Significance
Wetlands are unique ecosystems because they are in general sinks for carbon dioxide and sources of methane. Their climate footprint therefore depends on the relative sign and magnitude of the land–atmosphere exchange of these two major greenhouse gases. This work presents a synthesis of simultaneous measurements of carbon dioxide and methane fluxes to assess the radiative forcing of natural wetlands converted to agricultural or forested land. The net climate impact of wetlands is strongly dependent on whether they are natural or managed. Here we show that the conversion of natural wetlands produces a significant increase of the atmospheric radiative forcing. The findings suggest that management plans for these complex ecosystems should carefully account for the potential biogeochemical effects on climate.
Keywords: wetland conversion, methane, radiative forcing, carbon dioxide
Abstract
Significant climate risks are associated with a positive carbon–temperature feedback in northern latitude carbon-rich ecosystems, making an accurate analysis of human impacts on the net greenhouse gas balance of wetlands a priority. Here, we provide a coherent assessment of the climate footprint of a network of wetland sites based on simultaneous and quasi-continuous ecosystem observations of CO2 and CH4 fluxes. Experimental areas are located both in natural and in managed wetlands and cover a wide range of climatic regions, ecosystem types, and management practices. Based on direct observations we predict that sustained CH4 emissions in natural ecosystems are in the long term (i.e., several centuries) typically offset by CO2 uptake, although with large spatiotemporal variability. Using a space-for-time analogy across ecological and climatic gradients, we represent the chronosequence from natural to managed conditions to quantify the “cost” of CH4 emissions for the benefit of net carbon sequestration. With a sustained pulse–response radiative forcing model, we found a significant increase in atmospheric forcing due to land management, in particular for wetland converted to cropland. Our results quantify the role of human activities on the climate footprint of northern wetlands and call for development of active mitigation strategies for managed wetlands and new guidelines of the Intergovernmental Panel on Climate Change (IPCC) accounting for both sustained CH4 emissions and cumulative CO2 exchange.
For their ability to simultaneously sequester CO2 and emit CH4, wetlands are unique ecosystems that may potentially generate large negative climate feedbacks over centuries to millennia (1) and positive feedbacks over years to several centuries (2). Wetlands are among the major biogenic sources of CH4, contributing to about 30% of the global CH4 total emissions (3), and are presumed to be a primary driver of interannual variations in the atmospheric CH4 growth rate (4, 5). Meanwhile, peatlands, the main subclass of wetland ecosystems, cover 3% of the Earth’s surface and are known to store large quantities of carbon (about 500 ± 100 Gt C) (6, 7).
The controversial climate footprint of wetlands is due to the difference in atmospheric lifetimes and the generally opposite directions of CO2 and CH4 exchanges, which leads to an uncertain sign of the net radiative budget. Wetlands in fact have a great potential to preserve the carbon sequestration capacity because near water-logged conditions reduce or inhibit microbial respiration, promoting meanwhile CH4 production that may partially or completely counteract carbon uptake. Potential variations of the CO2/CH4 stoichiometry in wetlands exposed to climate and land-use change require the development of mitigation-oriented management strategies to avoid large climatic impacts.
The current and future contribution of wetlands to the global greenhouse gas (GHG) budget is still uncertain because of our limited knowledge of the combined and synergistic response of CH4 and CO2 land–atmosphere exchange to environmental variability (8, 9) and land-use change (e.g., wetland restoration, drainage for forestry, agriculture, or peat mining) (9, 10). Fluxes of CH4 and CO2 from natural wetlands show large spatiotemporal variations (11, 12), arising from environmental interactions controlling the production, transport, consumption, and release of CH4 (13, 14) as well as the dynamic balance between photosynthetic and respiratory processes that regulate the net accumulation of carbon in biomass and soil. Environmental factors such as variations in air and soil temperature, water table, and substrate availability for methanogenesis lead to a high spatial and temporal variation of CH4 emissions (15–17). The magnitude of emissions is also controlled by the balance between CH4 production and oxidation rates and by transport pathways: diffusion (18), ebullition (19), and aerenchyma transport (20).
Climate change influences the GHG balance of wetlands through thawing of the near-surface permafrost (21, 22) and thaw lakes (23), increased nitrogen availability due to accelerated decomposition of organic matter (24), and modification of the water tables with consequent shifts in CH4 emissions (1, 25). A review of carbon budgets of global peatlands concluded that these ecosystems may remain a small but persistent sink that builds a large C pool, reducing the atmospheric CO2 burden, whereas the stimulation of CH4 emissions induced by climate warming may be locally tempered or enhanced by drying or wetting (26). The climate footprint of wetlands can also be affected by anthropogenic activities such as the conversion of natural ecosystems to agricultural or forested land (10, 27). Draining peatlands for forestry may lead to a C loss and reduced CH4 emissions (10, 26), whereas land use for agriculture typically reduces the CH4 emissions and increases N2O emissions (26).
