Significance
Sleep in fruit flies shares all the essential features of mammalian sleep. Here, by using in vivo calcium imaging, we show for the first time, to our knowledge, that neuronal activity and reactivity decline during sleep and increase during wake simultaneously in many cells of the fly brain. Furthermore, we show that long wake reduces baseline and evoked neural activity and causes neurons to respond inconsistently to stimuli. The latter finding is reminiscent of the phenomenon of “local sleep in wake” described in rats, in which single cortical neurons unpredictably go “offline” during extended wake, leading to performance errors. Thus, these findings open the way to use Drosophila to study the molecular mechanisms underlying the cognitive deficits caused by sleep loss.
Keywords: Kenyon cells, sleep deprivation, mushroom bodies, GCaMP5
Abstract
Sleep in Drosophila shares many features with mammalian sleep, but it remains unknown whether spontaneous and evoked activity of individual neurons change with the sleep/wake cycle in flies as they do in mammals. Here we used calcium imaging to assess how the Kenyon cells in the fly mushroom bodies change their activity and reactivity to stimuli during sleep, wake, and after short or long sleep deprivation. As before, sleep was defined as a period of immobility of >5 min associated with a reduced behavioral response to a stimulus. We found that calcium levels in Kenyon cells decline when flies fall asleep and increase when they wake up. Moreover, calcium transients in response to two different stimuli are larger in awake flies than in sleeping flies. The activity of Kenyon cells is also affected by sleep/wake history: in awake flies, more cells are spontaneously active and responding to stimuli if the last several hours (5–8 h) before imaging were spent awake rather than asleep. By contrast, long wake (≥29 h) reduces both baseline and evoked neural activity and decreases the ability of neurons to respond consistently to the same repeated stimulus. The latter finding may underlie some of the negative effects of sleep deprivation on cognitive performance and is consistent with the occurrence of local sleep during wake as described in behaving rats. Thus, calcium imaging uncovers new similarities between fly and mammalian sleep: fly neurons are more active and reactive in wake than in sleep, and their activity tracks sleep/wake history.
The fundamental features that characterize mammalian sleep also define Drosophila melanogaster sleep (1–3). Most crucially, in both flies and mammals, sleep is distinguished from simple rest (quiet wake) by an increased arousal threshold, i.e., a reduced ability to respond to external stimuli. Moreover, in flies and mammals sleep is controlled homeostatically by the duration as well by the intensity of prior wake, suggesting basic similarities in the mechanisms of sleep regulation across species. Thus, in flies both sleep deprivation and a rich learning experience lead to a sleep rebound characterized by overall increased sleep time, increased arousal threshold, and longer sleep episodes (4–6). As in mammals, overall neuronal activity in flies is also high during wake and low during sleep (6–8). Specifically, a seminal study using local field potential (LFP) recordings from the Drosophila medial protocerebrum found high spike-like potentials that disappeared after the block of synaptic transmission in the mushroom bodies (MBs) (7). This high spike activity was present when flies were moving or had been quiescent for only a few seconds, but disappeared with sleep (i.e., after periods of immobility >5 min), when the overall LFP power in all frequencies also decreased by ∼60% (7). The study concluded that neural activity in the sleeping fly brain, or at least in a central region spanning the MBs, resembles that seen in mammals in several brainstem cell groups including noradrenergic neurons, whose firing strongly declines or stops completely during sleep (9). A more recent study in tethered flies able to walk on an air-suspended ball also found that periods of immobility >5 min are associated with increased arousal thresholds and with “flat” LFPs (6). LFPs, however, reflect the activity of thousands of cells (10), and the use of modified stereotrodes to resolve single-unit activity in flies is still in its infancy (11). Glass or tungsten microelectrodes, on the other hand, have recorded one neuron at a time in flies (e.g., refs. 12–14) and, to our knowledge, have not measured changes across sleep and wake.
Our goal was to study many fly neurons simultaneously while preserving single-cell resolution, to determine how sleep and wake affect spontaneous activity and the ability of neurons to react to stimuli. It was recently shown in rats that during sleep deprivation single cortical neurons may go unpredictably “offline,” as they normally do during sleep, with negative effects on performance (15). Thus, we also asked whether this phenomenon occurs in flies. We focused on the MBs, large areas of the Drosophila brain involved in olfactory learning (16, 17) and sleep regulation (18, 19), and used in vivo calcium imaging (20, 21) to measure spontaneous and evoked neuronal activity in the MB principal neurons, the Kenyon cells, during sleep, wake, and in response to different periods of sleep deprivation.
Results
Sleep and Wake States During Calcium Imaging.
