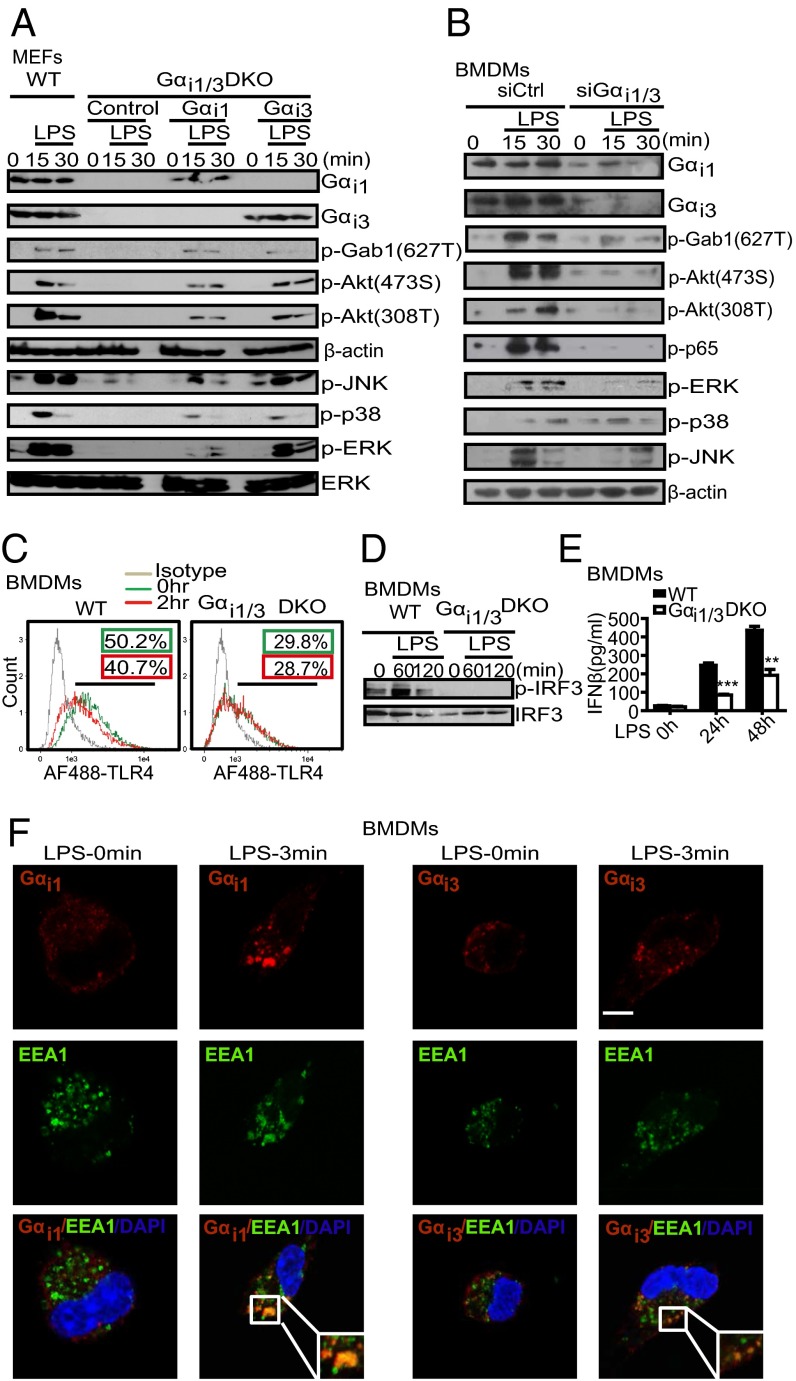
To examine roles for Gαi proteins in LPS-mediated signaling, we initially used mouse embryo fibroblasts (MEFs) derived from wild-type (WT) mice or Gαi1/3 DKO mice. Western blot assay results showed that MEFs lacking Gαi1 and Gαi3 (termed DKO-MEF cells) were severely impaired in LPS-induced phosphorylation of Gab1-627T, Akt-308T, Akt-473S, p38, JNK, and ERK. Moreover, expression of either Gαi1 or Gαi3 in DKO MEFs restored phosphorylation of p-Gab1, Akt-308T, Akt-473S, p38, JNK, and ERK in response to LPS (Fig. 2A).
Fig. 2.
Gαi1/3 deficiency impairs the TLR4 triggered NF-κB, MAPKs, and TRIF signaling pathways. (A) Exogenous Gαi1 or Gαi3 rescue the activation of Akt and MAPKs signaling in DKO MEFs in response to LPS. DKO MEFs were transfected with vectors encoding WT Gαi1 and Gαi3 (4 μg per well). After 48 h, cells were treated with LPS (1 μg/mL) for the indicated times. Gαi1, Gαi3, p-Gab1 (T627), p-Akt (473S), p-Akt (308T), β-actin, p-JNK, p-p38, p-ERK, and total ERK were detected by Western blot. (B) Knockdown of the expression of Gαi1/3 decreases the activation of Akt and MAPKs by LPS in BMDMs. BMDMs were transfected with control small interfering RNA (siCtrl) or Gαi1 and Gαi3-specific siRNA. After 48 h, cells were stimulated with LPS (100 ng/mL) for the indicated times. Gαi1, Gαi3, p-Gab1 (627T), p-Akt (473S), p-Akt (308T), p-ERK, p-p38, p-JNK, and β-actin were detected by Western blot. (C) BMDMs from WT and Gαi1/3 DKO mice were treated or not with LPS (100 ng/mL) for 2 h. Flow cytometry was then used to examine TLR4 endocytosis by determining the surface levels of TLR4. (D) BMDMs from WT and Gαi1/3 DKO mice were treated or not with LPS (100 ng/mL) for the times indicated. The presence of phosphorylated IRF3 in cell extracts was determined. (E) BMDMs were treated with LPS (100 ng/mL) for the indicated times and the amounts of secreted IFN-β were determined by ELISA. (F) BMDMs were stimulated with LPS (100 ng/mL) for the times indicated and processed for confocal microscopy to detect the presence of Gαi1, Gαi3, or EEA1. (Scale bar: 5 μm.) All images are representative of at least three independent experiments where over 500 cells were examined per condition and >95% of the cells displayed similar staining.