Several studies have analyzed the impact of northern peatlands on the Earth’s radiative budget either by computing the radiative forcing (RF) of sustained CH4 and CO2 fluxes (2) or by multiplying the annual ecosystem exchange of CO2 and CH4 with the global warming potentials of the two gases (28–30). However, although this latter approach is useful for comparison, its appropriateness in computing the actual RF has been questioned (31–33). An alternative approach for assessing the impact of peatland draining/drying on the RF has been applied by driving an atmospheric composition and RF model with pre- and postdrainage measured fluxes of CO2, CH4, and N2O (34).
Here, we ask, what is the climate cost of CH4 emissions compared with the benefit of net carbon sequestration? We assessed this question, using data from a network of wetland observational sites where direct and quasi-continuous CO2 and CH4 chamber and eddy covariance measurements are performed. Using the space for time analogy, flux observations at sites with contrasting land cover are combined with a sustained pulse–response model to predict the potential future RF of natural wetlands converted to agricultural or forested land.
Results and Discussion
As the land–atmosphere fluxes of CH4 and CO2 in wetlands can be opposite in sign and very different in magnitude, their net impact on the climate system is difficult to assess and predict. In particular, CH4 emissions from wetlands are continuous and thus add a positive term to the radiative balance (31) that can be partially or totally offset by a sustained carbon sequestration (35). The availability of consistent and simultaneous measurements of ecosystem CO2 and CH4 fluxes provides an opportunity to address these issues, using direct observations collected at 29 both natural and managed wetlands located in the Northern Hemisphere (Fig. 1A). Details on site locations, climate, vegetation type, measurement techniques, and yearly/seasonal GHG budgets are reported in SI Text, Site Analysis and SI Text, Measurement Techniques and Gap-Filling Methods (Tables S1–S5).
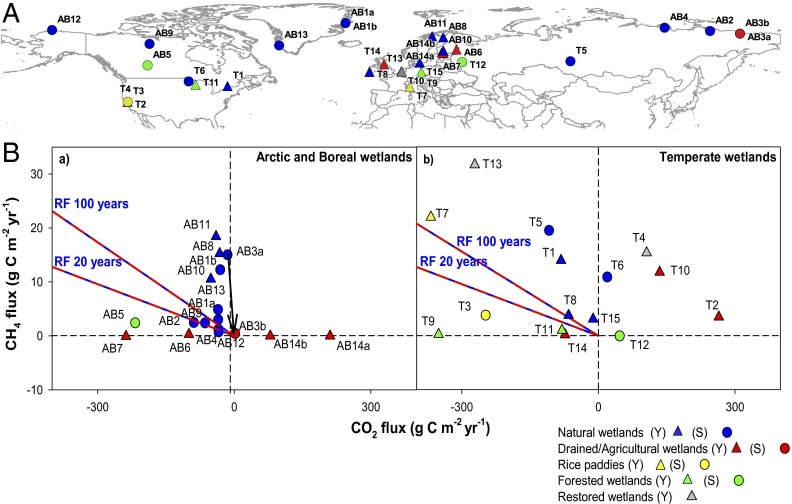
Fig. 1.
(A) Global distribution of the 29 measurement sites involved in the present analysis. Triangles represent sites with annual budgets (Y) and circles represent sites with growing season budgets (S). Site IDs and description are reported in SI Text, Site Analysis and Tables S1 and S2. (B) CH4 vs. CO2 flux (in grams C⋅m−2⋅y−1) for arctic/boreal and temperate wetlands relative to the modeled RF equilibrium lines. The two blue–red equilibrium lines represent the ratio of sustained CO2 and CH4 fluxes (grams CO2-C⋅m−2⋅y−1 per gram CH4-C⋅m−2⋅y−1) that would result in a zero cumulative RF over the period indicated for the line (20 y and 100 y). The slope of the line depends on the constant CO2 uptake rate that would be needed for compensating the positive RF of a unit CH4 emission at a fixed changing time. The arrow pointing down (AB3a to AB3b) indicates the carbon flux change at the specific site after a drainage experiment.