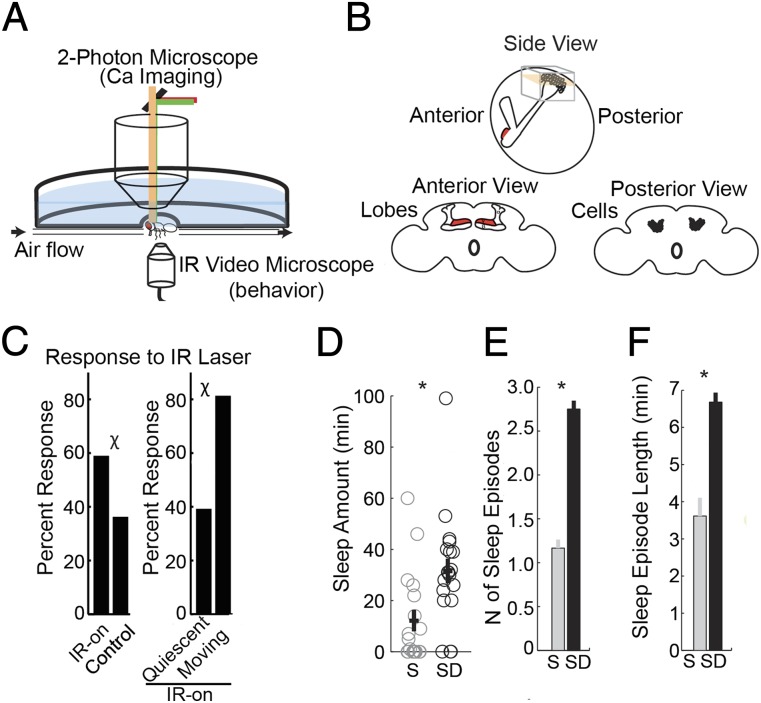
We expressed the genetically encoded calcium sensor GCaMP5 (22) in the Kenyon cells. First, we measured changes in GCaMP5 fluorescence across the sleep/wake cycle within the same fly. Before imaging, flies were allowed to sleep for 5 h during the first part of the 12-h dark period, when they normally sleep, or were sleep deprived to increase their chance to sleep under the microscope. Flies allowed to sleep were awake only 23 ± 1.7% (mean ± SE) of the 5 h before imaging, whereas sleep-deprived flies were awake 99 ± 0.0% of the time. Imaging occurred at the same circadian time in all flies. Under the two-photon microscope, flies were placed in a holder that fixed the head in place while legs and abdomen remained free to move and were recorded using an infrared (IR) digital microscope (Fig. 1A; details in Materials and Methods). MB cells expressing GCaMP5 are located in the periphery on the posterior side of the head (Fig. 1B). Imaging took place every 10 min over a 2-h period, with each stack of images taken at 0.7-µm intervals throughout the MB region over a 67-s time period.
Fig. 1.
Identification of sleep and wake states during calcium imaging. (A) Experimental setup. The fly is positioned between the two-photon microscope and the infrared digital microscope, with the head attached to a chamber holding oxygenated saline solution (blue). Airflow passes through a hose ending in front of the head while a vacuum in the back expels the airflow. (B) Diagram detailing MB position in the fly brain. Cell bodies and calyx region (gray box) were imaged from the back of the head either as a stack of images throughout the entire cell body and calyx region (gray box) or as a time series from a single plane (pink). (C) Probability of movement in response to the switching on of the laser light vs. control stimulation (Left) with laser light on in previously moving flies vs. previously quiescent flies (Right). Laser power was set at 100%, and the intensity was used to assess GCaMP5 fluorescence. χ = P < 0.05 between groups (Pearson’s χ2 test). (D–F) Quantity and quality of sleep during testing in flies that either slept (S) or were sleep-deprived (SD) before testing. *P < 0.05 between sleep and sleep-deprived groups (Mann–Whitney test). Number of flies = 17 (sleeping) and 18 (awake).
Sleep in Drosophila is defined as any period of immobility of more than 5 min, because in many experimental conditions, including in tethered flies (6), a quiescent period of that length is associated with a stable increase in arousal threshold. According to this definition, the first minute counted as sleep corresponds to the sixth minute of inactivity, because in previous experiments we found that arousal thresholds progressively increase during the first 5 min of quiescence (23). This period is therefore viewed as a transitional stage and counted as wake. Under the two-photon microscope, we used legs and abdomen movements to identify putative periods of sleep (>5 min of immobility). Thus, a putatively sleeping fly had to show no movements for more than 5 min before and during imaging, whereas a putatively awake fly had to move at least once during that time. The absence of movements alone, however, is not sufficient to define sleep unless associated with an increased arousal threshold. Thus, we measured whether putatively sleeping flies were less able to respond to a stimulus, namely the switching on of the IR laser light when imaging started. In the holder under the microscope, awake flies are not continuously in motion but tend to move in intense bouts of activity of 1–2 min over a 5-min period. We found that switching the laser on often triggered movements or, less frequently, caused the movements already present to become more intense. Specifically, across all imaging sessions, flies moved in 59% of cases (n = 191 sessions) when the laser light went on, but only in 36.3% of cases (n = 160) during control periods when the laser light was off, which were selected to occur half way (at the fifth min) between two stimulation periods (χ2 test, P = 2.31E-5) (Fig. 1C, Left). More importantly, awake flies responded more frequently to IR stimulation than those putatively asleep. Specifically, in flies that were already moving before imaging, movements occurred in 81.3% of the episodes (n = 101) when the laser was switched on (Fig. 1C, Right). By contrast, flies that were putatively asleep (quiescent) before imaging moved only 39.2% of the time (n = 90), and the difference in the response rate between the two groups was greater than chance (χ2 test, P = 4.00E-9) (Fig. 1C, Right). Thus, the IR laser light has a stimulatory effect on the great majority of awake flies, whereas in most cases (60.8%) flies presumed asleep did not move in response to the laser. We conclude that the arousal threshold is increased in the state that we previously called putative sleep, and from now on we will refer to that state as “sleep.”
