Significance
Anthropogenic emission of CO2 is causing global ocean acidification. For many species, biological responses to acidification often show limited impact at the level of the whole animal. Our integrative studies of whole-organism growth and metabolic rates, rates of protein synthesis and ion transport, enzyme activity, and gene expression show that although the organismal-level impact of acidification on developing sea urchins was minimal, dramatic compensation occurred at the cellular level. Increased rates of synthesis and ion transport resulted in 84% of available energy being allocated to those processes under acidification. Defining the limits of differential energy allocation for the maintenance of critical physiological functions in response to compounding stressors will help provide a mechanistic understanding of resilience potential to environmental change.
Keywords: ocean acidification, sea urchin, energetics, metabolic allocation, development
Abstract
Energy is required to maintain physiological homeostasis in response to environmental change. Although responses to environmental stressors frequently are assumed to involve high metabolic costs, the biochemical bases of actual energy demands are rarely quantified. We studied the impact of a near-future scenario of ocean acidification [800 µatm partial pressure of CO2 (pCO2)] during the development and growth of an important model organism in developmental and environmental biology, the sea urchin Strongylocentrotus purpuratus. Size, metabolic rate, biochemical content, and gene expression were not different in larvae growing under control and seawater acidification treatments. Measurements limited to those levels of biological analysis did not reveal the biochemical mechanisms of response to ocean acidification that occurred at the cellular level. In vivo rates of protein synthesis and ion transport increased ∼50% under acidification. Importantly, the in vivo physiological increases in ion transport were not predicted from total enzyme activity or gene expression. Under acidification, the increased rates of protein synthesis and ion transport that were sustained in growing larvae collectively accounted for the majority of available ATP (84%). In contrast, embryos and prefeeding and unfed larvae in control treatments allocated on average only 40% of ATP to these same two processes. Understanding the biochemical strategies for accommodating increases in metabolic energy demand and their biological limitations can serve as a quantitative basis for assessing sublethal effects of global change. Variation in the ability to allocate ATP differentially among essential functions may be a key basis of resilience to ocean acidification and other compounding environmental stressors.
Studies of biological responses to future scenarios of global change are of significant interest, given the most recent projections of future environmental conditions (1). In addition to important impacts in the atmosphere and on terrestrial systems, anthropogenic CO2 emission is causing acidification of the world’s oceans (2, 3). Determining the biological responses to ocean acidification is a critical component of the study of how marine ecosystems may be altered under future scenarios of anthropogenic global environmental change. Predicting the potential for evolutionary adaptation to global change requires an understanding of the biochemical mechanisms that maintain homeostasis of physiological systems (4, 5).
The developmental stages of many marine organisms have evolved cellular defenses to mitigate the impact of current environmental stressors (6). Whether these protective mechanisms can respond to future, rapid anthropogenic changes is still an open question. Marine invertebrate larvae, and particularly those with calcareous structures, have been used in numerous investigations of the biological impact of ocean acidification (2, 7–10). Although the magnitude of a response appears to be species specific, acidification can, to varying degrees, impact a wide range of biological processes in developmental forms (7–14). For instance, under near-future global mean CO2 conditions [720–1,000 µatm partial pressure of CO2 (pCO2)] (1), species of larval sea urchins generally are reduced in size by 10% or less (7, 9, 15–17), but studies of metabolic rate and ion regulation suggest that acidification may result in increased metabolic costs to maintain homeostasis (11, 12). By studying responses to seawater acidification at several levels of biological organization during the development of the sea urchin, Strongylocentrotus purpuratus—from whole-organism growth, to macromolecular synthesis rates, enzyme activities, and gene expressions—we show that, although the impact of acidification at the organismal level is minimal, dramatic compensation occurs at the cellular level. Specifically, growth is maintained by changes in energy allocation to accommodate the costs required to sustain increases in protein synthesis and ion transport. We conclude that measurements limited to morphological characteristics, metabolic rate, biochemical content, and gene expression do not reveal the major biochemical response mechanisms underlying the apparent resilience to acidification in developing sea urchins.
Results
Size and Metabolic Rate.
