Significance
Juvenile hormone (JH) controls many key processes during insect life cycles. Some of the effects of JH are mediated by a membrane-associated mechanism. In other circumstances, an intracellular JH receptor, methoprene-tolerant protein (MET), is activated upon binding of JH and directly regulates the expression of JH target genes. Here we use adult female mosquitoes as an example to demonstrate that both mechanisms are interconnected to coordinate hormonal responses. Our results indicate that the phospholipase C pathway is activated shortly after hormone exposure. Activation of this pathway induces phosphorylation of MET and profoundly increases the ability of MET to bind to JH target. This study establishes the link between the membrane-initiated JH signaling and the MET-mediated genomic action of JH.
Keywords: insect hormone, development, phospholipase C, protein kinase, transcription
Abstract
Juvenile hormone (JH) is a key regulator of a wide diversity of developmental and physiological events in insects. Although the intracellular JH receptor methoprene-tolerant protein (MET) functions in the nucleus as a transcriptional activator for specific JH-regulated genes, some JH responses are mediated by signaling pathways that are initiated by proteins associated with plasma membrane. It is unknown whether the JH-regulated gene expression depends on the membrane-mediated signal transduction. In Aedes aegypti mosquitoes, we found that JH activated the phospholipase C (PLC) pathway and quickly increased the levels of inositol 1,4,5-trisphosphate, diacylglycerol, and intracellular calcium, leading to activation and autophosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII). When abdomens from newly emerged mosquitoes were cultured in vitro, the JH-activated gene expression was repressed substantially if specific inhibitors of PLC or CaMKII were added to the medium together with JH. In newly emerged female mosquitoes, RNAi-mediated depletion of PLC or CaMKII considerably reduced the expression of JH-responsive genes, including the Krüppel homolog 1 gene (AaKr-h1) and the early trypsin gene (AaET). JH-induced loading of MET to the promoters of AaKr-h1 and AaET was weakened drastically when either PLC or CaMKII was inactivated in the cultured tissues. Therefore, the results suggest that the membrane-initiated signaling pathway modifies the DNA-binding activity of MET via phosphorylation and thus facilitates the genomic responses to JH. In summary, this study reveals an interplay of genomic and nongenomic signaling mechanisms of JH.
Juvenile hormones (JH) are a group of acyclic sesquiterpenoids produced in insects by the corpora allata, a pair of endocrine glands connected to the brain (1). They are important regulators in a wide variety of developmental and physiological events in insects, including development, reproduction, caste determination, behavior, diapauses, polyphenisms, and longevity (2–4).
Many effects of JH are mediated by the methoprene-tolerant (MET) protein, an intracellular JH receptor (5). MET contains a basic helix–loop–helix (bHLH) DNA-recognition motif near the N terminus, followed by two tandem Per-ARNT-Sim (PAS) domains, PAS-A and PAS-B (6). In vitro studies have demonstrated that JH-III binds MET with relatively high affinity and have identified a putative JH-binding pocket in the PAS-B domain of MET (7, 8). In the presence of JH, MET forms a heterodimer with a p160 steroid receptor coactivator (SRC), which also contains the bHLH-PAS domain (7, 9). The orthologs of SRC are called “Taiman” (TAI) in Drosophila melanogaster and “Ftz-F1-interacting steroid receptor coactivator” (FISC) in the yellow fever mosquito Aedes aegypti (10, 11). For simplicity, we will use a single name, Taiman, to describe all its orthologs in insects. TAI acts as the DNA-binding partner of MET; the MET–TAI complex recognizes an E-box–like sequence (5′-GCACGTG-3′) in the regulatory regions of JH-responsive genes, leading to the transcriptional activation of these genes (12). This function of MET–TAI in the JH-induced gene expression seems to be evolutionarily conserved in Ae. aegypti, D. melanogaster, the red flour beetle Tribolium castaneum, the silkworm Bombyx mori, and the cockroach Bombyx mori (9, 13–16).
The mechanisms by which JH exerts pleiotropic functions are manifold in insects. Several studies suggest that JH can act via a receptor on plasma membrane (3, 17). For example, development of ovarian patency during vitellogenesis is stimulated by JH in some insects via transmembrane signaling cascades that involve second messengers (18, 19). This nongenomic action of JH leads to rapid shrinkage of follicular epithelial cells, allowing vitellogenin from the hemolymph to pass through the large spaces between the follicular cells and to deposit in the developing oocytes (20). In vitro studies on Rhodnius prolixus have implied that JH regulates ovarian patency by binding to a specific protein on plasma membrane, which in turn activates a Na+/K+-ATPase through a PKC-dependent mechanism (21–23). In Heliothis virescens, JH-II and JH-III appear to invoke patency primarily via the diacylglycerol (DAG)/inositol triphosphate (IP3) signaling pathway, whereas JH-I acts through a G protein-coupled receptor (GPCR) and a cAMP-dependent pathway (18, 19). The putative membrane receptor of JH has not been isolated so far from any insect.
Some JH responses may require both the MET-mediated signaling pathway and the putative membrane receptor-initiated signaling cascade. JH-regulated protein synthesis in the male accessory glands of D. melanogaster is such an example. In vitro study with cultured male accessory glands has indicated that PKC and calcium play important roles in this JH-regulated event (24). Topical application of a JH analog causes a significant increase in protein synthesis in the accessory gland in wild-type flies but not in mutant flies in which the PKC activity is dramatically reduced (24). On the other hand, MET also is essential for this JH action. Met-null mutants accumulate fewer proteins in male accessory glands than do wild-type flies (25). It remains unknown whether the membrane-initiated signaling and the MET-mediated signaling interconnect in this or other JH responses. In this study we report activation of the PLC pathway by JH in Ae. aegyti mosquitoes. This activation increases the intracellular concentrations of DAG, IP3, and calcium. Moreover, activation of the PLC pathway led to enhanced binding of the MET–TAI complex to JH response elements (JHREs). This study significantly advances our understanding of the signaling network of JH and sheds light on the mechanisms underlying hormonal cross-talk involving JH.
Results
JH Causes an Increase in Second Messengers in Mosquito Cells.
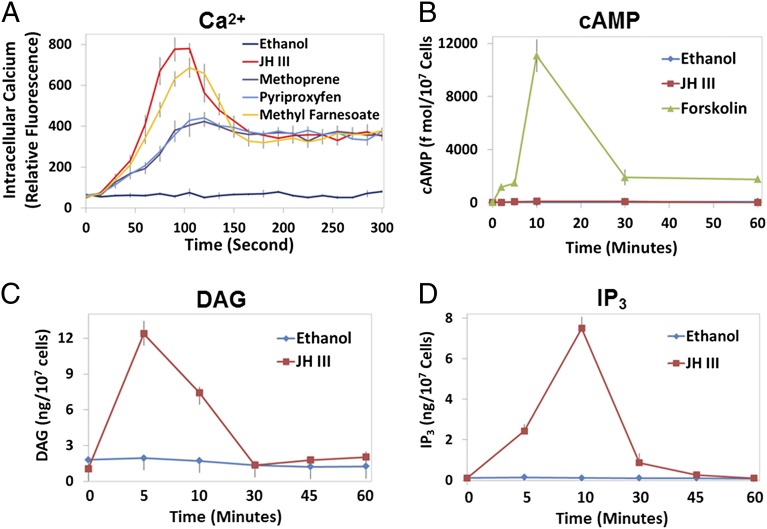
JH has been shown to induce expression of the Krüppel homolog 1 (Kr-h1) gene rapidly in cultured mosquito Aag2 cells (26). To examine the participation of second messengers in this JH response, we measured changes in the levels of calcium, cAMP, DAG, and IP3 in Aag2 cells after JH treatment (Fig. 1).
Fig. 1.
Effect of JH treatment on the intracellular levels of calcium, cAMP, DAG, and IP3 in mosquito Aag2 cells. Cells were treated with the indicated chemicals (1 µM) or ethanol (0.1%) as a solvent control. (A) Cytoplasmic calcium concentration was measured using the Fluo-8 AM fluorescent calcium indicator and a microplate reader. Values are expressed as relative fluorescence intensity. (B) Intracellular cAMP contents were assessed after the JH treatment. The adenylate cyclase activator forskolin was used as a positive control. (C and D) The amounts of DAG (C) and IP3 (D) were determined in cells 5, 10, 30, 45, and 60 min after the addition of JH-III. All experiments were repeated three times with similar results. Data are reported as the mean and SD of triplicate samples from a representative experiment.