We verified these observations with siRNA strategies in BMDMs. Combined knockdown of Gαi1 and Gαi3 inhibited phosphorylation of Akt-308T and Akt-473S in response to LPS (Fig. 2B). These data suggested that Gαi1/3 were required for TLR4-initiated signaling, including activation of NF-κB and MAPKs pathways. Because G proteins partition into lipid rafts which are involved in receptor endocytosis, we wondered whether Gαi1 and Gαi3 had a role in TLR4 endocytosis, a key step for the activation of the transcription factor IFN regulatory factor-3 (IRF3) through the adaptors TIR-domain-containing adapter-inducing interferon-β (TRIF)-related adaptor molecule (TRAM) and TRIF (13). Thus, we treated WT and Gαi1/3 DKO BMDMs with LPS for 0–120 min. As shown in Fig. 2C, LPS induced the rapid endocytosis of TLR4 in WT BMDMs, but not in Gαi1/3 DKO cells as analyzed by flow cytometry. Additionally, LPS-induced phosphorylation of IRF3, which occurs only when endocytosis is intact, was abolished in Gαi1/3 DKO BMDMs (Fig. 2D). We also found Gαi1/3 DKO BMDMs were defective for TRIF-mediated IFNβ production (Fig. 2E). Using confocal microscopy, we observed colocalization between the early endosomal marker EEA1 and Gαi1/Gαi3 within 3 min of LPS treatment (Fig. 2F).
Interaction Between Gαi1/3 Proteins and CD14/Gab1 Is Required for LPS-Dependent Phosphorylation of Gab1 and Its Interaction with p85.
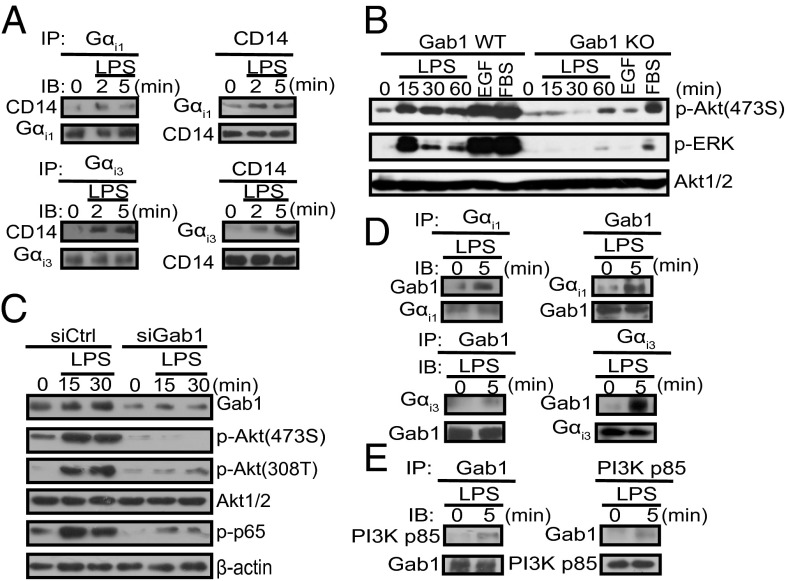
We further investigated how Gαi1/3 mediate TLR4-initiated signal transduction. In agreement with the report of Solomon et al. (14), we also found that Gαi1/3 coimmunoprecipitate with the LPS coreceptor CD14 (Fig. 3A). Grb2-associated binder 1 (Gab1), the scaffolding/adaptor protein, mediates signal transduction of many receptors. Phosphorylated Gab1 recruits downstream effectors such as p85 to activate downstream signaling effectors. In concert with the previous study (15), loss of Gab1 severely impaired Akt-308T and Akt-473S phosphorylation in response to LPS in both MEFs and BMDMs (Fig. 3 B and C), indicating that Gab1 lies downstream of Gαi proteins in mediating LPS signaling. As a matter of fact, we found LPS induced the association between Gab1 and Gαi proteins in BMDMs (Fig. 3D). In addition, LPS induced the association of Gab1 with p85 (Fig. 3E), indicating that Gαi proteins are required for the interaction between Gab1 and p85, and the subsequent activation of PI3K-Akt signaling. Overall, our findings suggest that in response to LPS, a Gαi-Gab1 association is required for subsequent PI3K-Akt activation.
Fig. 3.
Binding of Gαi1/3 proteins to CD14/Gab1 is required for LPS-dependent phosphorylation of Gab1 and its interaction with p85. (A) LPS induces the association of CD14 with Gαi1/3. BMDMs were treated with LPS (100 ng/mL) for the indicated times and were then lysed. CD14, Gαi1, or Gαi3 was immunoprecipitated as described in SI Materials and Methods. (B) Gab1 WT and Gab1 KO MEFs were treated with LPS (1 μg/mL). p-Akt (473S), p-ERK1/2, and Akt1/2 were detected by Western blot. (C) BMDMs from WT mice were transfected with control small interfereing RNA (siCtrl) or Gab1-specific siRNA. After 48 h, cells were stimulated with LPS (100 ng/mL) for the indicated times. Gab1, p-Akt (473S), p-Akt (308T), total Akt, p-p65, and β-actin were detected by Western blot. (D and E) LPS induces the association of Gαi1/3 with Gab1, followed by the association of Gab1 with PI3K p85. Gab1, Gαi1, Gαi3, or Gab1 was immunoprecipitated as described in SI Materials and Methods.