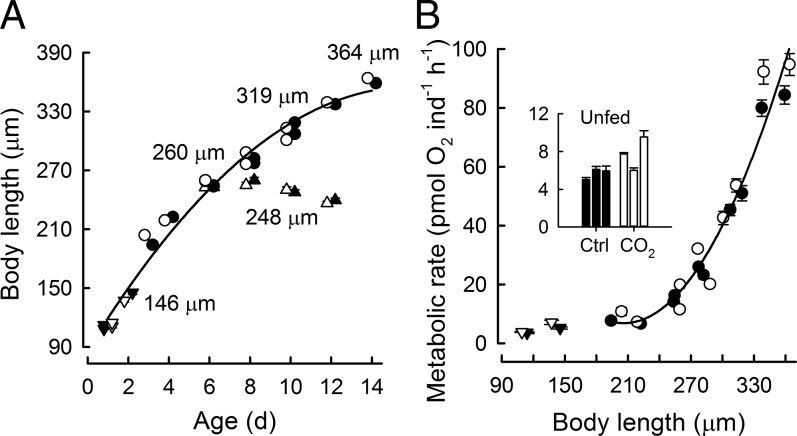
Body length (Fig. 1A) was measured during 14 d of development and growth in control and acidified seawater (Table S1). Midline body length (mean ± SEM) increased from 109.7 ± 0.6 µm on day 1 to 363.8 ± 3.1 µm on day 14 and did not differ between control and acidification treatments of larvae fed ad libitum (ANCOVA, P = 0.095, n = 2,081) (Fig. 1A) and unfed larvae (ANOVA, P = 0.537, n = 481). Metabolic rates (measured as oxygen consumption; see Discussion for oxyenthalpic equivalents) did not differ significantly between control and acidification treatments in fed larvae (ANCOVA, P = 0.326, n = 255) (Fig. 1B). Metabolic rates of embryos (Fig. 1B) and 6- to 10-d-old unfed larvae (Fig. 1B, Inset) were elevated under acidification (ANOVA, P < 0.001, n = 97). This increase in metabolic rates was 24% on average for embryos and 37% on average for unfed larvae.
Fig. 1.
Size (A) and metabolic rate (B) in developing sea urchins under control (closed symbols) and seawater acidification (open symbols) treatments. (A) Changes in diameters of embryos (inverted triangles), body lengths of larvae (circles) and unfed larvae (triangles). Each data point represents mean ± SEM (n = 50 individuals). Where not visible, error bars fall within the graphical representation of the data point. For visual clarity, data points for a given x-axis value are slightly offset when symbols overlap. Body lengths did not differ between control and acidification treatments for fed (ANCOVA, P = 0.095, n = 2,081) and unfed (P = 0.537, n = 481) larvae. (B) Metabolic rate (measured as O2 consumption) per individual as a function of body length. Error bars represent 1 SEM, n = 8–10 respiration assays. (Inset) Bar graph shows replicate measurements on 6-, 8-, and 10-d-old unfed larvae under control (Ctrl) and acidification (CO2) treatments. Metabolic rates did not differ significantly between control and acidification treatments for fed larvae (ANCOVA, P = 0.326, n = 255). Metabolic rates of embryos and unfed larvae were elevated under acidification (ANOVA, P < 0.001, n = 97).
Protein Content, Synthesis, and Turnover Rates.