The trade-off between CH4 net emission and CO2 net sequestration in wetlands is evident in Fig. 1B, where most sites are sources of CH4 (positive ecosystem fluxes) and CO2 sinks (negative values of net ecosystem exchange, NEE). Given that CH4 has a relatively short lifetime in the atmosphere (∼10 y) compared to CO2, the radiative balance of these two gases depends on the timeframe of the analysis. As an example of this dependence, the two red–blue equilibrium lines in Fig. 1B represent the ratio of sustained CO2 and CH4 fluxes that would result in a zero net cumulative radiative balance over 20 y and 100 y. The lines were simulated with a sustained pulse–response model (27) and used in this study also to calculate the RF of management options. The model generates the following flux ratios: −31.3 g and −19.2 g CO2-C⋅m−2⋅y−1 per gram CH4-C⋅m−2⋅y−1 for 20 y and 100 y, respectively. This implies that a continuous emission of 1 g CH4-C⋅m−2⋅y−1 and uptake of 31.3 g CO2-C⋅m−2⋅y−1 would have a positive cumulative RF (warming) for the first 20 y and a negative cumulative RF (cooling) after that. Sites that fall on the right side of the equilibrium lines have a positive radiative budget and those on the left side have a negative radiative budget for the specified 20-y or 100-y timeframe (Fig. 1B). Under the current climate, 59% of arctic and boreal sites’ and 60% of temperate sites’ observations have a positive radiative balance compared with both 20-y and 100-y equilibrium lines. All but one of the forested wetlands [arctic/boreal (AB)5, AB7, temperate (T)9, and T11] currently have a negative net radiative balance owing to their considerable CO2 uptake and relatively low CH4 emissions (Fig. 1B and Fig. S1). Sites located between the two lines have a positive or negative radiative budget, depending on the time span of the analysis (e.g., AB9, AB4, and T8, Fig. 1B).
Changes in the water level in wetlands substantially alter the ratio of CH4 and CO2 fluxes. Recent warming and drying in the Arctic has led to increased CO2 losses from the soil, in some cases switching arctic regions from a long-term carbon sink to a carbon source (36). In other cases, the drying of arctic and boreal wetlands reduces CH4 emission without generating larger CO2 emissions, owing to the compensation between accelerated decomposition of organic matter and an increase in net primary productivity (NPP) (37–39). As an example of management impacts, data show that the CO2 and CH4 emissions of the site AB3a dropped toward a near zero net radiative budget one year after drainage, whereas sites that were drained a long time ago, such as AB6 and AB7, have large carbon uptake rates (Fig. 1).
Different responses of CH4 and CO2 budgets at drained temperate wetlands compared with boreal or arctic wetlands mainly occur due to management activities. At these sites draining for agricultural use suppresses CH4 emissions and enhances CO2 efflux owing to accelerated peat degradation, exploitation through grazing, and carbon export (T2, T10, and T14). Conversely, rewetted former agricultural areas or restored wetlands typically emit CH4 (T13) at a rate that in the short term is not offset by the CO2 sink (T4). Although most of the studied temperate wetlands have a positive radiative budget, natural forested wetlands show significant carbon uptake driven by high rates of photosynthesis that offsets ecosystem respiration (T9 and T11). The long-term CH4 and CO2 balance of these ecosystems thus ultimately depends on the fate of the carbon stored in the trees.
At temperate latitudes, it is interesting to note that the two rice paddies (T3 and T7) that in general are known as major contributors to atmospheric CH4 (5% of the total emissions and about 10% of the anthropogenic emissions) (3) are also characterized by large CO2 uptake. However, the net GHG budget of this crop is further complicated by significant carbon imports (fertilization) and exports (harvest and dissolved organic carbon). Based on site observations, carbon losses due to harvest account for 67% and 70% of net ecosystem exchange at T3 (40) and T7, respectively, so that the net GHG balance from these ecosystems is strongly influenced by the carbon exports.
To quantify the effect of ecosystem management on the net climate impact of multiple GHG fluxes, we applied an analytical approach based on the concept of radiative forcing. RF is a widely used metric in climate change research to quantify the magnitude of an externally imposed perturbation to the incoming long-wave radiative component of the Earth’s atmospheric energy budget (41). Two types of human perturbations were considered: the conversion of natural wetlands to agricultural land and the conversion of natural forested wetlands to managed forested wetlands. Natural wetlands with full annual GHG budget were used as reference and paired in all possible combinations to managed sites (SI Text, Radiative Forcing Calculations and Table S6). Based on the difference between natural and perturbed ecosystems, we calculated the net RF due to CO2 and CH4 fluxes for 100 y, using a sustained pulse–response model (27) (SI Text, Radiative Forcing Calculations). The contribution of N2O fluxes to the RF was accounted for only in agricultural sites (AB6, AB14a,b, T10, and T14) where significant emissions of this GHG can be observed (3).