Gαi1/3 Regulate Macrophage Polarization.
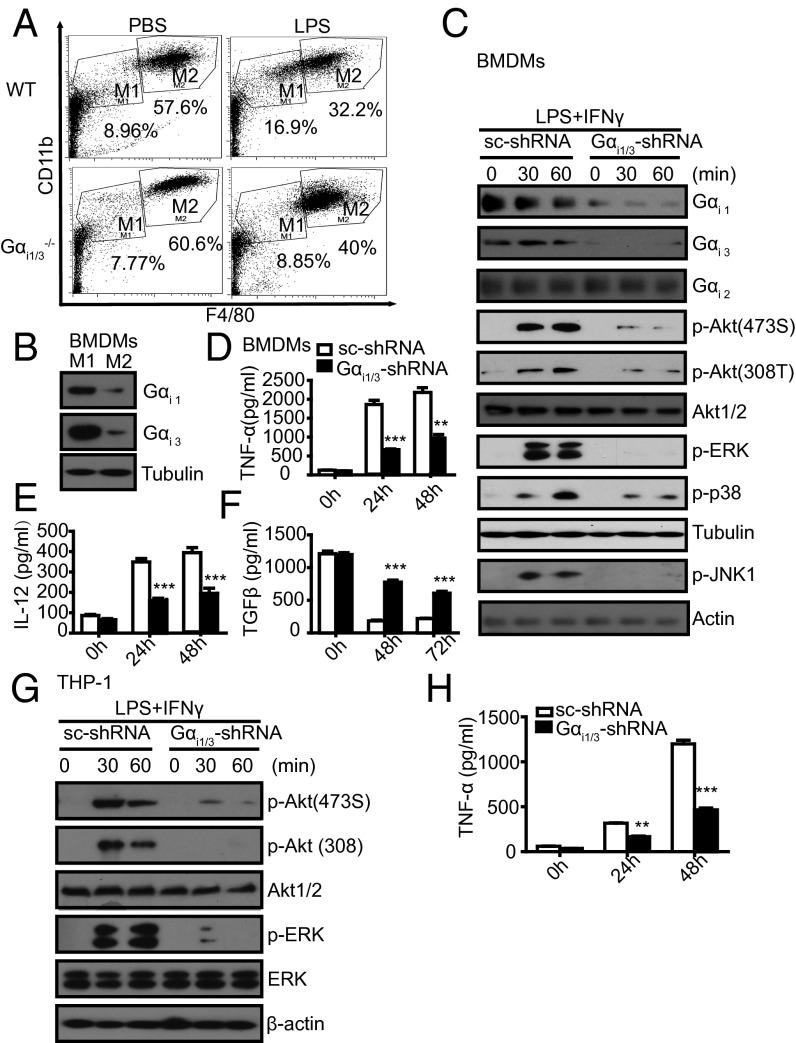
In light of our results that Gαi1/3 are important for LPS-induced signaling and cytokine release, we speculated that Gαi1/3 DKO macrophages might have an M2-like bias or antiinflammatory traits. To test this hypothesis, WT peritoneal macrophages were profiled by flow cytometry to assess macrophage subtypes at steady state (16), designating F4/80intCD11bhi as M1-like and F4/80hiCD11bhi as M2-like. WT mice exhibited a clear population shift away from the M2-like toward the M1-like phenotype in response to LPS. However, Gαi1/3−/− peritoneal macrophages presented reduced M1-like cells compared with WT macrophages (8.85% versus 16.9%) (Fig. 4A), suggesting that Gαi1/3 may participate in these shifting endotoxin responses.
Fig. 4.
Gαi1/3 regulate macrophage polarization. (A) Peritoneal cells extracted from WT and Gαi1/3 DKO mice were analyzed by flow cytometry for CD11b and F4/80 to discriminate macrophage subtypes. WT or Gαi1/3 DKO mice were treated i.p. for 6 h with 20 mg/kg LPS or PBS. Peritoneal macrophages were extracted and subpopulations of macrophages were analyzed by flow cytometry with F4/80 and CD11b. Population M1 (F4/80intCD11bint) and population M2 (F4/80hiCD11bhi) were gated. (B) Gαi1/3 protein expression in M1 and M2 macrophages. BMDMs were treated with LPS (5 ng/mL) plus IFN-γ (10 U/mL) or IL-4 (10 U/mL), respectively, and tested for the M1 and M2 macrophage formation. After 24 h, cells were lysed. Gαi1 and Gαi3 were detected by Western blot. (C) Knockdown of Gαi1/3 decreased the activation of Akt and MAPKs in M1 macrophages. BMDMs were transfected with control small RNA (Ctrl) or Gαi1 and Gαi3-specific siRNA. After 48 h, BMDMs were treated with LPS (5 ng/mL) plus IFN-γ (10 U/mL) for the indicated times and were then lysed. Gαi1, Gαi2, Gαi3, p-Akt (473S), p-Akt (308T), total Akt, p-ERK1/2, p-p38, Tubulin, p-JNK, and β-actin were analyzed by Western blot. (D–F) Knockdown of Gαi1/3 decreases the expression of M1 cytokines: TNF-α (D), IL-12 (E), but increase the expression of the M2 cytokine TGF-β (F). BMDMs were transfected with control small RNA (Ctrl) or Gαi1 and Gαi3-specific siRNA. After 48 h, BMDMs were treated with LPS (5 ng/mL) plus IFN-γ (10 U/mL) for the indicated times, and TNF-α, IL-12, and TGF-β in the supernatants were measured by ELISA. **P < 0.01, ***P < 0.001. (G) Knockdown of Gαi1/3 decreases the activation of Akt and ERK in the THP-1 cell line. THP-1 cells were treated with LPS (5 ng/mL) plus IFN-γ (10 U/mL) for the indicated times and were then lysed. P-Akt (473S), p-Akt (308T), total Akt, p-ERK, total ERK, and β-actin were analyzed by Western blot. (H) Knockdown of Gαi1/3 decreases the expression of the M1 cytokine TNF-α in THP-1 cells. THP-1 cells were transfected with control small interfering RNA (Ctrl) or Gαi1 and Gαi3-specific siRNA. The level of TNF-α in the supernatants was measured by ELISA. **P < 0.01, ***P < 0.001.