JH-III evoked a biphasic intracellular calcium ([Ca2+]i) response, with an initial large increase that peaked at ∼90 s and a moderate rise in [Ca2+]i that was sustained for more than 10 min (Fig. 1A and Fig. S1). Methyl farnesoate (MF), an unepoxidized precursor of JH-III, gave rise to similar biphasic [Ca2+]i changes. In cells treated with methoprene or pyriproxyfen, two biologically active mimics of JH, the increase in [Ca2+]i exhibited a different pattern. The JH mimics failed to induce the initial spike in [Ca2+]i that was observed in the JH-III–treated cells but were able to trigger the subsequent moderate increase of [Ca2+]i (Fig. 1A). JH-III seemed to have no marked effect on the concentration of cAMP, which remained at basal level regardless of the presence of JH-III (Fig. 1B). In contrast, JH-III induced drastic accumulations of DAG (Fig. 1C) and IP3 (Fig. 1D), which reached their peaks 5–10 min after the hormone treatment.
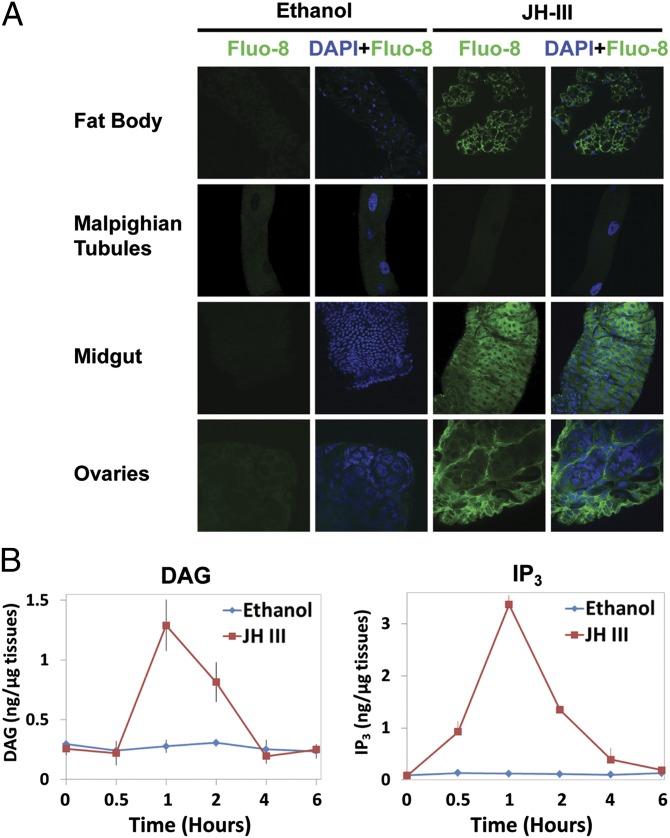
To validate these observations, we examined the second-messenger molecules in mosquito tissues. In adult female mosquitoes, the JH-III levels in the hemolymph increased shortly after adult emergence and peaked at about 36 h (27). Fat bodies, midguts, ovaries, and Malpighian tubules were collected from female mosquitoes within 30 min post eclosion (PE) before the rise of JH titers. The tissues were cultured in vitro in medium with JH-III or ethanol (solvent control). The [Ca2+]i levels increased substantially in fat bodies, midguts, and ovaries that were treated with JH-III as compared with those treated with ethanol (Fig. 2A). However, the [Ca2+]i levels in the Malpighian tubules were not affected by the JH treatment. In the cultured fat bodies, JH-III also increased the levels of DAG and IP3 by 4.7-fold and 27-fold over controls (P < 0.01), respectively, at 1 h after the hormone was added to the culture medium (Fig. 2B). Therefore, the measurements of second messengers in Aag2 cells and in the mosquito tissues implicated Ca2+, DAG, and IP3 in cellular responses to JH-III.
Fig. 2.
JH treatment causes a rise in intracellular levels of calcium, DAG, and IP3 in mosquito tissues. (A) JH-stimulated calcium responses in the fat body, Malpighian tubules, midgut, and ovaries. Tissues dissected from adult female mosquitoes within 30 min PE were preincubated with Fluo-8 AM in APS for 1 h, followed by incubation with 1 μM JH-III or ethanol for 15 min. Images were captured using a Zeiss LSM 510 confocal microscope at 1,000× magnification. Cell nuclei were stained blue with DAPI, whereas the binding of calcium to Fluo-8 AM greatly enhanced the green fluorescence intensity. Representative images were taken from one of three independent experiments with similar results. (B) JH treatment increased the production of DAG and IP3 in cultured fat bodies that were isolated from newly emerged mosquitoes. Fat bodies were incubated in the tissue culture medium with JH-III at a final concentration of 1 µM. Ethanol was used as a negative control. The experiment was repeated twice with three replicates per treatment.
JH Activates the Phospholipase C Pathway.
The increased levels of [Ca2+]i, DAG and IP3 in the mosquito cells after JH treatment imply an activation of the phospholipase C (PLC) pathway by JH-III. In animals, PLC is activated by some cell-surface receptors, including GPCRs and receptor tyrosine kinases (RTKs). PLC hydrolyses the membrane phospholipid PIP2 (phosphotidylinositol-4,5-bisphosphate) to form IP3 and DAG. Although DAG remains membrane-bound, IP3 diffuses to the endoplasmic reticulum (ER) and binds to its receptor (IP3R, a calcium ion channel), releasing Ca2+ from internal stores in the ER to the cytoplasm.
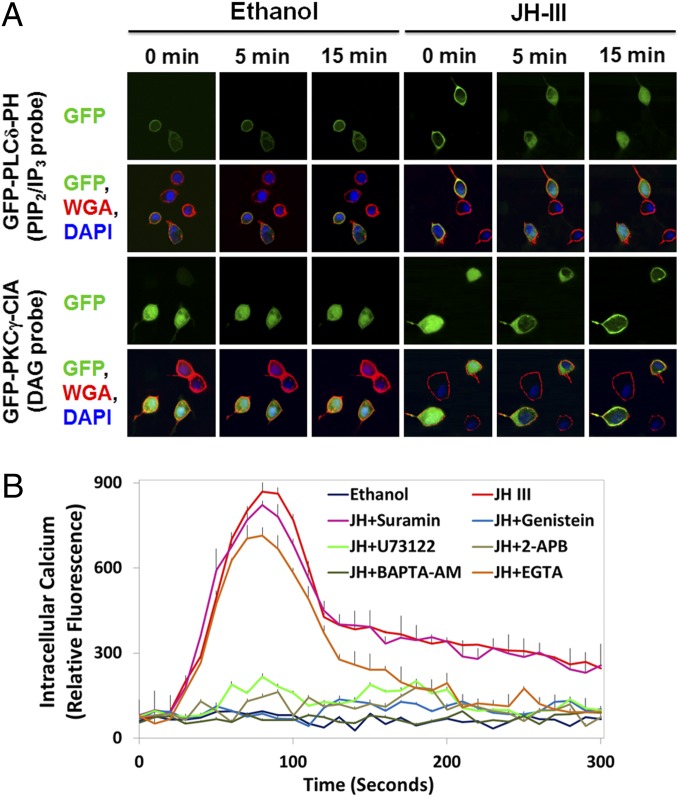
To verify the JH-induced hydrolysis of PIP2, we transfected Aag2 cells separately with two GFP-fusion reporters, GFP-PLCδ-PH and GFP-PKCγ-C1A. The Pleckstrin homology (PH) domain of human PLCδ binds with high affinity to PIP2 and IP3. In unstimulated cells, the GFP-PLCδ-PH probe was localized primarily to the plasma membrane (Fig. 3A). After the addition of JH-III to the culture medium, the GFP-PLCδ-PH probe was translocated from the plasma membrane to the cytoplasm within minutes, suggesting a considerable decrease in membrane PIP2 content. On the other hand, the C1 domain of rat PKCγ has a high affinity for DAG. A cytosol-to-membrane translocation of the GFP-PKCγ-C1A probe occurred shortly after JH treatment, indicating the production of DAG at the plasma membrane (Fig. 3A). This live-cell imaging experiment thus indicated that JH-III induces the activation of PLC in Aag2 cells.
