Abstract
The CO2 compensation point of the submersed aquatic macrophyte Hydrilla verticillata varied from high (above 50 microliters per liter) to low (10 to 25 microliters per liter) values, depending on the growth conditions. Plants from the lake in winter or after incubation in an 11 C/9-hour photoperiod had high values, whereas summer plants or those incubated in a 27 C/14-hour photoperiod had low values. The plants with low CO2 compensation points exhibited dark 14CO2 fixation rates that were up to 30% of the light fixation rates. This fixation reduced respiratory CO2 loss, but did not result in a net uptake of CO2 at night. The low compensation point plants also showed diurnal fluctuations in titratable acid, such as occur in Crassulacean acid metabolism plants. However, dark fixation and diurnal acid fluctuations were negligible in Hydrilla plants with high CO2 compensation points.
Exposure of the low compensation point plants to 20 micromolar 14CO2 resulted in 60% of the 14C being incorporated into malate and aspartate, with only 16% in sugar phosphates. At a high CO2 level, the C4 acid label was decreased. A pulse-chase study indicated that the 14C in malate, but not aspartate, decreased after a long (270-second) chase period; thus, the C4 acid turnover was much slower than in C4 plants.
Phosphoenolpyruvate carboxylase activity was high (330 micromoles per milligram chlorophyll per hour), as compared to ribulose bisphosphate carboxylase (20 to 25), in the plants with low compensation points. These plants also had a pyruvate, Pi dikinase activity in the leaves of 41 micromoles per milligram chlorophyll per hour, which suggests they are not C3 plants. NAD- and NADP+-malate dehydrogenase activities were 6136 and 24.5 micromoles per milligram chlorophyll per hour, respectively. Of the three decarboxylating enzymes assayed, the activities of NAD- and NADP+-malic enzyme were 104.2 and 23.7 micromoles per milligram chlorophyll per hour, while phosphoenolpyruvate carboxykinase was only 0.2.
Low compensation point Hydrilla plants fix some CO2 into C4 acids, which can be decarboxylated for later refixation, presumably into the Calvin cycle. Refixation would be advantageous in summer lake environments where the CO2 levels are high at night but low during the day. Hydrilla does not fit any of the present photosynthetic categories, and may have to be placed into a new group, together with other submersed aquatic macrophytes that have environmentally variable CO2 compensation points.
Full text
PDF

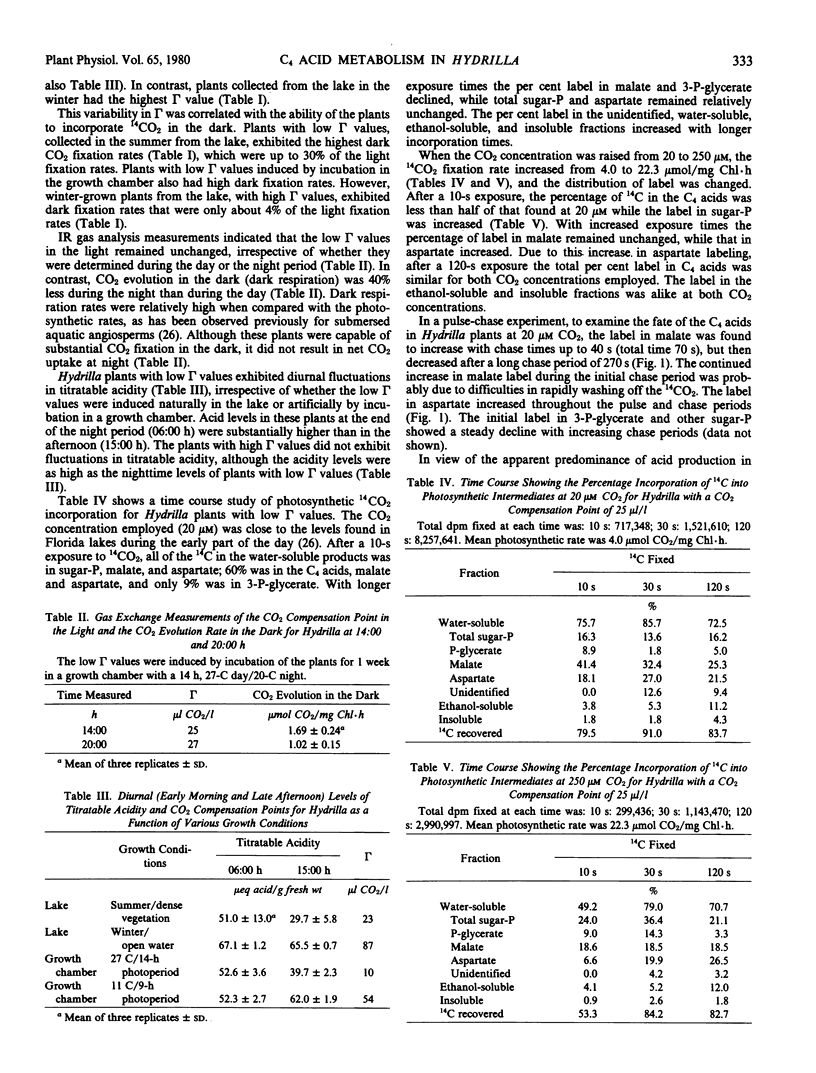
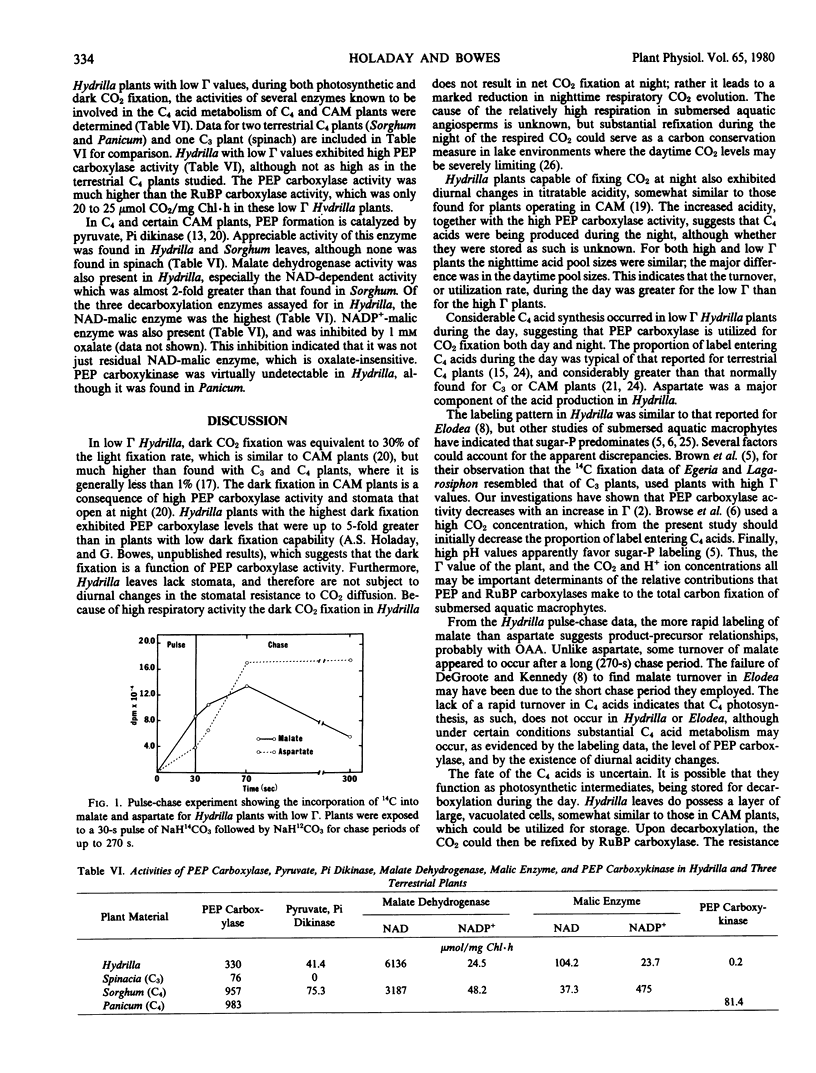