To understand the cellular mechanisms by which Gαi1/3 regulate macrophage polarization, we measured Gαi1 and Gαi3 expression in M1 and M2 macrophages induced by LPS/IFN-γ or IL-4, respectively. The results showed a significantly different expression of Gαi1 and Gαi3 in M1 and M2 macrophages (Fig. 4B). We next transfected BMDMs with siRNA specific for Gαi1/3 and stimulated with 5 ng/mL LPS plus 10 U/mL IFN-γ to induce the M1-like phenotype. The results indicated that the LPS-induced activation of the MAPKs and Akt signaling pathways was less in intensity and signaling in Gαi1/3-shRNA–transfected macrophage than in sc-shRNA transfected macrophages (Fig. 4C). Accordingly, LPS/IFN-γ–stimulated secretion of TNF-α and IL-12 was significantly reduced in Gαi1/3-shRNA expressing BMDMs (Fig. 4 D and E). Whereas TGF-β secretion was increased in Gαi1/3-shRNA–expressing BMDMs compared with sc-shRNA–expressing BMDMs (Fig. 4F). Similar results were also seen in human monocytic THP-1 cells (Fig. 4 G and H). These findings suggested that Gαi1/3-shRNA could suppress LPS/IFN-γ–induced M1-like macrophages, which presented a plausible mechanism for the resistance of Gαi1/3DKO mice to LPS-induced endotoxin shock.
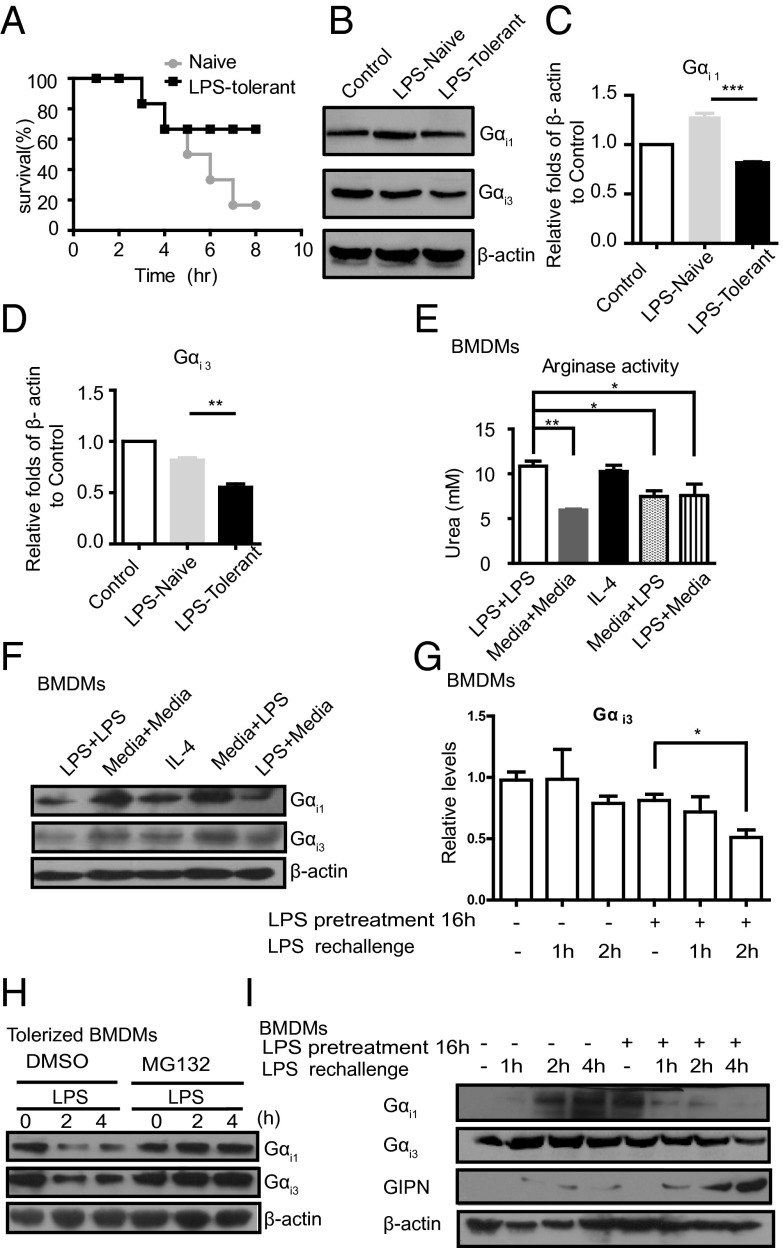
Gαi1/3 Degradation Is Involved in LPS Tolerance.