Fig. 3.
JH activates the phospholipase C–calcium pathway. (A) The amount of PIP2 at the plasma membrane decreased in Aag2 cells after exposure to JH-III. Aag2 cells were transfected with plasmids encoding GFP-PLCδ-PH or GFP-PKCγ-C1A and were stained with DAPI (blue) and the plasma membrane marker WGA (red). The PH domain of PLCδ binds with high affinity to PIP2 and IP3. The C1A domain of PKCγ has a high affinity for DAG. Subcellular translocation of the GFP reporters after JH treatment was captured using a confocal microscope at 1,000× magnification. Representative images are shown. (B) Intracellular calcium was quantitated after Aag2 cells were incubated with 1 µM of JH-III and the indicated chemicals that blocked the PLC–Ca2+ signaling pathway. Ethanol was used as a negative control. Data are presented as mean ± SD (n = 3).
To confirm that the increased [Ca2+]i resulted from activation of the PLC pathway, we treated Aag2 cells with JH-III and a number of inhibitors that targeted specific components of the PLC pathway. Inhibition of either PLC or IP3R by U73122 and 2-aminoethoxydiphenyl borate (2-APB), respectively, abolished the JH-induced elevation of [Ca2+]i (Fig. 3B). Although a membrane-permeable calcium chelator, 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM), completely eliminated the intracellular Ca2+ signal, EGTA (a non–cell-permeable calcium chelator) reduced the peak levels of [Ca2+]i by about 20% (P < 0.001) and brought [Ca2+]i back to the background level within about 5 min after the application of JH-III (Fig. 3B). The results indicated that the increased [Ca2+]i after JH treatment was released primarily from intracellular stores as a result of activation of the PLC pathway; influx of extracellular calcium also contributed to the increase, but to a lesser extent.
PLC often is activated by GPCRs and RTKs, leading to the production of IP3/DAG and to the release of Ca2+ from internal stores. Interestingly, the JH-induced increase in [Ca2+]i was blocked completely in Aag2 cells by the tyrosine kinase inhibitors Genistein and Tyrphostin A23 but not by the inhibitors of G protein signaling (Suramin and GDP-β-S) (Fig. 3B), suggesting that a member of the RTK family functions as the membrane receptor of JH.
In Drosophila S2 and Kc cells, JH also induced an increase in [Ca2+]i, with a pattern similar to that in mosquito Aag2 cells (Fig. S2). This elevation of [Ca2+]i required functional RTKs and PLC in the Drosophila cells, implying that this membrane protein-initiated JH signaling pathway is conserved in insects.
JH Activates Calcium/Calmodulin-Dependent Protein Kinase II.
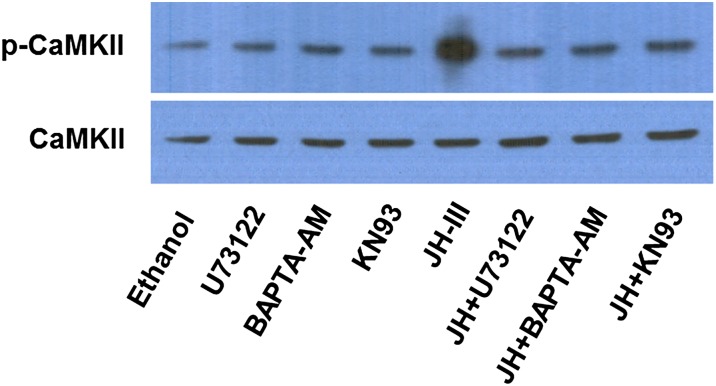
Calcium is involved in a wide variety of cellular processes in animals and plants. An increase in the cytosolic calcium ion could result in the activation of calcium/calmodulin-dependent protein kinase II (CaMKII), a serine/threonine kinase. The activated CaMKII can undergo further autophosphorylation at Thr286, allowing the kinase to remain active even after [Ca2+]i returns to basal level after stimulation (28). Phosphorylation at Thr286 was examined in mosquito CaMKII by Western blot. Autophosphorylation of CaMKII was enhanced significantly in the fat bodies that were cultured in vitro with JH-III as compared with the control group (Fig. 4). When the fat bodies were cultured in the presence of U73122, BAPTA-AM, or KN93 (a specific inhibitor of CaMKII), JH-III failed to induce the phosphorylation of CaMKII above the basal level (Fig. 4). This result indicated that the JH-induced activation of CaMKII is governed by the PLC–calcium pathway.
Fig. 4.
JH treatment leads to activation of CaMKII. Fat bodies from newly emerged adult female mosquitoes were cultured in vitro for 1 h in the presence of JH-III (1 µM), U73122 (1 µM), BAPTA-AM (10 µM), and/or KN93 (10 µM). Proteins then were extracted from the fat bodies and subjected to Western blot analysis using antibodies against CaMKII phosphorylated at threonine 286 (p-CaMKII; Cell Signaling Technology) and total CaMKII (Cosmo Bio USA).
The PLC–Calcium Signaling Pathway Is Required for JH-Induced Gene Expression.
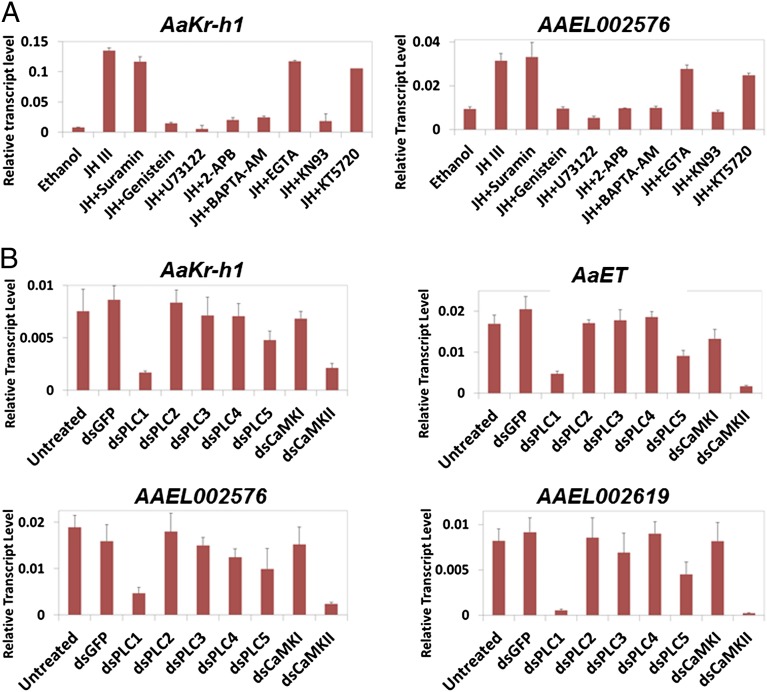
After demonstrating that JH-III activated the PLC–calcium pathway in mosquito cells, we examined whether JH-regulated gene expression relied on activation of the PLC pathway. The JH-induced expression of AaKr-h1 in Aag2 cells was repressed substantially (P < 0.001) when JH-III was added to the culture medium together with Genistein, U73122, 2-APB, BAPTA-AM, or KN93 (Fig. S3A). In contrast, the inhibitors for protein kinase A (KT5720) and G protein (Suramin) did not affect the up-regulation of AaKr-h1 in response to JH-III. To validate the roles of PLC and CaMKII in this JH response, expression of these enzymes was knocked down by RNAi in Aag2 cells (Fig. S4A). Five PLC isoforms were found in Ae. aegypti (Table S1). Depletion of PLC1 and PLC5 resulted in considerable decrease (P < 0.001) in the JH-induced expression of AaKr-h1, whereas the addition of dsRNA for PLC2, PLC3, or PLC4 seemed to have minor effects (Fig. S3B). Knockdown of CaMKII, but not CaMKI, reduced the JH-regulated expression of AaKr-h1 by more than 60% (P < 0.001).
In cultured fat bodies that were collected from newly emerged adult female mosquitoes, expression of AaKr-h1 and AAEL002576 was induced readily by JH-III. The JH-induced expression of both genes was diminished remarkably (P < 0.001) when the PLC pathway and CaMKII were inactivated by the Ca2+ chelator BAPTA-AM and specific inhibitors (Fig. 5A). Consistent with the results from Aag2 cells, Suramin, EGTA, and KT5720 did not have a substantial adverse effect on the JH-regulated expression.