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L. Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1972 Apr 10;247(7):2171–2176. [PubMed] [Google Scholar]
- Chen T. M., Brown R. H., Black C. C. Photosynthetic CO(2) Fixation Products and Activities of Enzymes Related to Photosynthesis in Bermudagrass and Other Plants. Plant Physiol. 1971 Feb;47(2):199–203. doi: 10.1104/pp.47.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote D., Kennedy R. A. Photosynthesis In Elodea canadensis Michx: Four-Carbon Acid Synthesis. Plant Physiol. 1977 Jun;59(6):1133–1135. doi: 10.1104/pp.59.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich P., Campbell W. H., Black C. C. Phosphoenolpyruvate carboxykinase in plants exhibiting crassulacean Acid metabolism. Plant Physiol. 1973 Oct;52(4):357–361. doi: 10.1104/pp.52.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Kagawa T. NAD malic enzyme in leaves with C-pathway photosynthesis and its role in C4 acid decarboxylation. Arch Biochem Biophys. 1974 Jan;160(1):346–349. doi: 10.1016/s0003-9861(74)80043-3. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1968 Jan;106(1):141–146. doi: 10.1042/bj1060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. The C4-dicarboxylic acid pathway of photosynthesis. Identification of intermediates and products and quantitative evidence for the route of carbon flow. Biochem J. 1969 Aug;114(1):127–134. doi: 10.1042/bj1140127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi C., Perchorowicz J. T., Gibbs M. Malate synthesis by dark carbon dioxide fixation in leaves. Plant Physiol. 1978 Apr;61(4):477–480. doi: 10.1104/pp.61.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradshahi A., Vines H. M., Black C. C. Carbon Dioxide Exchange and Acidity Levels in Detached Pineapple, Ananas comosus (L.), Merr., Leaves during the Day at Various Temperatures, Oxygen and Carbon Dioxide Concentrations. Plant Physiol. 1977 Feb;59(2):274–278. doi: 10.1104/pp.59.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamondon J. E., Bassham J. A. Glycolic Acid Labeling During Photosynthesis with CO(2) and Tritiated Water. Plant Physiol. 1966 Oct;41(8):1272–1275. doi: 10.1104/pp.41.8.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley R. A., Naylor A. W. Photosynthesis in Eurasian Watermilfoil (Myriophyllum spicatum L.). Plant Physiol. 1972 Jul;50(1):149–151. doi: 10.1104/pp.50.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T. K., Haller W. T., Bowes G. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiol. 1976 Dec;58(6):761–768. doi: 10.1104/pp.58.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]