Another essential feature of sleep is its homeostatic regulation, i.e., quality and quantity of sleep increase after sleep deprivation. Consistent with our conclusion that >5 min of quiescence qualifies as sleep in our experimental conditions, we found that during the 2-h imaging session flies that had previously been sleep-deprived spent more time asleep (Fig. 1D) and had more (Fig. 1E) and longer sleep episodes (Fig. 1F) than flies that slept before imaging. Moreover, quiescent flies that had previously been sleep-deprived for 29–34 h had a lower response rate to IR stimulation than quiescent flies that had previously been sleep-deprived for only 5–8 h (response rate: 13% ± 7.2 vs. 32% ± 9.1; χ2 test, P < 0.015; n of flies sleep-deprived (SD) 5–8 = 22, SD 29–34 = 21; Fig. S1B). Spontaneous motor activity during control periods with laser off (2 min before the laser was switched on) did not differ between the two SD groups (χ2 test, P = 0.2).
The mechanism underlying the arousal response due to the laser is unknown, but thermal stimulation appears a more likely candidate relative to acoustic or visual stimulation. Indeed, we found that the response rate increases with increasing laser power (Fig. S1A), whereas the noise produced during imaging by the galvo mirror system and shutters does not change with different laser intensities. Moreover, D. melanogaster cannot sense IR wavelengths directly, and insects that sense IR do so through an organ that uses thermoreception (24). By using a no-contact thermometer centered below the fly, we found that temperature increased by 0.18 ± 0.01 °C during stimulation (baseline = 22.16 ± 0.047 °C; during stimulation = 22.34 ± 0.046 °C, Mann–Whitney test P = 0.011, three flies, each fly tested five times) (Fig. S1C). The quick rise in temperature is consistent with the fact that the response to the laser is quick. Indeed, by dividing the stimulation period into 5-s epochs and scoring each epoch for activity we found that most responding flies (81.5%) moved within the first 5 s after the laser was turned on (14 awake flies, 79 trials; 100% laser power tested every 15 min for 60 s). Finally, Trp1A1 null flies, which have an impaired response to temperature gradients (25, 26), failed to show a significant difference in response rate when the laser was switched on relative to when it was off (Fig. S1D).
The laser-induced increase in temperature that we measured is small, but was detected across a region that included both head and body. It is likely that the temperature increased more over the head region, where the laser was focused and heat-sensitive neurons are located (26). Moreover, at least in larvae, Trp1A1 is necessary to show preference even within a narrow temperature range, 18 °C over 19 °C (25). Thus, although not conclusive, these results are consistent with heat being the most likely candidate to mediate the arousal response to the laser stimulation.
Calcium Levels in Individual MB Cells Change When Flies Fall Asleep and Wake Up.
Having established that flies can sleep under the two-photon microscope, calcium levels were measured at the wake-to-sleep and sleep-to-wake transitions. We focused on consolidated bouts of sleep and wake (>5 min) to measure changes starting from a stable baseline and used flies previously sleep-deprived for 8 h to increase their chance of sleep. As shown in Fig. 2A, individual brightly fluorescing cells could easily be identified in the stacked images and were defined as those neurons whose fluorescence intensity exceeded background fluorescence by at least two times. These bright cells were likely responding to environmental conditions such as the constant oxygen-enriched flow passed through the chamber during the 2-h imaging session. GCaMP5 fluorescence decreased in these cells at the wake-to-sleep transition. Specifically, a significant decline in GCaMP5 signal was seen between the last 5 min of wake relative to the first 10 min of sleep, as well as between the last 5 min of wake relative to the end of the sleep period (Fig. 2B). By contrast, GCaMP5 fluorescence increased in these cells at the sleep-to-wake transition (Fig. 2C). Of note, no changes in ΔF/F signal occurred from the beginning to the end of a 10-min period of either continuous sleep (Wilcoxon signed-rank test, P = 0.471) or wake (P = 0.082), demonstrating that the changes in calcium levels in these cells reflect changes in behavioral state.
Fig. 2.