Fig. 5.
The PLC/Ca2+/CaMKII pathway is essential for the JH-induced gene expression in Ae. aegypti. (A) Fat bodies from newly emerged mosquitoes were cultured in vitro with the indicated inhibitors for 1 h. After JH-III was added to the culture medium, the fat bodies were cultured for another hour and collected for RNA extraction. Expression of the JH target genes AaKr-h1 and AAEL002576 was analyzed by real-time PCR. Results are the mean ± SD of three independent experiments. (B) Female mosquitoes at 1 h PE were injected with 0.5 µg of dsRNA against GFP (a negative control), individual PLC isoforms, CaMKI, or CaMKII. At 72 h after the injection, the mosquitoes were collected for RNA extraction. Expression of the JH-regulated genes in adult mosquitoes was measured using real-time PCR. Results are the mean ± SD of three independent experiments.
To explore the function of PLC and CaMKII in JH signaling in vivo, dsRNAs for these proteins were injected into newly emerged mosquitoes (Fig. S4B). At 72 h PE, mRNA transcripts of several JH target genes (AaKr-h1, AaET, AAEL002576, and AAEL002619) were measured by quantitative RT-PCR (qRT-PCR). Depletion of PLC1 and CaMKII showed the most severe effects, reducing expression of those genes by more than 60% (P < 0.01) (Fig. 5B). Injection of dsRNA for PLC5 reduced the expression of JH target genes by 30–40% (P < 0.05), whereas knockdown of PLC2, PLC3, PLC4, or CaMKI did not affect the gene expression significantly (P > 0.10). Together, these results indicated that the JH-regulated gene expression in mosquito cells requires the participation of the PLC–Ca2+ pathway.
The PLC Pathway Modulates Transactivation Activity of the Intracellular JH Receptor.
The expression of many of the JH-regulated genes in previtellogenic mosquitoes relies on the function of the JH receptor MET (29). To examine whether the PLC pathway affects the transactivation activity of the MET–TAI complex, we carried out a transfection assay with Aag2 cells. The luciferase reporter gene under the control of a JHRE identified in AaET (AaET_JHRE1) was activated significantly (P < 0.001) in a JH-dependent manner by overexpression of AaMET and AaTAI (Fig. 6A). This JH-induced expression was lessened considerably (P < 0.001) when PLC or CaMKII was inactivated by specific inhibitors before the addition of JH-III. In a control experiment, the yeast GAL4 transcription factor was expressed in Aag2 cells to drive expression of a UAS-Luc reporter gene. The up-regulation of UAS-Luc by GAL4 was not markedly affected (P > 0.05) when the transfected cells were treated with JH-III and the inhibitors of the PLC pathway (Fig. 6B). These results suggested that the JH-activated PLC pathway specifically modulates the function of the MET–TAI complex on JHRE. In Drosophila Kc167 cells and S2 cells, transactivation by the mosquito JH receptor complex also relied on the JH-activated PLC pathway in a similar manner (Fig. S5).
Fig. 6.
Blocking the PLC/Ca2+/CaMKII pathway reduces the MET/TAI-mediated gene expression. (A) Aag2 cells were transfected with expression plasmids for AaMET and AaTAI, together with a 4×JHRE-luc firefly luciferase reporter construct and a constitutively expressing Renilla luciferase construct. Transfected cells were preincubated with the indicated inhibitors for 1 h followed by treatment with 1 µM JH-III for 4 h. Results are expressed as the ratio of firefly to Renilla luciferase activity and are the mean ± SD of at least three independent experiments. (B) Aag2 cells were transfected with an expression vector for GAL4, together with a 4×UAS-luc firefly luciferase reporter construct and a constitutively expressing Renilla luciferase construct. Transfected cells were treated with the indicated inhibitors and JH-III as described in A.
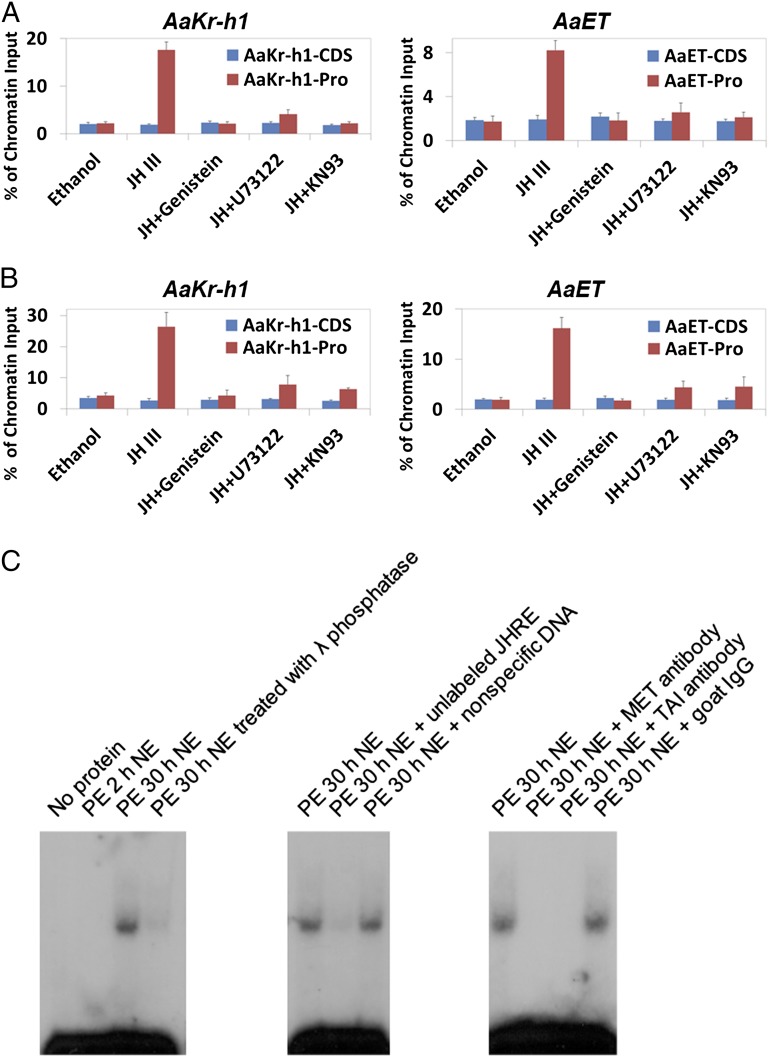
To test whether the PLC pathway affected the binding of MET–TAI to JHREs, we carried out ChIP experiments using in vitro-cultured abdomens that were dissected from newly emerged mosquitoes. Three hours after JH-III was added to the culture medium, binding of AaMET and AaTAI to the proximal promoter of AaKr-h1 was increased by 8.2-fold and 6.2-fold (P < 0.001), respectively, as compared with the ethanol-treated tissues (Fig. 7 A and B). Similar increase was observed on the promoter of AaET. When tyrosine kinases, PLC, or CaMKII were inactivated by their specific inhibitors, the protein levels of AaMET and AaTAI in the cultured abdomens (were not affected markedly Fig. S6). However, the JH-induced binding of AaMET-AaTAI to JHREs was repressed substantially (P < 0.01) (Fig. 7 A and B), implying that activation of the PLC pathway by JH is essential for binding of the MET–TAI complex to JHRE.
Fig. 7.
The PLC pathway affects binding of the MET/TAI complex to the JH-responsive promoters. Abdomens from newly emerged mosquitoes were cultured in vitro with Genistein, U73122, and KN93 for 1 h. After 1 µM JH-III was added to the medium, culture continued for additional 3 h. (A and B) ChIP assays were performed using antibodies against AaMET (A) and AaTAI (B). Precipitated DNA was examined using real-time PCR. For each JH target gene, two pairs of primers were designed to amplify the proximal promoter (Pro) region and the coding DNA sequence (CDS) region. Results are presented as a percentage of input chromatin and represent mean values ± SDs of two independent experiments. (C) Dephosphorylation inhibited the binding of MET/TAI to JHRE. Nuclear proteins were extracted from the abdomens of adult female mosquitoes at the indicated time points and were incubated in vitro with the [32P]-labeled JHRE from the AaET gene (AaET_JHRE1). For phosphatase treatment, nuclear proteins extracted from mosquitoes at 30 h PE were incubated with 1 U/μL λ phosphatase for 1 h on ice. For competition experiments, nuclear extracts (NE) were incubated with an approximate 100× molar excess of unlabeled probe or a nonspecific double-stranded oligonucleotide for 20 min before incubation with the labeled probe. Binding of AaMET/AaTAI to the probe was verified by adding polyclonal antibodies against AaMET and AaTAI directly to the binding reactions. Nonspecific goat IgG was used as a negative control.