To determine whether Gαi1/3 expression is changed in endotoxin tolerance, we preinjected mice with saline or a low dose of LPS, then challenged them with a lethal dose of LPS plus d-galactosamine 16 h later. As expected, preexposure to a low dose of LPS resulted in much better survival with lethal dose of LPS (Fig. 5A). We also found a decreased Gαi1/3 expression in peritoneal macrophages of LPS-tolerant mice compared with that in saline-treated mice (Fig. 5 B–D). We suggest that the decreased expression of Gαi1/3 inhibits the production of proinflammatory cytokines and dampens inflammatory responses to improve survival during acute sepsis. Further, we examined the arginase activity in macrophages that were first incubated for 16 h with medium alone, or with 100 ng/mL LPS, and then stimulated for 8 h with the same dose of LPS (100 ng/mL). We found increased arginase activity in BMDMs rechallenged with LPS, which resembles IL-4–stimulated M2 polarization (Fig. 5E). We also examined the expression of Gαi1/3 in BMDMs that were first incubated for 16 h with medium alone, or with LPS (100 ng/mL), and then stimulated for an 8-h time course with the same dose of LPS. We found that the expression of Gαi1/3 was increased in naive or medium-pretreated macrophages, whereas it was decreased in LPS-pretreated or IL-4 treated macrophages (M2-like) (Fig. 5F). Next, we examined whether there was a change in Gαi3 mRNA level. In concert with Gαi3 protein, there was a decrease of Gαi3 mRNA in LPS rechallenged BMDMs (Fig. 5G). Because elimination of G proteins by ubiquitination has been found in model organisms (17), we explored a role for the proteasome in degradation of Gαi1/3 in tolerance. Significantly, proteasome inhibition by MG-132 prevented Gαi1/3 loss in endotoxin-tolerized macrophages (Fig. 5H). A previous study reported that Gα-interacting protein (GAIP) N terminus interacting protein (GIPN) was a putative E3 ubiquitin ligase, which could promote Gαi3 down-regulation (18). We further investigated the expression of GIPN in vehicle-pretreated (naive) or LPS-pretreated (tolerized) BMDMs. The results revealed that GIPN increased significantly in LPS-pretreated BMDMs (Fig. 5I). Therefore, GIPN up-regulation might be responsible for Gαi3 degradation in LPS-pretreated cells.
Fig. 5.
Gαi1/3 degradation is involved in LPS tolerance. (A) Survival of mice (n = 6 per group) given saline (Naive) or preexposure to a low dose of LPS (LPS-tolerant) were challenged with a lethal dose of LPS (4 mg/kg. i.p.) plus d-galactosamine (500 mg/kg). Survival was monitored over 10 h. (B) Gαi1/3 expression in peritoneal macrophages (primary Mφ) isolated from mice treated as in A for 2 h. Gαi1, Gαi3 was detected by Western blot. (C and D) Expression of Gαi1/3 level was quantified from Western blot assays. The data are expressed as the mean ± SE of the ratios of indicated protein to β-actin. **P < 0.01, ***P < 0.001. (E) BMDMs (2 × 105 cells per well) were tolerized or not with 100 ng/mL LPS for 16 h, washed, and rechallenged with 100 ng/mL LPS. After 8 h of incubation, arginase activity was assessed by an assay of urea production from arginine substrate. *P < 0.05, **P < 0.01. (F) Expression of Gαi protein in BMDMs tolerized or not with 100 ng/mL LPS for 16 h, washed, and rechallenged with 100 ng/mL LPS. After 8 h of incubation, cells were lysed. Gαi1, Gαi3, and β-actin were assessed by Western blot. (G) BMDMs were vehicle-treated or tolerized with 100 ng/mL LPS for 16 h, washed, and rechallenged with 100 ng/mL LPS for the indicated times. Gαi3 mRNA expression was measured by QRT-PCR. *P < 0.05, **P < 0.01. (H) BMDMs tolerized overnight with LPS (100 ng/mL) were pretreated for 30 min with either vehicle only (DMSO) or MG132 (25 μM). Cells were subsequently stimulated with LPS (100 ng/mL) for the indicated times and lysed. Gαi1, Gαi3, and β-actin were assessed by Western blot. (I) BMDMs were vehicle-treated or tolerized with 100 ng/mL LPS for 16 h, washed, and rechallenged with 100 ng/mL LPS for indicated times. Gαi1, Gαi3, GIPN, and β-actin were assessed by Western blot.