Sleep/wake changes in calcium levels during baseline. (A) Representative images showing changes in GCaMP5 fluorescence across sleep and wake. Mean intensity levels are shown over the cell body region (Reg.) and in two individual cells. Images show the sum intensity from multiple layers primarily containing cell bodies. Individual cell bodies can be seen fluorescing above background level (arrows point to two individual cell bodies). Time line spanning 1 h of the 2-h protocol shows wake (green) and sleep (red) periods. (B) Decline in GCaMP5 fluorescence in single cells at the wake-to-sleep transition. The change in fluorescence [ΔF/mean(F)] was calculated by subtracting the mean fluorescence of the last 5 min of wake from either the mean fluorescence of the first 10 min of sleep (+10) or the mean fluorescence of the entire sleep bout (end), divided by the mean fluorescence of both wake and sleep periods (13 flies, 257 cells, 23 sleep episodes). (C) Increase in GCaMP5 fluorescence in single cells at the sleep-to-wake transition. The change in fluorescence [ΔF/mean(F)] was calculated by subtracting the mean fluorescence of the last 5 min of sleep from either the mean fluorescence of the first 10 min of wake (+10) or the mean fluorescence of the entire wake bout (end), divided by the mean fluorescence of both wake and sleep periods (15 flies, 267 cells, 26 wake episodes). In B and C, #P < 0.05 (Wilcoxon signed-rank test).
Sleep/Wake Changes in Calcium Levels in Response to Two Different Stimuli.
We then studied how sleep and wake affect the neuronal response evoked by acute exposure to oxygen and then to an oxygen–vinegar mix (Fig. S2). These stimuli were selected to test responsiveness to a weak stimulus (oxygen) and a strong stimulus (vinegar). The chances of measuring the response to two different stimuli in the same fly during both sleep and wake are low. Thus, we used two independent experimental groups, comparing the evoked response between flies that remained awake or slept from the start of the imaging session to when the stimuli were delivered. Analysis of single cells was done using stacked images acquired as in the previous experiment, but the testing period was shortened to 14 min to increase the number of flies that could be tested. In the stacks, cells were defined as “responders” if their fluorescence in response to oxygen or vinegar increased by at least 50% relative to baseline. The number of individual cells responding to oxygen and vinegar was larger in awake flies than in sleeping flies (Fig. 3A). Moreover, for both stimuli, the mean increase in fluorescence in each responding cell was larger in awake flies relative to sleeping flies (Fig. 3B). When the entire region of cell bodies was considered, the average response was also larger during wake than during sleep (Fig. 3C). In fact, sleeping flies showed no response to oxygen (Wilcoxon signed-rank test, P = 0.192), in contrast to awake flies. In response to vinegar instead, sleeping flies also showed a response (Wilcoxon signed-rank test P < 0.05), but smaller than that of awake flies (Fig. 3C). The overall weaker response to oxygen relative to vinegar is expected because prior studies have shown that there are no oxygen-specific sensors in the antennal lobe and the response to oxygen results from either the airflow itself (27) or a decrease in carbon dioxide concentration (28–30). Finally, we also measured the response to oxygen and vinegar using a time series optimized for rapid imaging of a single plane spanning the cell body region and the calyx (see Materials and Methods for details). The peak regional ΔF/F response to both stimuli tended to be larger in awake flies than in sleeping flies (Fig. 3D). In the body region, the latency to the peak response to vinegar was also faster in awake animals (14 ± 1.5 vs. 9 ± 1 s, P = 0.04), resulting in a significantly larger ΔF/F at the beginning of the stimulation in awake animals compared with sleeping animals (Fig. 3D, green circles). Fluorescence decreased at the end of the time series in the absence of stimulation, but this decline did not occur in the calyx or in the presence of stimuli, suggesting that it is unlikely due to photobleaching. Overall, these results using two different stimuli find that sleep is associated with reduced reactivity of Kenyon cells.
Fig. 3.
Evoked calcium responses during sleep and wake. (A) Number of responding cells (ΔF/F > 50%) to the first exposure to oxygen and vinegar. The number of flies tested were sleep = 18 and wake = 42. (B) ΔF/F in individual cells responding to oxygen and vinegar. The number of cell bodies tested were 147:654 (sleep:wake) during oxygen and 171:756 during vinegar. (C) ΔF/F in the cell body region. #P < 0.05 relative to no response (Wilcoxon signed-rank test). In A–C, *P < 0.05 between sleep and wake groups (Mann–Whitney test). (D) Mean change in fluorescence (ΔF/F) during the time series. The odor sequence is as follows: no airflow (light gray), oxygen-enriched airflow (gray), vinegar (black), oxygen-enriched airflow (gray), and no airflow (light gray). Wake points are circled when Mann–Whitney P values are <0.05 compared with sleep. Dotted line indicates SE.
Effects of Sleep/Wake History on Calcium Levels and Response to Stimuli.
In mammals, overall levels of neuronal activity depend on the behavioral state and also, albeit to a smaller extent, on sleep/wake history. In rats, for example, average firing rates in each behavioral state (sleep or wake) are higher or lower depending on whether the previous several hours were spent awake or asleep, respectively (31). Thus, we asked whether a previous history of sleep, short wake or long wake, affects calcium levels. For this analysis we used only flies that were awake during the entire imaging protocol, subdivided into three groups—sleep, short wake (5–8 h), and long wake (29–34 h)—based on their sleep/wake behavior in the previous 5–34 h (see Materials and Methods for details).