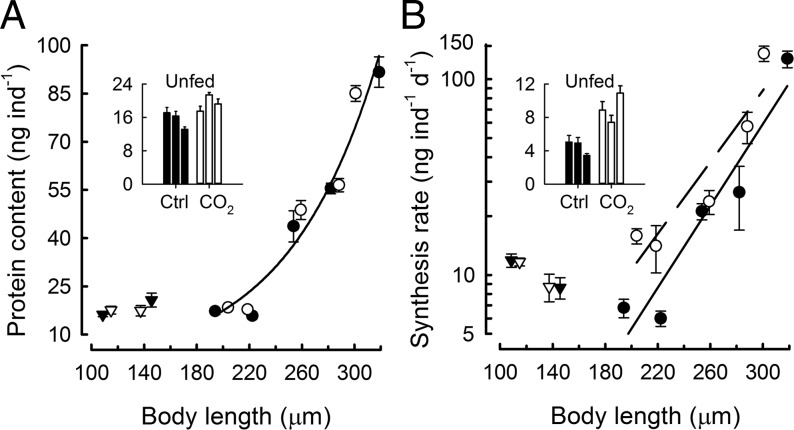
Acidification did not affect whole-body protein content of any developmental stage studied (embryos and unfed larvae, ANOVA, P = 0.063, n = 49; feeding larvae, ANCOVA, P = 0.847, n = 49) (Fig. 2A). The mole-percent amino acid composition of protein did not differ between control and acidification treatments (Table S2). In contrast, increases in absolute rates of protein synthesis were evident under acidification treatment from the earliest larval stage studied (194 µm, 3-d-old) (Fig. 2B). A regression of protein synthesis rate on size shows that growing sea urchin larvae under acidification had an average increase of 1.6-fold in size-specific rates of protein synthesis during the larval period tested (ANCOVA, P = 0.009, n = 20) (Fig. 2B). From a post hoc analysis of developmental stages with no growth (embryos and unfed larvae), seawater acidification resulted in a 2.0-fold increase in protein synthesis rates in 6- to 10-d-old unfed larvae (P < 0.001, n = 12; ANOVA in Table S3) (Fig. 2B, Inset) and showed no difference for 1- and 2-d-old embryos (P = 0.953, n = 8). These increases in rates of protein synthesis (Fig. 2B) represent turnover, because protein content did not change (Fig. 2A). Electrophoretic analyses of larval proteins showed that, even though the rate of protein synthesis increased under acidification (Fig. S1A), the size distribution and pattern of synthesized proteins (shown by35S-Met/Cys labeling) appear unchanged in 12-d-old, fed larvae (Fig. S1B). The analyses presented here support the conclusion that the increased rates of protein turnover involved all molecular weight classes of proteins observable at the resolution of 1D gel electrophoresis. The impact of seawater acidification on the processes of protein metabolism is illustrated in Fig. S2. Calculation of protein depositional efficiency (the ratio of protein accreted to protein synthesized) revealed that acidification resulted in a lower efficiency of protein deposition (21.2%) relative to controls (34.3%) for larvae of a given size. Combined, these analyses (Fig. 2 and Figs. S1 and S2) show that the balance between increased rates of protein synthesis and decreased protein depositional efficiency is an important mechanism for maintaining protein growth under simulated ocean acidification.
Fig. 2.
Protein content and synthesis in developing sea urchins under control (closed symbols) and seawater acidification (open symbols) treatments. (A) Protein content as a function of body length in embryos (triangles) and fed larvae (circles). Error bars indicate 1 SEM, n = 4–5 protein assays. Where not visible, error bars fall within the graphical representation of the data point. No statistical differences in protein content were observed between control and acidification treatments (embryos and unfed larvae, ANOVA, P = 0.063, n = 49; feeding larvae, ANCOVA, P = 0.847, n = 49). (Inset) The bar graph shows replicate measurements on 6-, 8-, and 10-d-old unfed larvae. (B) Each data point represents a protein synthesis rate, calculated from the combined slope (± SE) of duplicate six-point time-course assays of the amount of protein synthesized, corrected for intracellular specific activity of 14C-alanine in the free amino acid pool. Size-specific protein synthesis rates were significantly greater under acidification in growing larvae (ANCOVA, P = 0.009, n = 20) and (Inset) in unfed larvae (Post hoc test, P < 0.001, n = 12). See Fig. S2 for equations for the regression lines shown here in A and B.
Metabolic Cost of Protein Synthesis.