Losses of carbon due to harvest and natural disturbances (e.g., mainly fires, wind throw, and pests) were also taken into account in the RF calculation, either in the form of annual harvest (for agricultural land) or after each rotation for wood harvest, and assumed every 100 y for natural disturbances in forested wetlands (42–44). It was assumed that all of the removed biomass was emitted into the atmosphere as CO2 during the same year. The results of the RF simulations (Fig. 2) are thus dependent on the ecosystem and management type. Results show that at all timescales the net effect of GHG emissions in arctic and boreal natural wetlands converted into agricultural sites (Fig. 2A) is a large positive RF, whereas the conversion of drained wetlands into energy crops (AB6) results in a minor negative RF for the 100-y simulations. The temperate wetlands (Fig. 2B) that were converted into agriculture sites showed, in general, a positive RF with a large spread among sites induced by management intensity [e.g., intensive (T10) vs. extensive (T14) grazing]. Given that the carbon balance of forest ecosystems largely depends on the fraction of harvested biomass, we carried out an uncertainty analysis by perturbing the harvest rate of the accumulated NPP according to two Gaussian distributions for natural (50 ± 10%, observed harvest rate at AB7) and managed (67 ± 10%) (45) sites, respectively (SI Text, Radiative Forcing Calculations). To evaluate the uncertainty generated by our assumptions, NPP was estimated with two alternative methodologies: (i) applying average ratios of NPP/gross primary productivity derived from the partitioning of the observed NEE (46), based on a recent meta-analysis (NPP/GPP = 0.39 and 0.49 for boreal and temperate forests, respectively) (47), and (ii) summing the observed NEE to the soil respiration rates reported in the IPCC Wetland Supplement for natural and managed wetlands (48).
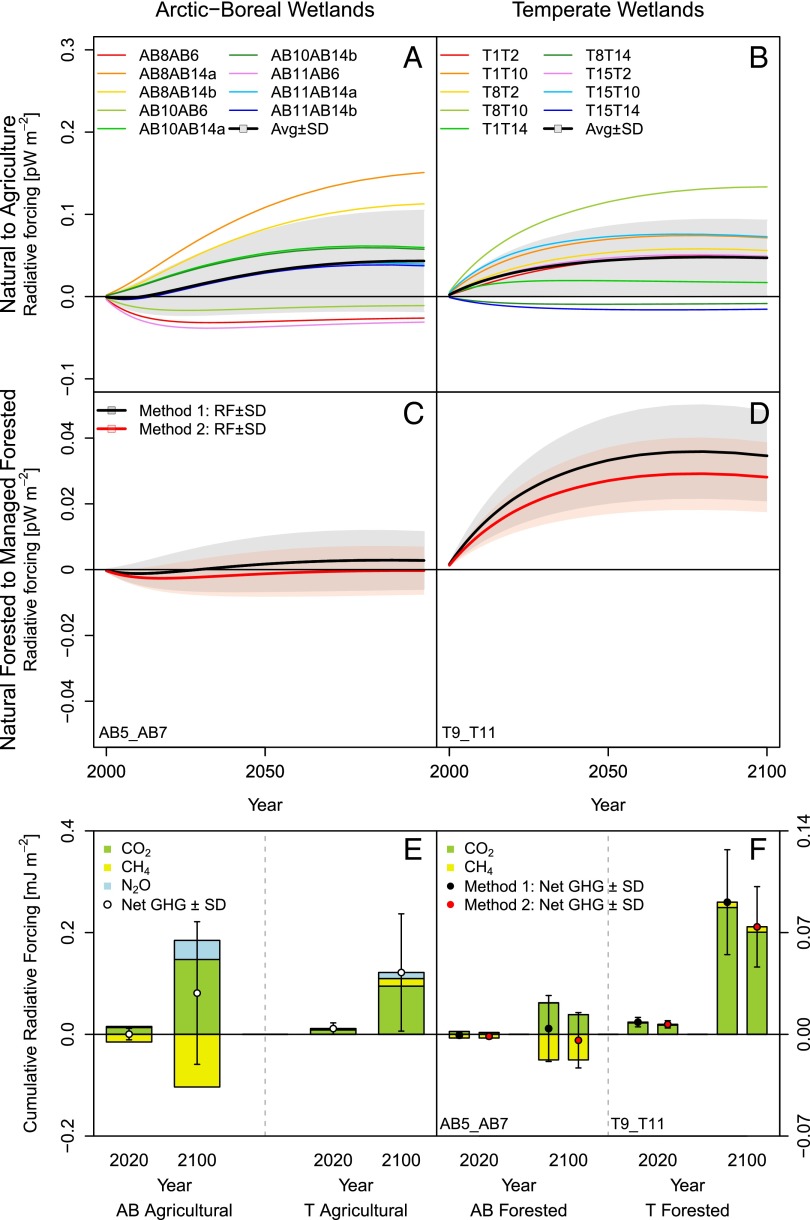
Fig. 2.