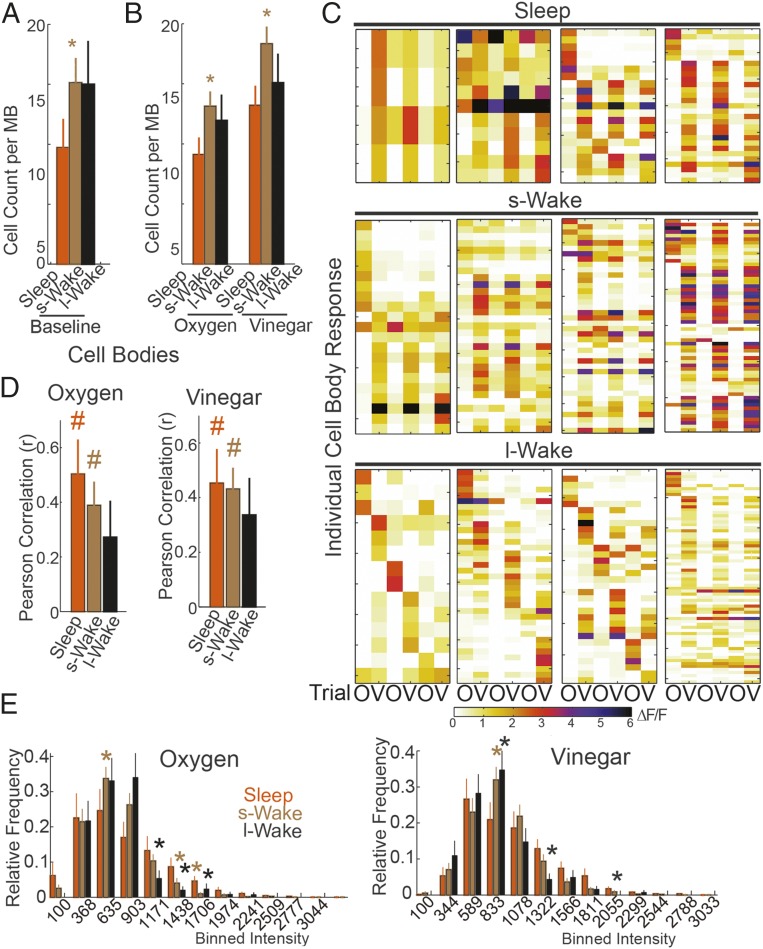
Analysis of stack images found that, compared with flies that slept before testing, flies previously awake for 5–8 h showed a larger number of bright cells during baseline, i.e., before any odor was presented (Fig. 4A), and more activated cells in response to both oxygen and vinegar (Fig. 4B). By contrast, flies previously awake for many hours (long-wake group) did not differ from flies previously asleep, neither in the number of bright cells at baseline nor in the number of cells responding to odors, in both cases due to increased variability (Fig. 4 A and B). Overall fluorescent intensity in the calyx or cell body region was not affected by the sleep/wake history, neither in the time series nor in stack images, although a trend was present, consistent with the changes seen in single cells (short wake > long wake > sleep). Overall, these results suggest that, as in rodents, the effects of sleep/wake history on neuronal activity are subtle and thus can be seen when measuring calcium levels in the few single cells that are active at baseline, but not when averaging GCaMP5 fluorescence across the entire region, where most cells are not active.
Fig. 4.
Effects of previous sleep or wake on baseline calcium levels and response to stimuli. (A) Number of spontaneously active cells during baseline. (B) Number of cells that responded to oxygen or vinegar throughout the three trials (at least in one of the three trials; stacked images). In A and B, the number of flies tested were sleep = 22, short-wake = 31, long-wake = 17. *P < 0.05 relative to sleep (post hoc Kruskal–Wallis test followed by Mann–Whitney test). All flies included were awake when tested. (C) Examples of cell response profiles from single flies that were asleep or awake for a short or long time before testing. Each row is a single cell, and columns indicate the ΔF/F response during the three trials for oxygen (O) and vinegar (V). Note that, because the number of responding cells varies across flies, the size of the panels and the width of the rows is not the same across flies. (D) Mean Pearson correlation (r) comparing the activation pattern in flies across trials (1–2, 2–3, 1–3) in response to oxygen (Left) and vinegar (Right). All flies were awake during testing and divided based on prior behavior: sleep, short wake, long wake. An r score of 1 would indicate a perfectly matched pattern. A “#” indicates that median values significantly differ from no correlation (Wilcoxon signed-rank test, P < 0.05). (E) Frequency plots describing the range in mean fluorescent response for each cell. Responses were subdivided based on their intensity into bins of 268 relative units, and the rate (number of responses/total number of cells) within each bin is plotted for the three groups. For each bin, an asterisk indicates a significant difference from the sleep group (Kruskal–Wallis test followed by Mann–Whitney test, P < 0.05). Note that the frequency of high-intensity responses is also reduced after short wake relative to sleep, but the overall increased number of responses compensates for this effect.