The cost of protein synthesis was determined for embryos and larvae of S. purpuratus reared under control and acidification treatments. The energy cost per unit protein synthesized is determined from concurrent measurements of changes in protein synthesis and metabolic rates in the presence and absence of emetine, a specific inhibitor of protein synthesis (18). Oxygen consumption was converted to energy using an oxyenthalpic equivalent of 484 kJ/mol O2, an average value based on lipids and proteins (19) that are the major biochemical constituents of developmental stages of S. purpuratus (20). From the simultaneous reduction in protein synthesis and metabolic rates, the cost of protein synthesis was calculated in 1-d-old blastulae (Fig. 3 A and B). This analysis was extended to a series of developmental stages, and the cost of protein synthesis in developing sea urchins was calculated to be 2.4 ± 0.21 J/mg protein synthesized (grand mean, n = 9) (Fig. 3C). Important for calculations of energy allocation to protein synthesis under control and acidification treatments is that the cost of synthesizing a unit-mass of protein did not differ between treatments (P = 0.304, t test, n = 3 for each treatment) (Fig. 3C; see SI Materials and Methods for details of biological and technical replications).
Fig. 3.
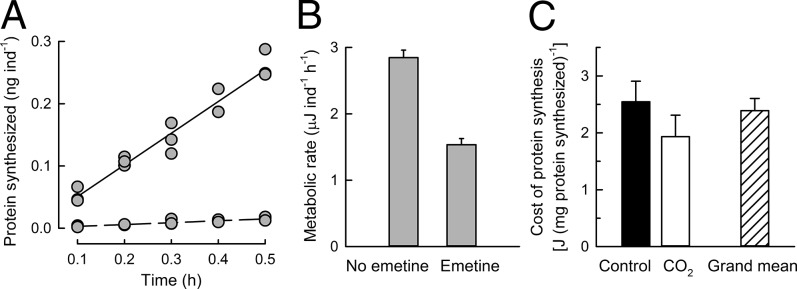
Energy cost of protein synthesis in developing sea urchins. (A) Protein synthesis of 1-d-old embryos in the absence (solid regression line, rate = 0.51 ± 0.032 nanograms per individual per hour, slope ± SE of slope) and presence (dashed regression line, rate = 0.03 ± 0.004 nanograms per individual per hour) of 100 µM emetine. Synthesis rates were calculated from triplicate, five-point time-course assays. (B) Metabolic rate measured as oxygen consumption, for the cohort of embryos in A, converted to energy equivalents (expressed in microJoules). Error bars represent 1 SEM, n = 8 respiration assays. (C) The cost of protein synthesis was calculated from the simultaneous decreases in protein synthesis and metabolic rate under inhibition by emetine. Error bars represent 1 SEM. The energy cost of protein synthesis did not differ significantly between control (black bar, n = 3) and seawater acidification (open bar, n = 3) treatments (t test, df = 4, t = 1.178, P = 0.304). The grand mean (hatched bar) of 2.4 ± 0.21 J/mg protein synthesized was calculated from all cost determinations (n = 9) for two pCO2 treatments and developmental stages spanning 1-d-old embryos to 10-d-old larvae.
Ion Transport Rate, Enzyme Activity, and Gene Expression of Na+,K+-ATPase.
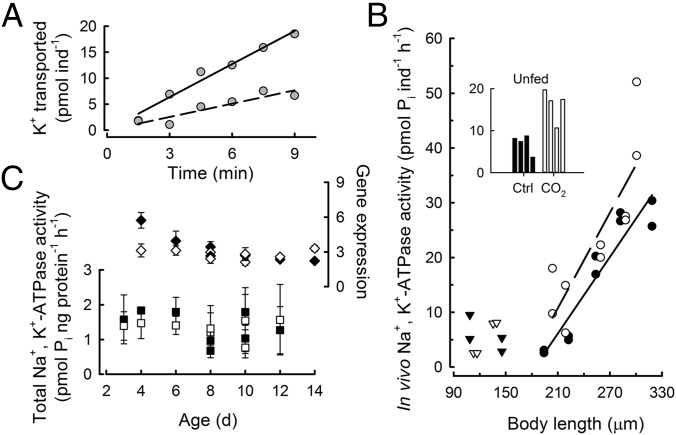
Measurements of Na+,K+-ATPase were made at three levels: in vivo physiological activity, total biochemical activity, and gene expression. The physiologically active fraction of total Na+,K+-ATPase (herein referred to as “ion transport,” i.e., in vivo Na+,K+-ATPase activity) was measured as the difference in transport rate of rubidium (86Rb+, a physiological analog of K+) in the presence and absence of ouabain (Fig. 4A). A regression of ion transport rates on size shows that growing sea urchin larvae under