The implication of CaMKII in the DNA binding of MET–TAI suggested that the MET–TAI complex is regulated posttranslationally by phosphorylation and that this modification positively regulates their DNA-binding properties. To test this hypothesis, a gel-shift assay was performed using AaET_JHRE1 as probe. Nuclear proteins were extracted from abdomens of mosquitoes at 2 and 30 h PE when endogenous JH was at the basal level and high level, respectively. Only the nuclear proteins from mosquitoes at PE 30 h contained a specific binding activity that recognized AaET_JHRE1 (Fig. 7C). Incubating the nuclear proteins with antibodies against either AaMET or AaTAI abolished this binding, indicating that both AaMET and AaTAI are components of the protein–DNA complex. When the nuclear proteins from 30 h PE were treated with λ protein phosphatase in vitro before incubation with the labeled probe, the specific binding activity decreased considerably (Fig. 7C), suggesting that phosphorylation of AaMET and/or AaTAI is critical for the binding of the two proteins to JHRE.
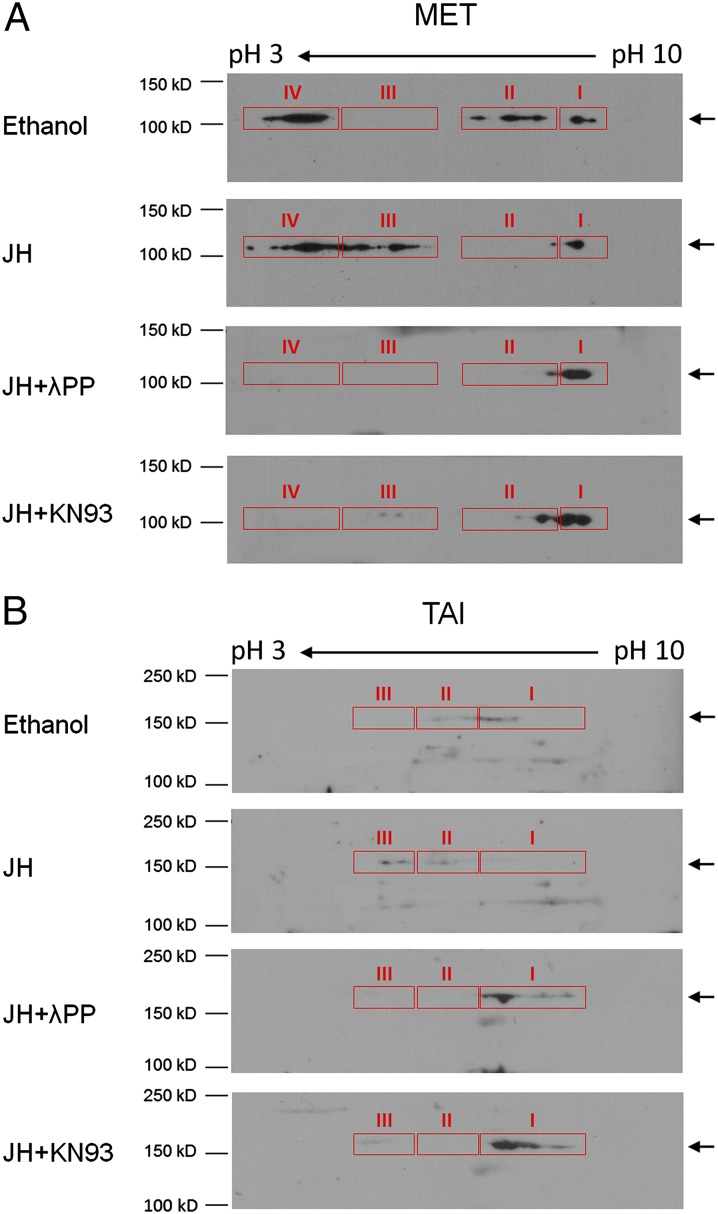
To examine the phosphorylation of AaMET and AaTAI, proteins were extracted from in vitro-cultured abdomens that were dissected from newly emerged mosquitoes. The proteins were analyzed using 2D gel electrophoresis, followed by immunoblotting with antibodies against AaMET and AaTAI. Multiple molecular forms of AaMET were detected and arbitrarily sorted into four clusters (I–IV) in descending order of isoelectric point (pI). Compared with the ethanol-treated sample, there were some minor changes in clusters I and IV of AaMET in the JH-treated tissues. Cluster II of AaMET mostly disappeared in the JH-treated tissues and was replaced with cluster III that migrated to lower pH. Treatment of the protein extracts with λ phosphatase before electrophoresis revealed that the more acidic forms of AaMET (clusters III and IV) were sensitive to phosphatase treatment, suggesting that they are phosphoproteins (Fig. 8A). The JH-induced phosphorylation (mainly in cluster III) was considerably abrogated in the cultured tissues when CaMKII was inactivated by KN93. Therefore, we conclude that JH-activated CaMKII promotes the phosphorylation of certain residues in AaMET, but some other residues in AaMET are phosphorylated even in the absence of JH. Similar experiments were carried out for the detection of AaTAI. As shown in Fig. 8B, AaTAI in the ethanol-treated tissues was present primarily in clusters I and II. In contrast, the major portion of AaTAI in the JH-treated sample was detected in cluster III, which had lower pI than clusters I and II, indicating that the JH treatment also enhanced the phosphorylation of AaTAI in the mosquito tissues. The JH-induced phosphorylation of AaTAI also was mediated by CaMKII (Fig. 8B).
Fig. 8.
JH treatment induces phosphorylation of AaMET and AaTAI in mosquitoes. Abdomens from newly emerged mosquitoes were cultured in vitro with ethanol or 1 µM JH-III for 2 h. To inactivate CaMKII, the tissues were preincubated with 10 µM KN93 for 1 h before JH-III was added to the culture. Cell extracts were prepared and analyzed by 2D gel electrophoresis followed by Western blot analysis as described in Materials and Methods. A portion of the protein extracts from the JH-treated sample was incubated with λPP (2.5 U/μL) before IEF and SDS/PAGE. AaMET (A) and AaTAI (B) were visualized by immunoblotting with specific antibodies against AaMET and AaTAI.
Discussion
It is intriguing that JH regulates a diverse array of biological processes at different stages of insect life cycles. In some cases, JH seems to act via membrane proteins, and the responses are fast. Some other JH responses involve the alteration of gene expression, giving rise to sustained JH effects. It has been postulated that JH uses multiple molecular mechanisms to exert its pleiotropic functions (17, 30). Our study with mosquitoes demonstrates that JH acts on plasma membrane and activates the PLC pathway, leading to the enhanced transactivation activity of the MET–TAI complex. To our knowledge, this study provides the first evidence indicating the collaboration between the intracellular receptor MET and the prospective membrane receptor in mediating JH responses.
The existence of a membrane JH receptor was proposed by Davey et al. (17) when they studied the role of JH in the development of ovarian patency. Membranes prepared from the follicle cells of R. prolixus and Locusta migratoria were found to bind JH with nanomolar affinity (21, 31), which is comparable to that of the intracellular receptor MET from D. melanogaster and T. castaneum (7, 8). The putative membrane JH receptor from R. prolixus selectively binds JH-II and JH-III but not JH-I (21). In contrast, the membrane preparation from L. migratoria preferentially binds JH-I, because JH-II and JH-III fail to compete with JH-I for the binding to membranes of follicle cells (31).
