Ebola viruses (EBOV) are zoonotic infectious agents that are highly pathogenic in humans, causing severe hemorrhagic fever with fatality rates of ∼50–70% (1). This genus of negative single-stranded RNA viruses consists of five known species that are part of the Filoviridae family. The current EBOV outbreak in western Africa began in March 2014 and has since resulted in >24,000 cases and >10,000 deaths (1). This 25th known EBOV outbreak is unprecedented in its magnitude, duration, and societal impact. Given the likelihood of future EBOV outbreaks, significant efforts are being devoted to develop vaccines that block EBOV transmission and novel therapeutic interventions to treat infected individuals (2, 3). Progress in these pursuits requires better understanding of what key elements of the immune response correlate with virus replication control and protection from disease. In PNAS, McElroy et al. report the results of their study of the cellular and humoral immune responses of four EBOV-infected people treated at Emory University (all of whom received experimental therapies) (4). Their data provide critical insight into aspects of the host response in humans to EBOV that have not previously been examined using contemporary immunologic methods, and provide the foundation for future studies, elucidating immune responses mediating effective virus control.
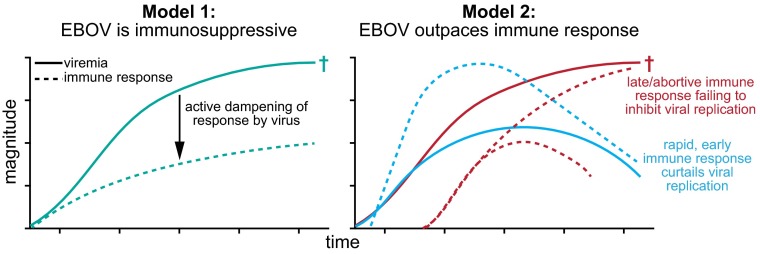
The high mortality rate of EBOV infections indicates that the immune system often fails to control viral replication. In fact, it has been suggested that a key pathogenic mechanism of EBOV is its ability to cause global immunosuppression (Fig. 1). Studies of the interaction of EBOV with cells in culture have shown that at least three Ebola virus-encoded proteins (viral protein 24, viral protein 35, and glycoprotein) act on cell-intrinsic resistance pathways, resulting in inhibition of type I IFN production, impairing the action of IFN on infected cells, and counteracting the effects of tetherin, a host factor that prevents virion release (5). Moreover, in vitro studies have indicated that Ebola virus impairs dendritic cell responses, including cytokine production, maturation, and ability to induce T-cell proliferation. The acute reduction in circulating T-cell counts seen during in vivo Ebola virus infection, which parallels the high levels of bystander lymphocyte apoptosis described in culture, has also been proposed to contribute to impaired adaptive immune responses (reviewed in refs. 6 and 7).
Fig. 1.
Schematic of viral replication and immune activation for two models accounting for the failure of the immune system to control EBOV in lethal infections. Model 1 proposes that EBOV leads to substantial immune suppression that prevents an effective immune response from being raised. Model 2 suggests that the timing and kinetics of viral replication and of the immune response is key to infection outcome, with a late or abortive immune response leading to death. New data from McElroy et al. (4) support the idea that EBOV infection can lead to extensive immune activation (Model 2), although it remains to be defined what key elements of the response (function, specificity, magnitude of T- and B-cell responses) are essential to viral clearance. †denotes patient death.
Because of the unpredictability of EBOV outbreaks, their often rural location, the priority to provide medical care, and the need for biosafety-level 4 biohazard containment of specimens obtained, detailed studies of human immune responses to EBOV infections have been very limited. To date, the main emphasis of human studies has been the production of pro/anti-inflammatory mediators, antibody production, and lymphocyte counts measured in the blood. From this work, the picture that emerges is one of a potent inflammatory response rather than global immune suppression (reviewed in ref. 8). In the case of lethal infections, the pace of emergence of host immune responses may be outstripped by very rapid viral replication, precipitating infection-associated tissue damage, including vascular leakage, dysregulated coagulation, and multiorgan failure (Fig. 1). Similarly, contrary to what in vitro work might suggest, some human studies have detected a type I IFN response, which studies in mice have emphasized as important in curtailing Ebola virus disease (9–11). Lower antibody levels and greater viremia have been associated with fatal infections and an early inflammatory response may be characteristic of less severe infection, consistent with mouse data (12, 13). Notably, only one study has thus far examined T-cell responses during Ebola virus infection and found significantly lower levels of activation in patients that died (14).
The new data from McElroy et al. clearly indicate that robust T- and B-cell responses are generated during the symptomatic phase of acute EBOV infection (4). Peak frequencies of activated T cells and plasmablasts were comparable to other acute viral infections studied (15, 16), rather than the delayed responses seen in some persistent infections, like hepatitis C (17). The CD8+ T-cell phenotype observed is suggestive of cytotoxic function, and cytokine production detected upon stimulation with EBOV peptides indicates their antigen-specific nature. Use of tetramers to track specific T cells during the course of infection will help discern the extent to which the high level of T-cell response is antigen-driven and fully functional. Given the high level of PD-1 expression on T cells, a receptor known to inhibit function in settings of very high or chronic antigen loads, it is possible that these emerging T cells may exhibit some functional impairment (18). Importantly, interrogation of the specificity, breadth, and functional capacity of antibodies from responding plasmablasts will also help illuminate the role humoral responses play in viral clearance [and if, as described for ZMapp, multiple simultaneous antibody engagements of EBOV glycoprotein are essential (3)]. Although the study by McElroy et al. (4) does not enable an assessment of the relationship between viremia, disease severity, and level of adaptive immune responses, the observation of extensive T- and B-cell activation suggests that if the immune response is given the opportunity to keep ahead of the rapidly increasing viral load, either by reducing viral replication therapeutically or by giving the immune system a head start through the generation of memory T and B cells by prior vaccination, the chances of survival might be substantially increased. Importantly, McElroy et al.’s study (4) also describes a second wave of activated T cells during the convalescent phase in three of the patients that, given its magnitude, suggests persistence of viral antigen (and potentially compartmentalized replication) after viremia has become undetectable in the blood. This finding is consistent with earlier observations that EBOV RNA can be detected in urine, sweat, and semen for several weeks following resolution of acute EBOV infection (19, 20).