Discussion
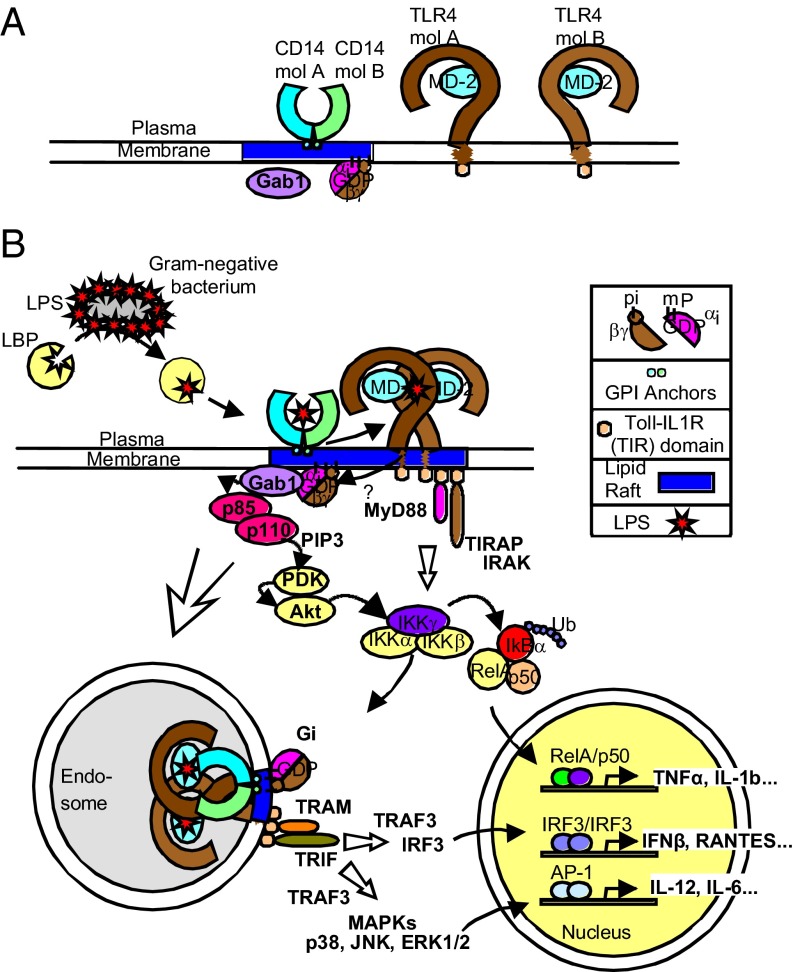
Our current study demonstrated that Gαi1/3 is involved in three major downstream signaling pathways of TLR4: (i) TLR4-induced production of proinflammatory cytokines, possibly by CD14–Gαi1/3–Gab1–PI3K–Akt–NF-κB complex formation; (ii) Gαi1/3-MAPK signaling; and (iii) the Gαi1/3-IRF3 signaling pathway (Fig. 6). A previous study had also shown that PI3K and Akt are activated by TLR4 in macrophage (15); however, the underlying mechanisms were not well characterized. Here, we demonstrated that, in response to LPS, Gαi1/3 recruited Gab1, which further interacted with PI3K regulatory subunit p85 to promote Akt activation in macrophages. Thus, Gαi1/3 may be one of the most important adaptors that trigger PI3K/Akt signaling in the LPS response. We also found that Gαi1/3 act at the endosome level and are involved in endocytosis of TLR4 in macrophages.
Fig. 6.
Model of Gαi1/3-mediated activation of TLR4 in response to LPS. (A) Distribution of CD14, TLR4, Gαi1, Gαi3, and Gab1 in rested macrophage. (B) LPS induces formation of a complex between CD14, Gαi1 and Gαi3. However, whether and how Gαi1 and Gαi3 combine to TLR4 is not clear. Subsequently, Gαi1 and Gαi3 interact with Gab1, which promotes its phosphorylation by an unknown kinase. Activated Gab1 interacts with regulatory subunit of PI3K (p85), which leads to the activation of the catalytic subunit of PI3K (p110). Active PI3K phosphorylates PIP2 (phosphatidylinositol 4,5-bisphosphate) to generate PIP3 (phosphatidylinositol 3,4,5-trisphosphate), which in turn interacts with the pleckstrin homology domain of Akt and PDK1, and PDK1 phosphorylates Akt on Thr308. When Akt is activated, it may phosphorylate IKKs, which further promotes NF-κB activation. Besides, Gαi1 and Gαi3 also involve in TLR4 endocytosis. We also propose that Gαi proteins may couple to the MAPK pathway. However, the exact mechanism merits further investigation.
Heterotrimeric G proteins serve as signal transducers for GPCRs at the plasma membrane (6). However, it is also known that G proteins reside intracellularly (19, 20), indicating that they may have cytoplasmic functions. Indeed, Ma et al. have reported that c-src and hck can be stimulated by a Gα protein (21). In this study, we found that LPS induces the formation of a complex among CD14, Gαi1, and Gαi3. Recruitment of Gαi1 and Gαi3 to the receptor may occur directly or through a GPCR.
Gab1 is characterized by an N-terminal PH domain, a central proline-rich domain interacting with an SH3 domain, and multiple conserved tyrosine residues favored by various SH2 domain-containing proteins (22). The PH domain binds to PIP3, the main product of PI3K. The phosphotyrosine-containing motifs form complexes with proteins having an SH2 domain, for example, the p85 regulatory subunit of PI3K, PLCγ and SHP-2, thus providing a platform for interaction of additional signaling protein(s). Here, we found that Gab1 is an intracellular target of Gαi proteins in response to LPS. Gαi1/3 interacted with Gab1 and promoted its phosphorylation by an unknown kinase. Activated Gab1 interacts with the regulatory subunit of PI3K (p85), which leads to the activation of the catalytic subunit of PI3K (p110). Active PI3K phosphorylates PIP2 (phosphatidylinositol 4,5-bisphosphate) to generate PIP3 (phosphatidylinositol 3,4,5-trisphosphate), which in turn interacts with the pleckstrin homology domains of Akt and PDK1, and PDK1 phosphorylates Akt on Thr308. When Akt is activated, it may phosphorylate IKKα, which further promotes NF-κB activation (23).