Our pharmacological studies using specific inhibitors imply that the membrane receptor for JH in mosquito cells is itself an RTK or is a membrane protein associated with a tyrosine kinase. It is interesting that the unidentified membrane receptor is able to differentiate JH-III and MF from methoprene and pyriproxyfen. The elevation of [Ca2+]i showed discrete magnitudes and patterns when Aag2 cells were exposed to the individual chemicals. The mechanisms and significance behind the observed difference remain to be determined. Previous studies have suggested that MF has a JH-like activity in D. melanogaster (32, 33). Our result indicates that MF mirrors JH-III in activating the PLC pathway in mosquito cells (Fig. S7). In the cultured mosquito tissues, MF also induces the expression of Kr-h1 (Fig. S7). This induced expression requires the enzymatic function of PLC. More detailed studies are needed to test whether the binding affinities of MF to MET and the putative membrane receptor are comparable to those of JH-III. This information may help us understand why insects bother to epoxidize MF to JH-III and how cells differentiate MF from JH-III.
JH induces an increase in DAG and IP3 in mosquito cells. DAG and IP3 reached maximum levels 5–10 min after JH-III was added to the Aag2 cells but peaked about 1 h after JH application to in vitro-cultured fat bodies. The Aag-2 cell line was derived from Ae. aegypti embryos, and the fat bodies were from adult mosquitoes (34). The observed discrepancy may stem from different compositions of the signaling complexes at distinct developmental stages. The slower response in the cultured fat bodies also may be attributed to poor oxygenation. Our tissue culture was incubated under regular atmosphere conditions (air), but many other researchers have found that it is critical to keep the cultured insect tissues under an atmosphere of 95% oxygen and 5% carbon dioxide (35). This possibility is being investigated currently.
Different types of membrane JH receptors may exist in insects to mediate various JH-regulated biological events. A recent study of Helicoverpa armigera, a lepidopteran insect, indicated that JH induces phosphorylation of an isoform of Broad, BrZ7, to prevent metamorphosis (36). This JH action is mediated by a GPCR/PLC/PKC pathway. In our study, inactivation of GPCRs appears to have no effect on the JH-induced gene expression in Aag2 cells or in the tissues from adult mosquitoes. Further investigation is warranted to examine whether more than one type of membrane JH receptor participates in JH signaling in a tissue- and stage-specific manner in a single insect species.
Many steroid hormones, including 17β-estradiol, use both membrane receptors and nuclear receptors to exert their effects (37). The primary estrogen effects are mediated by nuclear estrogen receptor alpha and estrogen receptor beta, which function as ligand-activated transcription factors. In addition, distinct membrane estrogen receptors are involved in ensuring rapid cell responses to the changing hormonal levels. The membrane estrogen receptors include the classical estrogen receptors that are associated with plasma membrane and a G protein-coupled estrogen receptor (38). Binding of 17β-estradiol to membrane estrogen receptors can rapidly activate many kinases in various signaling pathways and elicit calcium influx across the plasma membrane. The membrane-mediated mechanisms also can intersect with the genomic pathway, modulating activity of the nuclear estrogen receptors and other transcriptional factors via protein phosphorylation (38, 39).
JH also may exert its action by interacting with both membrane receptors and intercellular receptors in insects. In the JH-induced development of ovarian patency in R. prolixus, the follicle cells reduce their volumes within minutes. This membrane-mediated JH action does not require new protein synthesis (17). In mosquito cells, nongenomic and genomic responses are induced by JH. Production of DAG and IP3, as well as an increase in intracellular calcium, takes place shortly after exposure to JH. Furthermore, our study indicates that the membrane-mediated JH signaling constitutes a requisite for the MET-mediated transcriptional regulation of JH target genes. The JH-activated PLC pathway enhances DNA binding of the MET–TAI complex, presumably by activating CaMKII to phosphorylate MET and TAI directly or indirectly.
Multiple phosphorylated forms of AaMET and AaTAI were detected in our Western blot analysis after 2D electrophoresis, suggesting that quite a few serine, threonine, or tyrosine residues were phosphorylated by various protein kinases activated by JH. A bioinformatic analysis with the GPS 2.1 software (40) revealed 14 putative CaMKII phosphorylation sites in AaMET and 23 sites in AaTAI. Identification and characterization of phosphorylation sites in AaMET and AaTAI that are involved in JH signaling remains a main goal for future research.
In Ae. aegypti, MET also heterodimerizes in a JH-dependent manner with Cycle (CYC), another bHLH–PAS protein (41). The MET–CYC complex in newly emerged adult female mosquitoes is required for circadian expression of some JH-induced genes, including AaKr-h1 (41). It would be interesting to determine whether DNA binding of the MET–CYC complex also relies on the activation of the PLC pathway by JH.
In this study we found that the fat body, ovaries, and midgut in newly emerged adult mosquitoes increased intracellular calcium levels after exposure to JH. In contrast, the Malpighian tubules did not show similar response, suggesting that the putative membrane receptor is not expressed ubiquitously or that additional factors may act upstream of PLC and modulate the signaling initiated by the membrane receptor. If the membrane JH receptor and MET exhibit diverse temporal and spatial expression profiles, the presence of two distinct signaling pathways and their interaction may allow variable and heterogeneous cell responses and adaptation to different circumstances. Furthermore, the membrane JH receptor-activated PLC pathway potentially can modulate other signaling pathways in insects. Recent studies have shown that ultraspiracle (USP, a component of the functional ecdysteroid receptor complex) is phosphorylated by PKC in Drosophila (42). In H. armigera, it seems that the 20E-induced phosphorylation of USP relies on the function of PLC and Ca2+ influx (43). Although different isoforms of PKC and PLC may be involved in 20E and JH signaling, it is possible that JH uses the membrane-initiated signaling to influence the cell responses to 20E. This hypothesis is currently under investigation.
Materials and Methods
Cell Culture.
Ae. aegypti Aag2 cells (34, 44) were maintained at 28 °C in Schneider’s Drosophila medium (Life Technologies) supplemented with 10% (vol/vol) FBS (Atlanta Biologicals). At 80% confluence, the cells reached a density of 2 × 105 cells/cm2.
Drosophila S2 and Kc167 cells, obtained from the Drosophila Genomics Resource Center (Indiana University, Bloomington, IL), were maintained at 26 °C in Schneider’s Drosophila medium supplemented with 5% (vol/vol) FBS.
JH-III, methoprene, and pyriproxyfen were purchased from Sigma-Aldrich. MF was obtained from Echelon Biosciences. These chemicals were dissolved in ethanol. For inhibition experiments, cells were preincubated with inhibitors for 45–60 min before the addition of JH-III. Final concentrations of inhibitors in all cell-culture studies were as follows: 2-APB (EMD Millipore), 40 µM; BAPTA-AM (EMD Millipore), 10 µM; EGTA (MP Biomedicals), 2 mM; Genistein (MP Biomedicals), 20 µM; KN93 (EMD Millipore), 10 µM; KT 5720 (Santa Cruz Biotechnology), 10 µM; Suramin (Sigma-Aldrich), 20 µM; U73122 (EMD Millipore), 1 µM.
Mosquito Rearing and Tissue Culture.
Ae. aegypti mosquitoes were maintained under laboratory-controlled conditions (28 °C, 60–70% humidity, with a 14/10 h day/night cycle). Larvae were fed with ground fish food (TetraMin Tropical Flakes), and adults were supplied continuously with 10% (wt/vol) sucrose solution in a jar with a cotton wick. At 5–7 d PE, female mosquitoes were fed on anesthetized rats to initiate egg production. All dissections were performed in Aedes physiological saline (APS) (45).
Tissues used in this study were obtained from adult female mosquitoes within 30 min PE. In vitro tissue culture was performed as described previously for fat body culture (46, 47). Mosquito abdomens were cut open and floated on top of the fat body culture medium. The cuticle was exposed to air, and the tissues that remained attached to the inner wall of the abdomen cuticle were exposed to the medium. Abdomens dissected from five mosquitoes were incubated as a group in a single well of a tissue-culture plate. A total of 15 mosquitoes (three groups of five mosquitoes) were used for each treatment. In experiments in which inhibitors were used, the dissected abdomens were preincubated with the inhibitors for 1 h before the addition of JH-III.
Measurement of Second Messengers.