The magnitude, geographic reach, and severity of the current EBOV outbreak have engendered widespread recognition that new tools—including a preventative vaccine—are needed to prevent and control future outbreaks. Toward this end, vaccine development efforts have proceeded at an unprecedented pace over the past 6 months, and promptly transitioned from phase 1 clinical trials to phase 3 safety, efficacy, and effectiveness studies in affected countries in West Africa, where two candidate vaccines are now being evaluated (2). In addition, other candidate EBOV vaccines have been advanced into phase 1 clinical trials within the past 3 months. A number of vaccine candidates protect against high-dose challenge with virulent EBOV in nonhuman primate (NHP) models, which suggests that development of an efficacious EBOV vaccine might be possible (2). Despite the encouraging preclinical results and expeditious advancement of clinical studies, the recent significant decline in EBOV incident infec
The work of McElroy et al. provides a valuable step to help accelerate the development of EBOV vaccines and inform future efforts to develop immune-based therapies.
tion rates in Liberia (where a large phase 2/3 study is underway), and lower (but continuing) rates of infection in Guinea and SierraLeone (where phase 3 effectiveness studies have just or will soon begin), have raised the question of whether it will be possible to formally demonstrate vaccine efficacy in the setting of a waning EBOV epidemic (1). Because it is not yet clear if traditional public health measures alone will be effective at extinguishing the current outbreak, there remains a compelling interest in expediting the licensure and availability of promising EBOV vaccine candidates, not only to be better prepared for future outbreaks but also as an important additional public health tool to eliminate the current one. Toward this end, regulatory authorities have expressed interest in the possibility of novel alternative accelerated pathways to vaccine licensure that would be predicated on the identification of specific immune responses that are convincingly associated with the prevention, control of, or natural immunity to EBOV infection. In this way, specific immunologic correlates that predict protective immunity could define the magnitude, character, and specificity of responses that candidate vaccines would need to elicit. As data are acquired to support such an accelerated pathway, the bridging to NHP models is one important option. However, available NHP models may not reflect human infection (in route or magnitude of most EBOV exposures) and the levels and specificities of the immune responses elicited by candidate vaccines might not be entirely congruent between NHPs and humans. For these reasons, there is also a pressing need to develop an improved, high-resolution understanding of the nature of cellular and humoral immune responses that emerge following acute EBOV in humans and that are associated with containment of virus replication, protection from lethal pathology, ultimate viral clearance, and long-term protection from reinfection. Toward this end, the work of McElroy et al. (4) provides a valuable step to help accelerate the development of EBOV vaccines and inform future efforts to develop immune-based therapies.
Footnotes
Conflict of interest statement: M.B.F. is an employee of Merck & Company, Inc., which is developing a candidate Ebola virus vaccine.
See companion article on page 4719.
References
- 1.WHO 2015. Ebola Situation Reports. Available at apps.who.int/ebola/en/current-situation/ebola-situation-report. Accessed March 19, 2015.
- 2.Kanapathipillai R, et al. Ebola vaccine—An urgent international priority. N Engl J Med. 2014;371(24):2249–2251. doi: 10.1056/NEJMp1412166. [DOI] [PubMed] [Google Scholar]
- 3.Qiu X, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McElroy AK, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci USA. 2015;112:4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misasi J, Sullivan NJ. Camouflage and misdirection: The full-on assault of ebola virus disease. Cell. 2014;159(3):477–486. doi: 10.1016/j.cell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong G, Kobinger GP, Qiu X. Characterization of host immune responses in Ebola virus infections. Expert Rev Clin Immunol. 2014;10(6):781–790. doi: 10.1586/1744666X.2014.908705. [DOI] [PubMed] [Google Scholar]
- 7.Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4(8):487–498. doi: 10.1016/S1473-3099(04)01103-X. [DOI] [PubMed] [Google Scholar]
- 8.Zampieri CA, Sullivan NJ, Nabel GJ. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat Immunol. 2007;8(11):1159–1164. doi: 10.1038/ni1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bray M. The role of the Type I interferon response in the resistance of mice to filovirus infection. J Gen Virol. 2001;82(Pt 6):1365–1373. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
- 10.Villinger F, et al. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson KL, Rollin PE. Cytokine and chemokine expression in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S357–S363. doi: 10.1086/520611. [DOI] [PubMed] [Google Scholar]
- 12.Leroy EM, et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet. 2000;355(9222):2210–2215. doi: 10.1016/s0140-6736(00)02405-3. [DOI] [PubMed] [Google Scholar]
- 13.Mahanty S, et al. Protection from lethal infection is determined by innate immune responses in a mouse model of Ebola virus infection. Virology. 2003;312(2):415–424. doi: 10.1016/s0042-6822(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez A, et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: Cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78(19):10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wrammert J, et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J Virol. 2012;86(6):2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JD, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Dustin LB, Rice CM. Flying under the radar: The immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71–99. doi: 10.1146/annurev.immunol.25.022106.141602. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 19.Kreuels B, et al. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014;371(25):2394–2401. doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 20.Rowe AK, et al. Clinical, virologic, and immunologic follow-up of convalescent Ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(Suppl 1):S28–S35. doi: 10.1086/514318. [DOI] [PubMed] [Google Scholar]