Nishida et al. have demonstrated that TLR4 is one of two receptors of pertussis toxin (PTX) (24), thus PTX is not a specific inhibitor of receptor-Gαi signaling. As a consequence, here we did not use PTX as inhibitor to study the action of Gαi1 and Gαi3 on LPS stimulation. Fan et al. demonstrated that constitutively active Gαi2 and Gαi3, which incorporate a Q204L mutation that impairs their GTPase activities, potentiated TLR4-induced ERK1/2 phosphorylation (10), indicating that TLR4 may transactivate a GPCR to promote signaling (25). Moreover, Dauphinee et al. demonstrated that Gαi proteins modulate endothelial TLR signaling independent of TRAF6 (26). Thus, we propose a signaling model of Gαi1 and Gαi3-mediated activation of the Gab1-PI3K-Akt-NF-κB pathway in response to LPS. Gαi1 and Gαi3 also contribute to the endocytosis of TLR4. A model depicting interaction cascades that are mediated by CD14 in order to promote TLR4 signaling is shown in Fig. 6. CD14 chaperones LPS molecules to the plasma membrane localized complex of TLR4 and MD2, which signals through the Gαi1/3 and TIRAP-MyD88 adaptors to activate inflammatory cytokine expression. CD14 and Gαi1/3 then transport TLR4 to endosomes, where TRAM-TRIF signaling activates IRF3, which further initiates the expression of IFNs. The shift in macrophage polarization is now recognized as a relevant event in tumorigenesis, wound healing, and resolution of inflammation, and its deregulation underlies both tumor progression and chronic inflammatory diseases (27, 28). We have shown that Gαi1/3 play an important role in maintaining homeostasis of macrophage responses and are required for normal polarization. This polarization was associated with enhanced NF-κB responses and constitutive expression of polarized markers. In concert with our results, Rudolph et al. reported that Gαi2-deficient mice with elevated Gαi1/3 expression develop colitis due to unresolved inflammations (29) and Fan et al. also found that splenocytes from Gαi2−/− mice exhibit augmented IFN-γ and IL-12 production by LPS, compared with WT mice (30). Therefore, Gαi1/3 expression seems to be a common participant favoring M1 macrophages, and a critical contributor to their effector functions. Moreover, as Gαi1 is primarily found in the brain, and Gαi3 is also expressed in brain, it implicates that Gαi1/3 could be involved in chronic inflammation diseases such as obesity, type 2 diabetes, and aging, especially because NF-κB in the brain has been appreciated to be a central cause for neural inflammation and diseases as reported by Zhang et al. and Yan et al. (31, 32). Inhibition of inflammation could revert aging related-degenerative symptoms (33). We also confirmed decreased Gαi1/3 expression in LPS-tolerant macrophage, which was consistent with the study by Makhlouf et al. (34, 35). Because there is no report of Gαi1-specific E3 ubiquitin ligase, we only demonstrated the mechanism of Gαi3 degradation, involving altered gene transcription and proteasomal degradation. However, the contribution of altered gene transcription and protein degradation to LPS tolerance merits further investigation.
Collectively, our study suggested that LPS unresponsiveness occurs by programming macrophages to the M2 phenotype. Given the importance of Gαi1/3 in controlling inflammation, we propose that controlling Gαi expression or targeting associated ubiquitin E3 ligases may represent approaches to control inflammation.
Materials and Methods
Mice.
Gαi1/3−/−(Gnai1/3−/−) mice on 129SvEv background were generated by breeding homozygous DKO mice (36). Studies used 6- to 8-wk-old Gαi1/3−/− and age-matched 129SvEv WT mice for all of the experiments. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with the approval of Center for New Drug Safety Evaluation and Research, China Pharmaceutical University.
Cell Lines and Cell Culture.
WT and Gαi1/3 DKO MEFs were derived from WT, and Gαi1/3 doubly deficient mouse embryonic day 14.5 embryos. The MEFs (5 × 105 to 10 × 105) were then immortalized by transfection with the total SV40 genome (plasmid pSV40WT) and subcultured several times with DMEM supplemented with 10% (vol/vol) FCS (37). WT and Gab1-deficient MEFs were used as previously described (38). BMDMs were obtained as described (39) and were maintained in DMEM supplemented with 10% (vol/vol) FBS and 10% (vol/vol) supernatants of L929 mouse fibroblasts as conditioned medium, providing macrophage colony-stimulating factor at 37 °C in humidified air with 5% CO2 for 6 d. THP-1 cells were maintained in medium 1640 containing 10% (vol/vol) heat-inactivated FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37 °C under 5% CO2 in air.
Statistical Analysis.
Statistical significance was determined with a Student t test, and P < 0.05 is considered to be statistically significant.
Standard methods were used for bone marrow macrophage isolation and culture, transfections, and Western blot analyses. For further details including primer sequences, see SI Methods and Materials.
Supplementary Material
Acknowledgments
We are very grateful to Dr. Gen-Sheng Feng at UC San Diego for providing Gab1 WT and Gab1 KO MEFs. This work was supported in part by NIH Intramural Research Program Project Z01 ES101643 (to L.B.); National Science Foundation of China Grants 81473230, 81403020, 81273547, and 91129731; and Natural Science Foundation of Jiangsu Province Grants BK2012025 and BK2011632. This work was also supported in part by Department of Defense Grant PR054819 (to W.-M.C.), NIH Grant AI054128 (to W.-M.C.), and the Leukemia and Lymphoma Society (W.-M.C.), and by NIH Grant DK069771 (to M.J.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503779112/-/DCSupplemental.