Intracellular calcium levels in Aag2 cells were determined by using the fluorescent probe Fluo-8 AM (AAT Bioquest). Fluo-8 elicits a strong increase in fluorescence intensity upon the binding of calcium. For calcium quantification, Aag2 cells were seeded overnight at 50,000 cells per 100 µL per well in a 96-well black wall/clear bottomed plate. Before the assay, growth medium was replaced with HBSS buffer (1× HBSS with 20 mM Hepes buffer, pH 7.3). An equal volume of Fluo-8 NW dye-loading solution was added in each well. Then the plate was incubated successively at 28 °C for 15 min and at room temperature for 30 min. After JH-III was added to the cells, fluorescence intensities were measured using a SpectraMax M5e plate reader (Molecular Devices) with a filter set of Ex/Em = 490/525 nm or were recorded with a fluorescence microscope using an FITC filter.
Intracellular cAMP concentrations were measured using the cAMP Direct Biotrak enzyme immunoassay system (GE Healthcare). Aag2 cells were seeded in a 96-well plate overnight in complete medium. After JH treatment, the cells were lysed with the lysis reagent provided in the kit. The lysates were analyzed according to the manufacturer's instructions. The optical density at 450 nm was determined using a SpectraMax M5e plate reader.
Quantitative determination of DAG was performed by using a radioenzymatic assay as described by Bollag and Griner (48). In brief, total cell lipids were extracted, and DAG in the lipids was converted in vitro to [32P]-labeled phosphatidic acid by Escherichia coli DAG kinase. [32P]-phosphatidic acid was extracted and subsequently separated from other lipids by TLC, using phosphatidic acid (Sigma-Aldrich) as a reference. The amount of phosphatidic acid was determined by radioautography using a Typhoon FLA 7000 phosphorimager (GE Healthcare Life Sciences).
IP3 was measured by using a Rat Inositol Triphosphate ELISA kit (Blue Gene). The assay was performed according to the manufacturer's instructions.
Confocal Imaging.
To visualize calcium signaling in mosquito tissues, fat bodies, Malpighian tubules, midguts, and ovaries were dissected from newly emerged mosquitoes and were incubated immediately in APS solution containing Fluo-8 AM and DAPI for 1 h at 27 °C. After three washings with APS, tissues were cultured in medium containing JH-III or ethanol for 15 min, a predetermined time point. The tissues then were mounted on slides, and images were captured using a confocal microscope.
The plasmids GFP-C1-PLCδ-PH and GFP-C1-PKCγ-C1A were obtained from Tobias Meyer (Stanford University, Stanford, CA) through Addgene. To track IP3 and DAG signals, Aag2 cells were seeded sparsely on a 24-well chambered coverslip (0.1 mm thickness; MatTek) and were transfected with the expression plasmids for the GFP-fusion proteins. JH-III or ethanol was added to cell culture medium 2 d after transfection. Plasma membranes were stained with wheat germ agglutinin (WGA)-Alexa 594 (red), and nuclei were stained with DAPI (blue). All image recordings were performed under a 100× oil immersion objective on an LSM 510 confocal microscope (Carl Zeiss). Images were processed using Zen 2009 software (Carl Zeiss).
qRT-PCR.
Total RNA was extracted from Aag2 cells or mosquito tissues by using TRIzol reagent (Life Technology). A Maxima First Strand cDNA Synthesis Kit (Thermo Scientific) and an oligo(dT) primer were used for cDNA synthesis. qPCR was carried out by using the GoTaq qPCR Master Mix (Promega) on an ABI 7300 system (Applied Biosystems) according to the manufacturer's protocol. PCR reactions were performed in triplicate. Transcript abundance was normalized to that of RpS7. Primers for RT-PCR are listed in Table S2.
dsRNA-Induced Gene Silencing.
Synthesis of dsRNAs and microinjection were performed as described previously (49). Briefly, 0.5 μg dsRNA was injected into newly emerged female Ae. aegypti mosquitoes within 1 h PE. dsRNA for GFP was used as control. The injected mosquitoes were maintained in the insectary under normal conditions and were dissected 3–4 d after injection. mRNA extracted from the abdomen was examined by qRT-PCR.
For the cultured Aag2 cells, 3 µg of dsRNA was added directly to each well of a 12-well plate. Cells were collected 48 h after the addition of dsRNA. qRT-PCR was performed to determine the mRNA levels of the targeted genes.
Luciferase Reporter Assay.
pCMA, pCMA-GAL4, pCMA-GAD, pCMA-GBD, and UAS×4-188-cc-Luc were from Lucy Cherbas (Indiana University, Bloomington, IA) (50). The construction of pCMA-AaMET (amino acids 1–977), pCMA-AaTAI (amino acids 1–1,488), and 4×JHRE1-luc has been described previously (9, 12). The Renilla luciferase construct pRL-CMV (Promega) was included as an internal control to normalize for variations in transfection efficiency.
Transfection was performed as described by Li et al. (12). At 24 h after transfection, inhibitors and JH-III were added to the culture medium. The transfected cells were harvested 4 h later. Luciferase activity was measured using a Dual-Luciferase Reporter Assay System (Promega). Results were presented as the ratio of firefly to Renilla luciferase activity.
ChIP Assay.
Polyclonal antibodies for AaMET and AaTAI have been reported previously (11, 51). In vitro cultured abdomens were homogenized in PBS on ice, followed by the addition of formaldehyde to a final concentration of 1% and incubation at 37 °C for 10 min. ChIP assays were performed using a SimpleChIP Plus Enzymatic ChIP kit (Cell Signaling Technology) according to the manufacturer's instructions. Mock immunoprecipitations using preimmune sera for each antibody were included as negative controls. The precipitated DNA and DNA inputs were analyzed by using qRT-PCR. PCR primers are listed in Table S2.
EMSA.
Abdomens were collected from 200 adult female Ae. aegypti mosquitoes for each time point. Nuclear protein extraction and the EMSA experiments were carried out as described by Li et al. (9). The nucleotide sequence of the probe was 5′-CCATCCCACACGCGAAGACGATAAAACCA-3′ (AaET_JHRE1).
2D Gel Electrophoresis.
Proteins were extracted from the cultured mosquito tissues using lysis buffer [20 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, and 0.1 mM PMSF]. For the dephosphorylation experiment, the protein extracts from the JH-treated sample were incubated with lambda protein phosphatase (λPP) (New England Biolabs) at 2.5 U/µL for 1 h on ice. Proteins from each treatment were precipitated using ice-cold trichloroacetic acid/acetone and then were redissolved in rehydration buffer [7 M urea, 2 M thiourea, 2% (wt/vol) CHAPS, 0.5% ampholytes, and 20 mM DTT]. Equal amounts of proteins (300 µg) from each sample were loaded on an immobilized pH gradient (IPG) strip with a pH range of 3–10 (Life Technologies). Isoelectric focusing (IEF) was performed using the ZOOM IPGRunner System (Life Technologies) following the manufacturer's instructions. After IEF, IPG strips were equilibrated in a reducing solution [50 mM DTT, 2% (wt/vol) lithium dodecyl sulfate (LDS), and 140 mM Tris⋅HCl, pH 8.5] and an alkylating solution [125 mM iodoacetamide, 2% (wt/vol) LDS, and 140 mM Tris⋅HCl, pH 8.5] for 12 min each in succession. The equilibrated strips were placed on top of the vertical 4–12% Bis-Tris ZOOM gel (Life Technologies) to perform 2D SDS/PAGE. After electrophoresis, proteins were transferred to PVDF membranes for probing with antibodies against AaMET and AaTAI.
Supplementary Material
Acknowledgments
We thank Dr. Lucy Cherbas (Indiana University Bloomington) for providing the pCMA-GAL4 and UAS×4-188-cc-Luc plasmids. This work was supported by National Institutes of Health Grant R01 AI099250 (to J.Z.) and in part by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, US Department of Agriculture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423204112/-/DCSupplemental.