We then studied the effects of sleep/wake history on the response to repeated stimulation. Image stacks were taken during exposure to oxygen followed by oxygen–vinegar, and this sequence was repeated three times to assess the consistency of the evoked responses. Cells that responded to oxygen and/or oxygen–vinegar (>50% ΔF/F) during any of the three trials were identified, and the correlation across the activation patterns of all trials was compared. Fig. 4C shows raw data for representative experiments: each of the 12 panels refers to one fly, with each row representing the response of one individual cell to the three trials for oxygen and for oxygen–vinegar (OVOVOV). Across individual flies, the responding patterns across trials correlated significantly after sleep and after short wake (Wilcoxon signed-rank test, P < 0.05) (Fig. 4D). After long wake, however, activation patterns were no longer correlated (Fig. 4D) due to a reduced number of high-intensity responses compared with sleep (Fig. 4E). As shown in Fig. 4C, this decline could occur in any of the trials, not necessarily in the last one. Indeed, the lack of correlation after long wake was also present when trials 1–2, 2–3, and 1–3 were considered separately, and mean raw intensity for the responders did not decrease from the first to the last trial, indicating that habituation does not account for this result.
Discussion
Several groups have recorded GCaMP fluorescence from the MBs in tethered flies (27, 32–35), but none of the previous studies tested the effects of sleep and wake. To do so, it was first necessary to prove that flies can indeed sleep during calcium imaging. We show here that, when flies are immobile under the two-photon microscope for >5 min, their ability to behaviorally respond to a stimulus—e.g., switching on the laser light—is reduced. We call this state of reduced arousal threshold “sleep,” applying the same criteria used in previous studies that found that tethered flies, when asleep, have reduced neuronal activity (6, 7). We also show, again consistent with previous results (6), that sleep as defined in the current study is homeostatically regulated by the duration of previous wake. Thus, we conclude that the state of quiescence as defined in our experimental setup qualifies as sleep and not simply as rest.
State-Dependent Effects.
The most stringent condition under which to study the effects of sleep and wake on calcium levels is to compare GCaMP5 fluorescence within the same fly while the animal spontaneously transitions from wake to sleep and back in the absence of acute stimulation. By doing so, we found that calcium levels in individual MB cells increased when the fly woke up and declined when it fell asleep, consistent with a decrease in spontaneous activity during sleep relative to wake as reported using LFP recordings (6, 7) (Fig. 2). In addition to spontaneous activity, evoked activity in response to oxygen and vinegar was also higher in wake than in sleep. Both the number of MB cells responding to these stimuli and their mean response were greater in awake flies relative to sleeping flies (Fig. 3). A decline in the electrical response of neurons to different kinds of stimuli is a classical marker of sleep, documented in mammals (e.g., ref. 36) as well as in bees (37). Thus, we were able with calcium imaging to detect sleep/wake-dependent changes in both spontaneous and evoked activity of Kenyon cells.
In the mammalian cortex, most of sleep [non-rapid eye movement (NREM) sleep] is associated with a decrease in glutamate and acetylcholine levels, an increase in GABA levels (38), and an overall decrease in the level of activity of arousal promoting neuromodulatory systems such as the noradrenergic, orexinergic, and dopaminergic systems (39, 40). All these factors are likely to contribute to the decline in neuronal activity and excitability during sleep. Similarly, it is likely that several mechanisms exist to make Kenyon cells more active and more responsive to stimuli during wake than during sleep. The neurons projecting from the antennal lobe to the calyx are cholinergic. In addition to acetycholine, receptors for GABA, glutamate, octopamine, and dopamine are also expressed in the MBs (41). Direct evidence for sleep/wake changes in the brain levels of any of these neurotransmitters is missing in flies. However, both dopamine (42, 43) and octopamine (44, 45) promote arousal, although they do so by affecting brain regions other than the MBs (43–45). Moreover, both dopamine and octopamine modulate calcium and cAMP levels (46) in response to cholinergic inputs and play an important role in modulating MB neuronal activity during associative olfactory learning (47–50), suggesting that they could affect the excitability of Kenyon cells. Also, suppressing GABA activity reduces sleep time in flies, and mutations in the GABAA receptor confer resistance to GABA antagonists that promote wakefulness (51). As for dopamine and octopamine, the sleep-promoting effect of GABAA receptors is mediated outside the MBs by the pigment-dispersing factor neurons (52). However, GABAergic inputs through the anterior paired lateral neurons modulate the odor-specific activation pattern in the MBs (53). Reducing GABAA receptor expression increases the number of cells included in the pattern, and suppressing GABAB expression increases calcium influx in the responding cells. Thus, an increase in GABAergic transmission mediated by both GABAA and GABAB receptors could explain why sleep is associated with a decrease both in the number of responders and in the extent of their response to stimuli.
History-Dependent Effects.
Analysis of stack images found that, compared with flies that slept before testing, flies previously awake for 5–8 h showed more bright cell bodies during baseline, i.e., before any odor was presented (Fig. 4A), and more activated cells in response to both oxygen and vinegar (Fig. 4B). This result is consistent with the finding in mammals that spontaneous neuronal activity increases with time spent awake and decreases after sleep (31).