References
- 1.Zanoni I, et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell. 2011;147(4):868–880. doi: 10.1016/j.cell.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabhann JN, et al. (2014) Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS ONE 9(1):e85834. [DOI] [PMC free article] [PubMed]
- 6.Milligan G, Kostenis E. Heterotrimeric G-proteins: A short history. Br J Pharmacol. 2006;147(Suppl 1):S46–S55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serezani CH, Aronoff DM, Sitrin RG, Peters-Golden M. FcgammaRI ligation leads to a complex with BLT1 in lipid rafts that enhances rat lung macrophage antimicrobial functions. Blood. 2009;114(15):3316–3324. doi: 10.1182/blood-2009-01-199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiege K, et al. Gαi2 is the essential Gαi protein in immune complex-induced lung disease. J Immunol. 2013;190(1):324–333. doi: 10.4049/jimmunol.1201398. [DOI] [PubMed] [Google Scholar]
- 9.Cao C, et al. Galpha(i1) and Galpha(i3) are required for epidermal growth factor-mediated activation of the Akt-mTORC1 pathway. Sci Signal. 2009;2(68):ra17. doi: 10.1126/scisignal.2000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan H, Peck OM, Tempel GE, Halushka PV, Cook JA. Toll-like receptor 4 coupled GI protein signaling pathways regulate extracellular signal-regulated kinase phosphorylation and AP-1 activation independent of NFkappaB activation. Shock. 2004;22(1):57–62. doi: 10.1097/01.shk.0000129759.58490.d6. [DOI] [PubMed] [Google Scholar]
- 11.Fan H, et al. Heterotrimeric Gα(i) proteins are regulated by lipopolysaccharide and are anti-inflammatory in endotoxemia and polymicrobial sepsis. Biochim Biophys Acta. 2011;1813(3):466–472. doi: 10.1016/j.bbamcr.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li P, et al. Toll-like receptor-induced inflammatory cytokines are suppressed by gain of function or overexpression of Gα(i2) protein. Inflammation. 2012;35(5):1611–1617. doi: 10.1007/s10753-012-9476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9(4):361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon KR, et al. Heterotrimeric G proteins physically associated with the lipopolysaccharide receptor CD14 modulate both in vivo and in vitro responses to lipopolysaccharide. J Clin Invest. 1998;102(11):2019–2027. doi: 10.1172/JCI4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, et al. Scaffolding adaptor protein Gab1 is required for TLR3/4- and RIG-I-mediated production of proinflammatory cytokines and type I IFN in macrophages. J Immunol. 2010;184(11):6447–6456. doi: 10.4049/jimmunol.0901750. [DOI] [PubMed] [Google Scholar]
- 16.Ghosn EE, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci USA. 2010;107(6):2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Dohlman HG. Regulation of G protein and mitogen-activated protein kinase signaling by ubiquitination: Insights from model organisms. Circ Res. 2006;99(12):1305–1314. doi: 10.1161/01.RES.0000251641.57410.81. [DOI] [PubMed] [Google Scholar]
- 18.Fischer T, De Vries L, Meerloo T, Farquhar MG. Promotion of G alpha i3 subunit down-regulation by GIPN, a putative E3 ubiquitin ligase that interacts with RGS-GAIP. Proc Natl Acad Sci USA. 2003;100(14):8270–8275. doi: 10.1073/pnas.1432965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koelle MR. Heterotrimeric G protein signaling: Getting inside the cell. Cell. 2006;126(1):25–27. doi: 10.1016/j.cell.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Marrari Y, Crouthamel M, Irannejad R, Wedegaertner PB. Assembly and trafficking of heterotrimeric G proteins. Biochemistry. 2007;46(26):7665–7677. doi: 10.1021/bi700338m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102(5):635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Rohrschneider LR. The gift of Gab. FEBS Lett. 2002;515(1-3):1–7. doi: 10.1016/s0014-5793(02)02425-0. [DOI] [PubMed] [Google Scholar]
- 23.Ozes ON, et al. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401(6748):82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 24.Nishida M, et al. Pertussis toxin up-regulates angiotensin type 1 receptors through Toll-like receptor 4-mediated Rac activation. J Biol Chem. 2010;285(20):15268–15277. doi: 10.1074/jbc.M109.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2(4):338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 26.Dauphinee SM, Voelcker V, Tebaykina Z, Wong F, Karsan A. Heterotrimeric Gi/Go proteins modulate endothelial TLR signaling independent of the MyD88-dependent pathway. Am J Physiol Heart Circ Physiol. 2011;301(6):H2246–H2253. doi: 10.1152/ajpheart.01194.2010. [DOI] [PubMed] [Google Scholar]
- 27.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 28.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 29. Rudolph U, et al. (1995) Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nature Genetics 10(2):143–150. [DOI] [PubMed]
- 30.Fan H, et al. Differential regulation of lipopolysaccharide and Gram-positive bacteria induced cytokine and chemokine production in macrophages by Galpha(i) proteins. Immunology. 2007;122(1):116–123. doi: 10.1111/j.1365-2567.2007.02619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan J, et al. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat Med. 2014;20(9):1001–1008. doi: 10.1038/nm.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G, et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497(7448):211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernando LP, et al. Tolerance to LPS decreases macrophage G protein content. Shock. 1999;11(5):330–335. doi: 10.1097/00024382-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Makhlouf M, et al. Alterations in macrophage G proteins are associated with endotoxin tolerance. Biochim Biophys Acta. 1996;1312(2):163–168. doi: 10.1016/0167-4889(96)00019-5. [DOI] [PubMed] [Google Scholar]
- 36.Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA. 2001;98(6):3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu JY, Abate M, Rice PW, Cole CN (1991) The ability of simian virus 40 large T antigen to immortalize primary mouse embryo fibroblasts cosegregates with its ability to bind to p53. J Virol 65(12):6872–6880. [DOI] [PMC free article] [PubMed]
- 38. Sun Y, et al. (2004) Role of Gab1 in UV-induced c-Jun NH2-terminal kinase activation and cell apoptosis. Mol Cell Biol 24(4):1531–1539. [DOI] [PMC free article] [PubMed]
- 39. Xu H, et al. (2012) Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol 13(7):642–650. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.