References
- 1.Tobe SS, Stay B. Structure and regulation of the corpus allatum. In: Berridge MJ, editor. Advances in Insect Physiology. Vol 18. Academic; London: 1985. pp. 305–432. [Google Scholar]
- 2.Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27(10):999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 3.Goodman W, Cusson M. In: The Juvenile Hormones. Insect Endocrinology. Gilbert L, editor. Elsevier; Amsterdam, The Netherlands: 2012. pp. 310–365. [Google Scholar]
- 4.Nijhout HF. Insect Hormones. Princeton Univ Press; Princeton: 1994. [Google Scholar]
- 5.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 6.Ashok M, Turner C, Wilson TG. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA. 1998;95(6):2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles JP, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108(52):21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene -tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 2005;272(5):1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA. 2011;108(2):638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103(7):1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Chen L, Sun G, Raikhel AS. The competence factor beta Ftz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol Cell Biol. 2006;26(24):9402–9412. doi: 10.1128/MCB.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, et al. A steroid receptor coactivator acts as the DNA-binding partner of the methoprene-tolerant protein in regulating juvenile hormone response genes. Mol Cell Endocrinol. 2014;394(1-2):47–58. doi: 10.1016/j.mce.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayukawa T, et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. 2012;109(29):11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayukawa T, Tateishi K, Shinoda T. Establishment of a versatile cell line for juvenile hormone signaling analysis in Tribolium castaneum. Sci Rep. 2013;3:1570. doi: 10.1038/srep01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem. 2011;286(10):8437–8447. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozano J, Kayukawa T, Shinoda T, Belles X. A role for Taiman in insect metamorphosis. PLoS Genet. 2014;10(10):e1004769. doi: 10.1371/journal.pgen.1004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davey KG. The modes of action of juvenile hormones: Some questions we ought to ask. Insect Biochem Mol Biol. 2000;30(8-9):663–669. doi: 10.1016/s0965-1748(00)00037-0. [DOI] [PubMed] [Google Scholar]
- 18.Pszczolkowski MA, Olson E, Rhine C, Ramaswamy SB. Role for calcium in the development of ovarial patency in Heliothis virescens. J Insect Physiol. 2008;54(2):358–366. doi: 10.1016/j.jinsphys.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Pszczolkowski MA, Peterson A, Srinivasan A, Ramaswamy SB. Pharmacological analysis of ovarial patency in Heliothis virescens. J Insect Physiol. 2005;51(4):445–453. doi: 10.1016/j.jinsphys.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Davey KG. Hormonal control of the follicular epithelium during vitellogenin uptake. Invertebr Reprod Dev. 1996;30(1–3):249–254. [Google Scholar]
- 21.Ilenchuk TT, Davey KG. The binding of juvenile hormone to membranes of follicle cells in the insect Rhodnius prolixus. Can J Biochem Cell Biol. 1985;63(2):102–106. [Google Scholar]
- 22.Ilenchuk TT, Davey KG. Effects of various compounds on Na/K-ATPase activity, JH-I binding capacity and patency response in follicles of Rhodnius prolixus. Insect Biochem. 1987;17(7):1085–1088. [Google Scholar]
- 23.Sevala VL, Davey KG. Action of juvenile hormone on the follicle cells of Rhodnius prolixus - evidence for a novel regulatory mechanism involving protein kinase C. Experientia. 1989;45(4):355–356. [Google Scholar]
- 24.Yamamoto K, Chadarevian A, Pellegrini M. Juvenile hormone action mediated in male accessory glands of Drosophila by calcium and kinase C. Science. 1988;239(4842):916–919. doi: 10.1126/science.3124270. [DOI] [PubMed] [Google Scholar]
- 25.Wilson TG, DeMoor S, Lei J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant(27) mutant phenotype. Insect Biochem Mol Biol. 2003;33(12):1167–1175. doi: 10.1016/j.ibmb.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Cui Y, Sui Y, Xu J, Zhu F, Palli SR. Juvenile hormone regulates Aedes aegypti Krüppel homolog 1 through a conserved E box motif. Insect Biochem Mol Biol. 2014;52:23–32. doi: 10.1016/j.ibmb.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro AB, et al. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J Insect Physiol. 1986;32(10):867–877. [Google Scholar]
- 28.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3(3):175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- 29.Zou Z, et al. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci USA. 2013;110(24):E2173–E2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler DE, Nijhout HF. A perspective for understanding the modes of juvenile hormone action as a lipid signaling system. BioEssays. 2003;25(10):994–1001. doi: 10.1002/bies.10337. [DOI] [PubMed] [Google Scholar]
- 31.Sevala VL, Davey KG, Prestwich GD. Photoaffinity-labeling and characterization of a juvenile hormone binding protein in the membranes of follicle cells of Locusta migratoria. Insect Biochem Mol Biol. 1995;25(2):267–273. [Google Scholar]
- 32.Harshman LG, et al. Bioassays of compounds with potential juvenoid activity on Drosophila melanogaster: Juvenile hormone III, bisepoxide juvenile hormone III and methyl farnesoates. J Insect Physiol. 2010;56(10):1465–1470. doi: 10.1016/j.jinsphys.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones D, Jones G. Farnesoid secretions of dipteran ring glands: What we do know and what we can know. Insect Biochem Mol Biol. 2007;37(8):771–798. doi: 10.1016/j.ibmb.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Lan Q, Fallon AM. Small heat shock proteins distinguish between two mosquito species and confirm identity of their cell lines. Am J Trop Med Hyg. 1990;43(6):669–676. doi: 10.4269/ajtmh.1990.43.669. [DOI] [PubMed] [Google Scholar]
- 35.Riddiford LM, Curtis AT, Kiguchi K. Culture of the epidermis of the tobacco hornworm Manduca sexta. Tissue Culture Association Manual. 1979;5:975–985. [Google Scholar]
- 36.Cai MJ, et al. Juvenile hormone prevents 20-hydroxyecdysone-induced metamorphosis by regulating the phosphorylation of a newly identified broad protein. J Biol Chem. 2014;289(38):26630–26641. doi: 10.1074/jbc.M114.581876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wendler A, et al. Position paper: Rapid responses to steroids: Current status and future prospects. Eur J Endocrinol. 2010;162(5):825–830. doi: 10.1530/EJE-09-1072. [DOI] [PubMed] [Google Scholar]
- 38.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 40.Xue Y, et al. GPS 2.1: Enhanced prediction of kinase-specific phosphorylation sites with an algorithm of motif length selection. Protein Eng Des Sel. 2011;24(3):255–260. doi: 10.1093/protein/gzq094. [DOI] [PubMed] [Google Scholar]
- 41.Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and Cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc Natl Acad Sci USA. 2012;109(41):16576–16581. doi: 10.1073/pnas.1214209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang S, Wang J, Sun Y, Song Q, Li S. PKC-mediated USP phosphorylation at Ser35 modulates 20-hydroxyecdysone signaling in Drosophila. J Proteome Res. 2012;11(12):6187–6196. doi: 10.1021/pr3008804. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Cai MJ, Zheng CC, Wang JX, Zhao XF. Phospholipase Cγ1 connects the cell membrane pathway to the nuclear receptor pathway in insect steroid hormone signaling. J Biol Chem. 2014;289(19):13026–13041. doi: 10.1074/jbc.M113.547018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peleg J. Growth of arboviruses in monolayers from subcultured mosquito embryo cells. Virology. 1968;35(4):617–619. doi: 10.1016/0042-6822(68)90293-6. [DOI] [PubMed] [Google Scholar]
- 45.Hagedorn HH, et al. Postemergence growth of the ovarian follicles of Aedes aegypti. J Insect Physiol. 1977;23(2):203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- 46.Deitsch KW, Chen JS, Raikhel AS. Indirect control of yolk protein genes by 20-hydroxyecdysone in the fat body of the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1995;25(4):449–454. doi: 10.1016/0965-1748(94)00082-a. [DOI] [PubMed] [Google Scholar]
- 47.Raikhel AS, Deitsch KW, Sappington TW. Culture and analysis of the insect fat body. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman & Hall; London: 1997. pp. 507–522. [Google Scholar]
- 48.Bollag WB, Griner RD. Measurement of cellular diacylglycerol content. Methods Mol Biol. 1998;105:89–98. doi: 10.1385/0-89603-491-7:89. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J, Chen L, Raikhel AS. Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2003;100(23):13338–13343. doi: 10.1073/pnas.2234416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu X, Cherbas L, Cherbas P. Transcription activation by the ecdysone receptor (EcR/USP): Identification of activation functions. Mol Endocrinol. 2003;17(4):716–731. doi: 10.1210/me.2002-0287. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Busche JM, Zhang X. Identification of juvenile hormone target genes in the adult female mosquitoes. Insect Biochem Mol Biol. 2010;40(1):23–29. doi: 10.1016/j.ibmb.2009.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.