acidification had an 1.4-fold increase in the size-specific ion transport rate (ANCOVA, P = 0.016, n = 20) (Fig. 4B). A post hoc analysis of developmental stages with no growth (embryos and unfed larvae) showed that the ion transport rates increased 2.3-fold in unfed larvae under acidification (P = 0.001, n = 8; ANOVA in Table S3) (Fig. 4B, Inset) and showed no difference in 1- and 2-d-old embryos (P = 0.852, n = 8). In contrast to the significant increases in physiological rates of ion transport under acidification, no concurrent changes in total enzyme activity (ANOVA, P = 0.877, n = 38) (Fig. 4C) were observed in prefeeding or fed larvae. Additionally, Na+,K+-ATPase gene expression did not reflect the direction of measured changes in rates of ion transport. There is a marginally significant effect of acidification on Na+,K+-ATPase gene expression (ANOVA, P = 0.049, n = 48) (Fig. 4C). Post hoc analysis showed that this effect was driven only by changes in 4-d-old larvae (P < 0.001, n = 3). All other ages showed no differences in gene expression under acidification relative to controls. Although differences were seen in 4-d-old larvae, the ranking was reversed: Gene expression was lower under acidification relative to controls, in contrast to the increased physiological rate of ion transport. Under our experimental conditions, regulation of Na+,K+-ATPase takes place primarily at the physiological level rather than at the biochemical or molecular biological levels.
Fig. 4.
Ion transport rate, total enzyme activity, and gene expression of Na+,K+-ATPase in developing sea urchins. (A) In vivo Na+,K+-ATPase activity was determined from 86Rb+ transport rates, corrected for the specific activity of K+ in seawater, in the absence (solid regression line, rate = 2.1 ± 0.19 picomoles K+ per individual per minute, slope ± SE of slope) and presence (dashed regression line, rate = 0.9 ± 0.18 picomoles K+ per individual per minute) of 2 mM ouabain. (B) In vivo Na+,K+-ATPase activity under control (closed symbols) and seawater acidification (open symbols) treatments in embryos (triangles) and larvae (circles) as a function of body size. Each data point was calculated from time-course assays as shown in A. (Inset) The bar graph shows duplicate measurements on 6- and 8-d-old unfed larvae. Seawater acidification treatment significantly increased in vivo Na+,K+-ATPase activity in growing larvae (ANCOVA, P = 0.016, n = 20) as well as in unfed larvae (Inset). Post hoc test, P = 0.001, n = 8. (C) Relative Na+,K+-ATPase gene expression and total enzyme activity. Error bars indicate 1 SEM, n = 3–4 assays. Where not visible, error bars fall within the graphical representation of the data point. No statistical differences in gene expression or total enzyme activity occurred between control and acidification treatments, with the exception of 4-d-old larvae (∼220 µm). For this stage, gene expression did not predict the changes in physiological rates of ion transport (B), because gene expression was higher in the control relative to acidification treatment (post hoc test, P < 0.001).
Changes in Energy Allocation in Response to Seawater Acidification.