We found that, after long wake, Kenyon cells were no longer able to respond consistently to repeated exposure to the same stimulus. It is unlikely that this result is due to habituation because the correlation among activation patterns did not decline in the last two trials relative to the first two trials. Instead, the results are more in line with recent findings in mammals suggesting that sleep may not be as global as previously thought. For example, intracranial recordings in epileptic patients have shown that the slow waves and spindles of NREM sleep often occur in some cortical areas but not in others (54) and frequently involve only small groups of neurons (55, 56), a finding that was confirmed in rats (15). Recordings of evoked responses in the rat barrel cortex after whisker stimulation also suggest the possible occurrence of “local wake during sleep”: responses sometimes appear sleep-like in one cortical column but wake-like in another (57). Finally, by using intracortical multiarray recordings, we recently found that, when rats stay awake longer than usual to explore and learn, small groups of cortical neurons can go offline while the rest of the brain is awake (15), a phenomenon called “local sleep during wake.” Thus, the few Kenyon cells that unpredictably fail to respond to stimuli after long wake may reflect the occurrence of local sleep in awake flies. In rats, the occurrence of local sleep during wake leads to performance errors (15) and is likely to account for some of the cognitive deficits associated with sleep deprivation in humans (58). The MBs have a pivotal function in olfactory memory processing (59, 60), and sleep deprivation either before or after training (4, 61) impairs olfactory memory, whereas blocking neurotransmission in the MBs during extended wake before training can rescue memory impairments (61). Thus, it is possible that at least one of the mechanisms by which extended wake impairs olfactory memory involves the occurrence of local sleep in Kenyon cells.
Materials and Methods
Fly Strains, Husbandry, and Sleep/Wake Recordings Before Calcium Imaging.
Flies tested were generated by crossing Canton-S (CS) wild type (Barry Ganetzky, Laboratory of Genetics, University of Wisconsin-Madison) to w1118; UAS-GCaMP5.003; P{w[+mW.hs]=GawB}OK107. The P{w[+mW.hs]=GawB}OK107 (stock no. 854) transgene and TrpA11 mutant (stock no. 26504) were ordered from the Bloomington Drosophila Stock Center. The UAS-GCaMP5 transgene was provided by Vivek Jayaraman, Howard Hughes Medical Institute, Janelia Research Campus. Flies were reared at 20 °C on standard cornmeal molasses. Virgin females were harvested within 12 h after eclosure and placed in clear 5- × 65-mm tubes with standard cornmeal molasses food at one end and a cotton-sealed cap in the other. Behavior was monitored in Drosophila activity monitors (DAM5, Trikinetics) at 20 °C and 68% humidity. Sleep deprivation using a mechanical agitator was performed as previously described (23).
Supplementary Material
Acknowledgments
We thank Dr. Vivek Jayaraman (Howard Hughes Medical Institute, Janelia Research Campus) for providing the GCAMP5 stock and advice on fly preparation for calcium imaging and Dr. Glenn Turner (Cold Spring Harbor Laboratory) for advice on calcium imaging. This research was funded by National Institute of Mental Health Grant R01MH099231 (to C.C. and G.T.) and National Institute of Neurological Disorders and Stroke Grant P01NS083514 (to C.C. and G.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419603112/-/DCSupplemental.
References
- 1.Cirelli C. Searching for sleep mutants of Drosophila melanogaster. BioEssays. 2003;25(10):940–949. doi: 10.1002/bies.10333. [DOI] [PubMed] [Google Scholar]
- 2.Shaw P. Awakening to the behavioral analysis of sleep in Drosophila. J Biol Rhythms. 2003;18(1):4–11. doi: 10.1177/0748730402239672. [DOI] [PubMed] [Google Scholar]
- 3.Ho KS, Sehgal A. Drosophila melanogaster: An insect model for fundamental studies of sleep. Methods Enzymol. 2005;393:772–793. doi: 10.1016/S0076-6879(05)93041-3. [DOI] [PubMed] [Google Scholar]
- 4.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313(5794):1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 5.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: Structural evidence in Drosophila. Science. 2011;332(6037):1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Alphen B, Yap MH, Kirszenblat L, Kottler B, van Swinderen B. A dynamic deep sleep stage in Drosophila. J Neurosci. 2013;33(16):6917–6927. doi: 10.1523/JNEUROSCI.0061-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr Biol. 2002;12(22):1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 8.van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal states in Drosophila. Curr Biol. 2004;14(2):81–87. [PubMed] [Google Scholar]
- 9.Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. Plenum Press; New York: 1990. [Google Scholar]
- 10.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents: EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong C, et al. Multi-unit recording with iridium oxide modified stereotrodes in Drosophila melanogaster. J Neurosci Methods. 2014;222:218–229. doi: 10.1016/j.jneumeth.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303(5656):366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 13.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat Neurosci. 2010;13(3):393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 14.Chiappe ME, Jayaraman V. Performing electrophysiology and two-photon calcium imaging in the adult Drosophila central brain during walking behavior. In: Martin J-R, editor. Genetically Encoded Functional Indicators (Neuromethods) Vol 72. Springer; 2012. pp. 83–101. [Google Scholar]
- 15.Vyazovskiy VV, et al. Local sleep in awake rats. Nature. 2011;472(7344):443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4(4):266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 17.Berry J, Krause WC, Davis RL. Olfactory memory traces in Drosophila. Prog Brain Res. 2008;169:293–304. doi: 10.1016/S0079-6123(07)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441(7094):753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 19.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441(7094):757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 20.Tian L, Hires SA, Looger LL. 2012. Imaging neuronal activity with genetically encoded calcium indicators. Cold Spring Harb Protoc 2012 Jun(6):647–656.