Trends of radiative forcing (RF, period 2000–2100) for paired sites and ecosystem types. (A and B) Net RF for CO2, CH4, and N2O in natural wetlands converted to agricultural land. (C and D) Net RF for the conversion of natural forested wetland to managed forests (AB5→AB7 and T9→T11). For each of the two pairs an uncertainty analysis on the effect of the harvest rate is presented. (E and F) Cumulative RF of individual gases at 20 y and 100 y for all site pairs, with their net RF (circles ± SD). The forcing units refer to the mean global impact of 1 m2 of wetland area (SI Text, Radiative Forcing Calculations). Site IDs can be found in SI Text, Site Analysis and Tables S1 and S2.
Results for the boreal site pair (AB5→AB7) show that the confidence intervals cross the x axis and therefore the ultimate sign of the RF depends on the harvest rate. In addition, with both methods used for the calculation of NPP, at average harvest rates the RF is not statistically different from zero (Fig. 2C). In contrast, for the temperate site pair (T9→T11) RF is positive, independently of the management intensity and of the applied methodology (Fig. 2D). Our analysis demonstrates that, to assess the RF of wetland management, both CH4 fluxes and the concomitant changes in CO2 emissions have to be accounted for. This is especially true at the decadal timescales for boreal wetlands converted to forest or agricultural land (Fig. 2 E and F).
Conclusions
The recent availability of simultaneous and continuous ecosystem observations of CH4 and CO2 fluxes in wetlands provides fundamental insights into the climate footprint of these ecosystems to support the development of sustainable mitigation strategies based on ecosystem management. Careful accounting of both CO2 and CH4 fluxes (and N2O fluxes where significant) is essential for an accurate calculation of the climate impact of wetlands. We also stress the importance of direct and quasi-continuous chamber or eddy covariance flux measurements over annual timescales for the observation of ecosystem responses to environmental drivers and management (e.g., flooding, drainage, and land use change) that may be missed with intermittent manual chamber measurements.
The net GHG budget of these ecosystems is spatially and temporally variable in sign and magnitude due to the generally opposite direction of CH4 (emission) and CO2 (uptake) exchange and, therefore, can be easily altered by both natural and anthropogenic perturbations (SI Text, Site Analysis and Table S3). Management and land use conversions in particular play a critical role in determining the future GHG balance of these ecosystems. Our results prove that management intensity strongly influences the net climate footprint of wetlands and in particular the conversion of natural ecosystems to agricultural land ultimately leads to strong positive RF. These considerations suggest that future releases of GHG inventories based on IPCC guidelines for wetlands should indeed address the relationship between the fluxes of CH4 and CO2, the management intensity, and the land use/land cover change on the net GHG balance as well as on the RF of these complex ecosystems.
Materials and Methods
This study is based on measurements of net ecosystem exchange of CO2 and CH4 trace gas exchange performed with eddy covariance and/or chamber methods (SI Text, Site Analysis and Tables S1 and S2). Most of the included study sites are part of FLUXNET, an international network of sites where energy and GHG fluxes are continuously monitored with a standardized methodology (49). The RF due to wetlands management was calculated for CO2, CH4, and, where significant (agricultural sites AB6, AB14a,b, T10, and T14), N2O fluxes, using a sustained pulse–response model (27). Annual concentration pulses were derived from the flux differences between pristine wetlands, taken as reference, and wetlands converted to either cropland or forests.