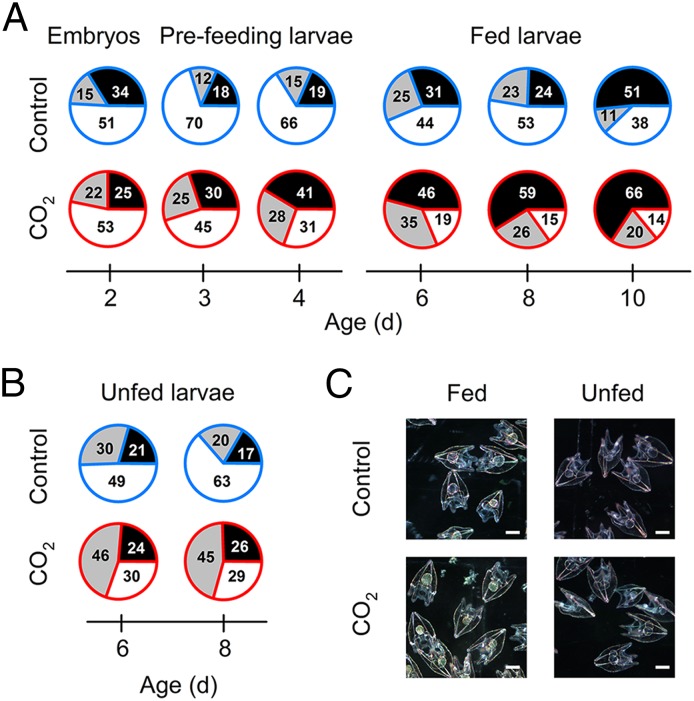
Protein synthesis and Na+,K+-ATPase together accounted for the majority of the allocation of metabolic energy throughout the period of growth and development studied (Fig. 5). The data and calculations that form the basis of Fig. 5 are given in Table S4 and in SI Calculations and Estimations. The proportion of ATP used for these two major energy-requiring processes increased with the transition to exogenous feeding and the commencement of growth, as well as with acidification treatment for all stages tested (with the exception of the earliest developmental stages tested, 2-d-old embryos). For embryos and prefeeding and unfed larvae—stages at which no growth occurred—an average of 40% of total ATP was devoted to protein synthesis and ion transport under control conditions (Fig. 5 A and B). In contrast, the allocation of total ATP for protein synthesis and ion transport increased to 55% in fed growing larvae. Importantly, acidification increased the average ATP allocation to protein synthesis and ion transport from 40 to 62% for prefeeding stages and unfed larvae and from 55 to 84% for feeding larvae (Fig. 5). A noteworthy pattern is evident in which the unaccounted fraction of total ATP decreased gradually from 53% in 2-d-old larvae to 14% in10-d-old larvae under continuous exposure to seawater acidification (Fig. 5A). This decrease in the unaccounted fraction of total ATP can be considered energy that is no longer available to support the demands of routine maintenance metabolism or the ability to respond to other stressors. Again, we emphasize that the major changes in ATP allocation in response to seawater acidification are not reflected by midline body lengths (Fig. 1A), gross morphological characteristics (Fig. 5C), metabolic rate (Fig. 1B), protein content (Fig. 2B), enzyme activity (Fig. 4C), or gene expression (Fig. 4C).
Fig. 5.
Changes in ATP allocation to protein synthesis (black), in vivo Na+,K+-ATPase activity (gray), and the unaccounted fraction of total ATP (white) in developing sea urchins. (A and B) Metabolic energy budgets for embryos, prefeeding, and fed larvae (A) and unfed larvae (B) under control (outlined in blue) and seawater acidification and CO2 (outlined in red) treatments. Values within each pie chart indicate the proportion (%) of the total metabolic rate allocated to each category. Data used for calculation of ATP allocation are given in Table S4. (C) Images of 6-d-old fed and unfed larvae under control and acidification treatments. (Scale bars: 100 µm.)
Discussion
The developmental stages of sea urchins have been used routinely to study biological responses to ocean acidification and to other environmental stressors (6, 9, 10). Often, additional metabolic costs are invoked as an explanation for physiological responses to ocean acidification (11, 12, 21). In the present study we show that, for sea urchins growing at the lower range (∼800 µatm) of the near-future pCO2 level projected by the Intergovernmental Panel on Climate Change (IPCC) (RCP6.0 in ref. 1; further details are given in Table S1), no changes were observed in growth rate, metabolic rate, biochemical composition, or the amount of Na+,K+-ATPase. The response to acidification was dramatic but occurred at other levels of biological analysis. Our results for larval size are in general agreement with another study showing that S. purpuratus exposed to 900 µatm pCO2 had an ∼5% reduction in body length but reporting notable changes in allele frequency and gene expression (17). In our study, we also noted no changes in body length but did observe an acidification-induced increase of ∼50% in the rates of protein synthesis and ion transport. Protein growth was maintained by changing the balance between increased rates of protein synthesis and decreased protein depositional efficiency. Our findings support prior work showing that ocean acidification does not affect protein content (22) but highlight the critical need to study the mechanisms and dynamics of biosynthesis, which undergo dramatic changes to compensate for the maintenance of biochemical content during growth. Similarly, our results show that increases in ion transport by Na+,K+-ATPase and the concomitant increase in ATP demand were not predicted by changes in the expression of the gene coding for that protein or by changes in the amount of total enzyme.