- 21.Riemensperger T, Pech U, Dipt S, Fiala A. Optical calcium imaging in the nervous system of Drosophila melanogaster. Biochim Biophys Acta. 2012;1820(8):1169–1178. doi: 10.1016/j.bbagen.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Akerboom J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32(40):13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber R, et al. Sleep homeostasis in Drosophila melanogaster. Sleep. 2004;27(4):628–639. doi: 10.1093/sleep/27.4.628. [DOI] [PubMed] [Google Scholar]
- 24.Evans G. Infrared radiation sensors of Melanophila acuminata (Coleoptera: Buprestidae): A thermopneumatic model. Ann Entomol Soc Am. 2005;98(5):738–746. [Google Scholar]
- 25.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci. 2008;11(8):871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 26.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454(7201):217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamiya A, Beshel J, Xu C, Zhong Y. Neural representations of airflow in Drosophila mushroom body. PLoS ONE. 2008;3(12):e4063. doi: 10.1371/journal.pone.0004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott K. Out of thin air: Sensory detection of oxygen and carbon dioxide. Neuron. 2011;69(2):194–202. doi: 10.1016/j.neuron.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin HH, Chu LA, Fu TF, Dickson BJ, Chiang AS. Parallel neural pathways mediate CO2 avoidance responses in Drosophila. Science. 2013;340(6138):1338–1341. doi: 10.1126/science.1236693. [DOI] [PubMed] [Google Scholar]
- 30.Vermehren-Schmaedick A, Ainsley JA, Johnson WA, Davies SA, Morton DB. Behavioral responses to hypoxia in Drosophila larvae are mediated by atypical soluble guanylyl cyclases. Genetics. 2010;186(1):183–196. doi: 10.1534/genetics.110.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vyazovskiy VV, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24(29):6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honegger KS, Campbell RA, Turner GC. Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J Neurosci. 2011;31(33):11772–11785. doi: 10.1523/JNEUROSCI.1099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Li Y, Lei Z, Wang K, Guo A. Transformation of odor selectivity from projection neurons to single mushroom body neurons mapped with dual-color calcium imaging. Proc Natl Acad Sci USA. 2013;110(29):12084–12089. doi: 10.1073/pnas.1305857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin AC, Bygrave AM, de Calignon A, Lee T, Miesenböck G. Sparse, decorrelated odor coding in the mushroom body enhances learned odor discrimination. Nat Neurosci. 2014;17(4):559–568. doi: 10.1038/nn.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291(5816):554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser W, Steiner-Kaiser J. Neuronal correlates of sleep, wakefulness and arousal in a diurnal insect. Nature. 1983;301(5902):707–709. doi: 10.1038/301707a0. [DOI] [PubMed] [Google Scholar]
- 38.Vanini G, Lydic R, Baghdoyan HA. GABA-to-ACh ratio in basal forebrain and cerebral cortex varies significantly during sleep. Sleep. 2012;35(10):1325–1334. doi: 10.5665/sleep.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BE. From waking to sleeping: Neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26(11):578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cayre M, Buckingham SD, Yagodin S, Sattelle DB. Cultured insect mushroom body neurons express functional receptors for acetylcholine, GABA, glutamate, octopamine, and dopamine. J Neurophysiol. 1999;81(1):1–14. doi: 10.1152/jn.1999.81.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15(13):1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Lebestky T, et al. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64(4):522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crocker A, Sehgal A. Octopamine regulates sleep in Drosophila through protein kinase A-dependent mechanisms. J Neurosci. 2008;28(38):9377–9385. doi: 10.1523/JNEUROSCI.3072-08a.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65(5):670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomchik SM, Davis RL. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64(4):510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23(33):10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riemensperger T, Völler T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15(21):1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 49.Schroll C, et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 2006;16(17):1741–1747. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, et al. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488(7412):512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 51.Agosto J, et al. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11(3):354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parisky KM, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60(4):672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lei Z, Chen K, Li H, Liu H, Guo A. The GABA system regulates the sparse coding of odors in the mushroom bodies of Drosophila. Biochem Biophys Res Commun. 2013;436(1):35–40. doi: 10.1016/j.bbrc.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 54.Nobili L, et al. Dissociated wake-like and sleep-like electro-cortical activity during sleep. Neuroimage. 2011;58(2):612–619. doi: 10.1016/j.neuroimage.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 55.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrillon T, et al. Sleep spindles in humans: Insights from intracranial EEG and unit recordings. J Neurosci. 2011;31(49):17821–17834. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047(1):45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Hung CS, et al. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep. 2013;36(1):59–72. doi: 10.5665/sleep.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263(5147):692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 60.Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411(6836):476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Yu F, Guo A. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep. 2009;32(11):1417–1424. doi: 10.1093/sleep/32.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.