Natural-managed site pairs were defined for all possible combinations of similar ecosystem types with available annual CO2 and CH4 budgets within each climatic or management-related category (arctic/boreal or temperate regions, cropland or forest; SI Text, Radiative Forcing Calculations and Table S6). These site pairs were selected to represent plausible and representative wetland conversions, and thus part of the sites were excluded from this analysis (e.g., rice fields). In the simple pulse–response RF model used here the perturbations to the tropospheric concentrations of CO2, CH4, and N2O were derived by integrating the effect of a series of consecutive annual mass pulses that correspond to the mean annual balances of these gases (27) (SI Text, Radiative Forcing Calculations). Different radiative efficiencies and atmospheric residence times of CO2, CH4, and N2O were taken into account, as well as the annual variation of their background concentrations. RF was calculated for a 100-y period starting from 2000, assuming that the background concentrations increase as in the A2 scenario of the Special Report on Emissions Scenarios (SRES). The RF methodology is described in detail in SI Text, Radiative Forcing Calculations. The data reported in this paper are tabulated in SI Text and part is archived in the FLUXNET database and/or published in peer-review articles as shown in SI Text references.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge support from JRC-IES-H07 ClimEcos project (995) and FP7 ICE-ARC (603887-2). Data collection and analysis were supported by the following grants: National Science Foundation (NSF) Project DEB-0845166 (T11); Natural Sciences and Engineering Research Council of Canada and the Canadian Foundation for Climate and Atmospheric Sciences Grants 313372 (AB9) and 246386-01 (AB5 and T1); Early Career Scheme, Research Grants Council of the Hong Kong Special Administrative Region, China, Project CUHK 458913 (T1); NSF Proposal 1204263 (AB12); Irish Environmental Protection Agency’s STRIVE (Science, Research, Technology and Innovation for the Environment) programme (project CELTICFLUX; 2001-CD-C2-M1) and the European Union (EU) 6th Framework Project CarboEurope-IP (505572), NitroEurope-IP (017841) (T7), and 017841/2 (T14); Helmholtz Association [Helmholtz Young Investigators Group, Grant VH-NG-821, and the Helmholtz Climate Initiative “Regional Climate Change” (Regionale Klimaänderungen REKLIM)] (AB4); the Nordic Centre of Excellence, DEFROST (Impact of a changing cryosphere - Depicting ecosystem-climate feedbacks from permafrost, snow and ice), under the Nordic Top-Level Research Initiative, Academy of Finland Centre of Excellence program (Project 1118615) and the Academy of Finland ICOS (Integrated Carbon Observation Systems) Projects (263149, 281255, and 281250) (AB7, AB8, and AB14a,b); Greenland Ecosystem Monitoring Programme; the Danish Energy Agency and the Nordic Center of Excellence DEFROST (AB1b and AB13); the Nordic Center of Excellence DEFROST and EU-GREENCYCLES (512464) (AB11) and the Swedish Research Councils FORMAS (T15); Dutch–Russian Scientific Cooperation Grant 047.017.037 (Nederlandse Organisatie voor Wetenschappelijk Onderzoek NWO); Darwin Center Grant 142.16.3051 and Terrestrial Carbon Observation System TCOS-Siberia (EVK2-CT-2001-00131) (AB2); TCOS-Siberia European Union Project 2002–2004 (EU Project N EVK2-2001-00143) (AB3); NSF Grant ATM-9006327 (T6); The Finnish Funding Agency for Technology and Innovation (Tekes); University of Eastern Finland Grant 70008/08 (AB6); CarboEurope-IP (GOCE-CT-2003-505572); Dutch National Research Programme Climate Changes Spatial Planning (ME2 project) and the province of North Holland (T10); Russian Science Foundation, Grant 14-27-00065 (T12); and Academy of Finland (125238) (AB10).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416267112/-/DCSupplemental.