A major conclusion from our work is that the primary metabolic mechanism to respond to ocean acidification in growing sea urchin larvae is a change in allocation within a fixed amount of total ATP to support increased protein synthesis and ion transport. Combined, protein synthesis and ion transport accounted for an average of 84% of the metabolic rate of feeding larvae under acidification, compared with 55% in controls. This ∼30% difference in the allocation of metabolic energy could reduce an organism’s ability to respond to additional energy-demanding environmental stressors and has more general implications for the ability to sustain fundamental biochemical processes (23). For instance, even a relatively small energy requirement for responses to environmental toxicants (24) and other macromolecular synthesis requirements [e.g., RNA (25)] could be constrained by changes in ATP allocation. A comprehensive accounting of the variations in each of the major processes supported by total ATP would improve predictions from models attempting to understand biological responses to ocean acidification and other compounding environmental stressors.
Biochemical Rate Compensation Under Acidification.
Protein synthesis.
Ocean acidification increased the rates of protein synthesis, described in this study as the linear regressions of size-specific synthesis rates with parallel slopes but with different intercepts (Fig. 2B). Because there were no differences in protein growth between treatments (Fig. 2A), this ∼50% elevation in protein synthesis throughout the developmental and growth period studied represents an increase in protein turnover. This increase in protein turnover was analyzed further by calculating protein depositional efficiency (Fig. S2). The size-specific protein depositional efficiency of 34% in larvae of S. purpuratus (270 µm) (Fig. S2) under control conditions agrees well with previously published values for another species of sea urchin, Lytechinus pictus, which range from 21% to 37% depending on developmental stage (18). Additionally, the value we report of ∼2%/h for fractional rates of protein turnover in embryonic stages of S. purpuratus (SI Calculations and Estimations) is consistent with earlier measurements (26), confirming the validity of our techniques and measurements. This increase in protein synthesis under acidification was not limited to a specific size-class of proteins (Fig. S1B). What might be the basis for an up-regulation of synthesis and turnover for so many different proteins? Future research to elucidate the cellular mechanisms of acidification-induced increases in protein synthesis and turnover could focus on the degradation of newly synthesized proteins (27), polypeptide elongation rates (and possible early truncation) on isolated ribosomes (28), or the precision of translation and folding of polypeptides (29). Each of these processes requires considerable amounts of ATP (30). Whatever the specific mechanism, the regulation of protein synthesis and turnover clearly are primary drivers of the changes in ATP allocation under ocean acidification.
Compensating for decreased depositional efficiency (Fig. S2) and increased protein turnover necessitated higher rates of protein synthesis (Fig. 2B), with significant consequences for use of ATP. The essential first step in being able to quantify these impacts was to measure the cost of protein synthesis for early developmental stages of S. purpuratus (Fig. 3). The value we report of 2.4 J/mg protein synthesized is within the range of published values for protein synthesis costs in animals (18, 31–36). The integrative analysis of biochemical and physiological processes we present in this study permits the calculation of stage-specific ATP allocation to protein synthesis during sea urchin development (Fig. 5). Importantly, the cost of protein synthesis in S. purpuratus has a fixed value that is independent of the developmental stages studied and the levels of pCO2 tested (Fig. 3C). Developmental stages responded to ocean acidification by increasing protein synthesis rates and hence the allocation of ATP to protein synthesis but not by altering the cost of synthesizing a unit-mass of protein.
Ion regulation.
A second mechanism contributing to increased ATP demand under acidification is the regulation of ion transport (Figs. 4 and 5). For all the developmental stages and feeding treatments investigated, ion transport accounted for between 11% and 30% of the metabolic rate, a range that increased to 20–46% under acidification (Fig. 5). The increase of in vivo Na+,K+-ATPase activity may be related to acid–base regulation (37). For marine invertebrates, acid–base regulation is an important response mechanism to ocean acidification (12, 38). Additionally, higher in vivo Na+,K+-ATPase activity may be related to enhanced sodium-dependent amino acid transport (39) to support increased protein synthesis under acidification.