References
- 1.Gorham E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol Appl. 1991;1(2):182–195. doi: 10.2307/1941811. [DOI] [PubMed] [Google Scholar]
- 2.Frolking S, Roulet NT. Holocene radiative forcing impact of northern peatland carbon accumulation and methane emissions. Glob Change Biol. 2007;13:1079–1088. [Google Scholar]
- 3.Ciais P, et al. Carbon and other biogeochemical cycles. In: Stocker TF, et al., editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2013. pp. 505–510. [Google Scholar]
- 4.Bousquet P, et al. Contribution of anthropogenic and natural sources to atmospheric methane variability. Nature. 2006;443(7110):439–443. doi: 10.1038/nature05132. [DOI] [PubMed] [Google Scholar]
- 5.Nisbet EG, Dlugokencky EJ, Bousquet P. Atmospheric science. Methane on the rise—again. Science. 2014;343(6170):493–495. doi: 10.1126/science.1247828. [DOI] [PubMed] [Google Scholar]
- 6.Limpens J, et al. Peatlands and the carbon cycle: From local processes to global implications – a synthesis. Biogeosciences. 2008;5:1475–1491. [Google Scholar]
- 7.Yu ZC. Northern peatland carbon stocks and dynamics: A review. Biogeosciences. 2012;9:4071–4085. [Google Scholar]
- 8.Sturtevant C, Oechel WC. Spatial variation in landscape-level CO2 and CH4 fluxes from arctic coastal tundra: Influence from vegetation, wetness, and the thaw lake cycle. Glob Change Biol. 2013;19(9):2853–2866. doi: 10.1111/gcb.12247. [DOI] [PubMed] [Google Scholar]
- 9.Zona D, et al. Methane fluxes during the initiation of a large-scale water table manipulation experiment in the Alaskan arctic tundra. Global Biogeochem Cycles. 2009;23(2):GB2013. [Google Scholar]
- 10.Minkkinen K, Korhonen R, Savolainen I, Laine J. Carbon balance and radiative forcing of Finnish peatlands 1900-2100 – the impact of forestry drainage. Glob Change Biol. 2002;8:785–799. [Google Scholar]
- 11.Drösler M, Freibauer A, Christensen TR, Friborg T. 2008. Observation and status of peatland greenhouse gas emission in Europe. The Continental-Scale Greenhouse Gas Balance of Europe, Ecological Studies, eds Dolman H, Valentini R, Freibauer A (Springer, New York), Vol 203, pp 237–255.
- 12.Harazono Y, et al. Temporal and spatial differences of methane flux at arctic tundra in Alaska. Memoirs of National Institute of Polar Research. 2006;59(Special Issue):79–95. [Google Scholar]
- 13.Matthews E. Wetlands. In: Khalil MAK, editor. Atmospheric Methane: Its Role in the Global Environment. Springer; Berlin: 2000. pp. 202–233. [Google Scholar]
- 14.Vourlitis GL, Oechel WC. 1997. The role of northern ecosystems in the global methane budget. Global Change and Arctic Terrestrial Ecosystem, Ecological Studies, eds Oechel WC, et al. (Springer, New York), Vol 124, pp 266–289.
- 15.Moore TR, Knowles R. CH4 emissions from fen, bog and swamp peatlands in Quebec. Biogeochemistry. 1990;11:45–61. [Google Scholar]
- 16.Whalen SC, Reeburgh WS. Interannual variations in tundra CH4 emissions: A four-year time series at fixed sites. Global Biogeochem Cycles. 1992;6(2):139–159. [Google Scholar]
- 17.Dise NB. Methane emissions from Minnesota peatlands: Spatial and seasonal variability. Global Biogeochem Cycles. 1993;7(1):123–142. [Google Scholar]
- 18.Kip N, et al. Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat Geosci. 2010;3:617–621. [Google Scholar]
- 19.Kellner E, Waddington JM, Price JC. Dynamics of biogenic gas bubbles in peat: Potential effects on water storage and peat deformation. Water Resour Res. 2005;41:W08417. [Google Scholar]
- 20.Öquist MG, Svensson BH. Vascular plants as regulators of CH4 emissions from subarctic mire ecosystem. J Geophys Res. 2001;107(D21):4580. [Google Scholar]
- 21.Lawrence DM, Slater A, Romanovsky VE, Nicolsky DJ. Sensitivity of a model projection of near-surface permafrost degradation to soil column depth and representation of soil organic matter. J Geophys Res Earth Surface. 2008;113(F2):000883. [Google Scholar]
- 22.Lupascu M, et al. High Arctic wetting reduces permafrost carbon feedbacks to climate warming. Nature Climate Change. 2014;4:51–55. [Google Scholar]
- 23.Anthony KM, et al. A shift of thermokarst lakes from carbon sources to sinks during the Holocene epoch. Nature. 2014;511(7510):452–456. doi: 10.1038/nature13560. [DOI] [PubMed] [Google Scholar]
- 24.Keuper F, et al. A frozen feast: Thawing permafrost increases plant-available nitrogen in subarctic peatlands. Glob Change Biol. 2012;18:1998–2007. [Google Scholar]
- 25.Nykänen H, Alm J, Silvola J, Tolonen JK, Martikainen PJ. Methane fluxes on boreal peatlands of different fertility and the effect of long term experimental lowering of the water table on flux rates. Global Biogeochem Cycles. 1998;12(1):53–69. [Google Scholar]
- 26.Frolking S, et al. Peatlands in the Earth’s 21st century climate system. Environ Rev. 2011;19:371–396. [Google Scholar]
- 27.Lohila A, et al. Forestation of boreal peatlands: Impacts of changing albedo and greenhouse gas fluxes on radiative forcing. J Geophys Res. 2010;115:G04011. [Google Scholar]
- 28.Roulet NT. Peatlands, carbon storage, greenhouse gases, and the Kyoto protocol: Prospects and significance for Canada. Wetlands. 2000;20(4):605–615. [Google Scholar]
- 29.Whiting GJ, Chanton JP. Greenhouse carbon balance of wetlands: Methane emission versus carbon sequestration. Tellus. 2001;53B:521–528. [Google Scholar]
- 30.Friborg T, et al. Siberian wetlands: Where a sink is a source. Geophys Res Lett. 2003;30(21):2129–2132. [Google Scholar]
- 31.Frolking S, Roulet NT, Fuglestvedt J. How northern peatlands influence the Earth’s radiative budget: Sustained methane emission versus sustained carbon sequestration. J Geophys Res. 2006;111:G01008. [Google Scholar]
- 32.Neubauer SC. On the challenges of modeling the net radiative forcing of wetlands: Reconsidering Mitsch et al. (2013) Landscape Ecol. 2014;29:571–577. [Google Scholar]
- 33.Bridgham S, Moore T, Richardson C, Roulet NT. Errors in greenhouse forcing and soil carbon sequestration estimates in freshwater wetlands: A comment on Mitsch et al. (2013) Landscape Ecol. 2014;29(6):1–5. [Google Scholar]
- 34.Laine J, Minkkinen K. Effect of forest drainage on the carbon balance of a mire: A case study. Scand J For Res. 1996;11:307–312. [Google Scholar]
- 35.Tarnocai C, et al. Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochem Cycles. 2009;23(2):GB2023. [Google Scholar]
- 36.Oechel WC, et al. Recent change of arctic tundra ecosystems from a net carbon sink to a source. Nature. 1993;361:520–526. [Google Scholar]
- 37.Sulman BN, Desai AR, Cook BD, Saliendra N, Mackay DS. Contrasting carbon dioxide fluxes between a drying shrub wetland in Northern Wisconsin, USA, and nearby forests. Biogeosciences. 2009;6:1115–1126. [Google Scholar]
- 38.Flanagan LB, Syed KH. Stimulation of both photosynthesis and respiration in response to warmer and drier conditions in a boreal peatland ecosystem. Glob Change Biol. 2011;17:2271–2287. [Google Scholar]
- 39.Merbold L, et al. Artificial drainage and associated carbon fluxes (CO2/CH4) in a tundra ecosystem. Glob Change Biol. 2009;15:2599–2614. [Google Scholar]
- 40.Hatala JA, et al. Greenhouse gas (CO2, CH4, H2O) fluxes from drained and flooded agricultural peatlands in the Sacramento-San Joaquin Delta. Agric Ecosyst Environ. 2012;150:1–18. [Google Scholar]
- 41.Ramaswamy V, et al. Radiative forcing of climate change. In: Houghton JT, et al., editors. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge Univ Press; Cambridge, UK: 2001. pp. 349–416. [Google Scholar]
- 42.Vanderwel MC, Coomes DA, Purves DW. Quantifying variation in forest disturbance, and its effects on aboveground biomass dynamics, across the eastern United States. Glob Change Biol. 2013;19(5):1504–1517. doi: 10.1111/gcb.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters BE, Wythers KR, Bradford JB, Reich PB. Influence of disturbance on temperate forest productivity. Ecosystems. 2013;16(1):95–110. [Google Scholar]
- 44.Krankina ON, et al. Effects of climate, disturbance and species on forest biomass across Russia. Can J For Res. 2005;35:2281–2293. [Google Scholar]
- 45.Callesen I, Østergaard H. 2008 Energy efficiency of biomass production in managed versus natural temperate forest and grassland ecosystems. 16th IFOAM Organic World Congress, Modena, Italy, June 16–20. Available at orgprints.org/view/projects/conference.html.
- 46.Reichstein M, et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algo- rithm. Glob Change Biol. 2005;11:1424–1439. [Google Scholar]
- 47.Tang J, et al. Steeper declines in forest photosynthesis than respiration explain age-driven decreases in forest growth. Proc Natl Acad Sci USA. 2014;111(24):8856–8860. doi: 10.1073/pnas.1320761111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. IPCC (2014) 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands, eds Hiraishi T, et al. (IPCC, Geneva, Switzerland)
- 49.Baldocchi DD, et al. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor and energy flux densities. Bull Am Meteorol Soc. 2001b;82:2415–2434. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.