Importantly, these significant changes in the demand for ATP to support increased rates of ion transport were not detectable by either total enzyme assays or changes in gene expression (Fig. 4C). There is growing evidence that transcript levels often are uncoupled from the amounts of proteins they encode. For example, recent large-scale transcriptomic and proteomic studies of the expression of ∼5,000 genes and their corresponding proteins have shown that changes in gene expression are not good measures of protein abundance (40). Even when mRNA predicts protein abundance, physiologically relevant protein activity can vary substantially from patterns of gene expression or protein abundance (41–44). For comparative studies of marine organisms that lack comprehensive and simultaneous quantification of transcriptomes and proteomes (40), analyses that infer physiological activity from measurements of gene expression should be interpreted with caution.
Allocation of metabolic energy.
We base our calculations of total metabolic expenditure on measurements of oxygen consumption. There is an extensive literature on the calculations of energetic and biochemical equivalents of respiratory oxygen consumption (thermodynamic and oxyenthalpic energy equivalents) (19). Measurements conducted using direct calorimetry (heat-dissipation rates) and indirect calorimetry (rates of oxygen consumption) on 31 species, including early stages of marine animals, have shown that under normoxic conditions the oxyenthalpic equivalent is fully aerobic, at 463 ± 39 kJ/mol O2 (45). Hence, under normoxic condition, a measured rate of oxygen consumption accounts for all interconvertible energy equivalents, independent of the specific biochemical pathways involved. Metabolic energy budgets in the current study were calculated based on the oxyenthalpic equivalent of 484 kJ/mol O2, an average value for lipid and protein (19, 20). Here, we use ATP as the energy equivalent for the calculation of metabolic allocation (Fig. 5 A and B).
A dramatic finding from this analysis of metabolic allocation is that 84% of the available ATP pool is accounted for by the cost of protein synthesis and ion regulation under acidification conditions (Fig. 5). This surprising capacity to respond to sublethal environmental stresses for an extended period (tested for 10 d) eclipses the fact that the ATP allocation in control conditions is consistent with existing literature. For instance, the summary diagram of ATP-demanding processes given in Hochachka and Somero (ref. 23, p. 28) shows that ∼30% of ATP is allocated to protein synthesis and ∼30% to regulate sodium–potassium flux. These values are similar to those we report for developmental stages in this article: On average, we found that protein synthesis accounted for 27% of ATP, and sodium–potassium pump activity accounted for 19% (Fig. 5). Notably, our measurements reported here are consistent with the range of previously reported values for these processes during sea urchin development (18, 46). The sustainability of the high allocation of ATP under environmental stress, the long-term impact on organismal performance, and the ability to support other essential physiological processes are important themes for future research.
Feeding State Alters Metabolic Rates Under Acidification.
Metabolic responses of unfed larvae.
When larvae developed to the feeding stage (4 d old) but subsequently were unfed through day 10, metabolic rates did increase in response to acidification (Fig. 1B, Inset). It is noteworthy that an increase in metabolic rate—an increase driven entirely by protein synthesis and ion transport—occurred under acidification only for larvae under conditions of food limitation (SI Calculations and Estimations). In the absence of growth in unfed larvae, such metabolic responses likely represent the costs of maintenance under acidification. The mechanisms underlying the differential energy response to the stress of ocean acidification between fed and unfed larvae require further study but likely are related to different anabolic (growth) and catabolic (starvation) states. The difference in metabolic response between fed and unfed larvae suggests that nutritional and physiological states impact the response to many environmental stressors, including ocean acidification (47). Such increases in metabolic demand have important implications for long-term resistance to starvation of larval forms with minimal energy reserves (48, 49).
Metabolic responses of fed larvae.
Once larvae reach feeding competence and are provided with food, rapid growth ensues (Fig. 1A). The metabolic cost of such growth is high in the developmental stages. For instance, in another species of sea urchin, L. pictus, up to 75% of the available ATP pool is allocated to protein synthesis during growth (18). Clearly the large energy requirements for growth, even under ad libitum feeding conditions, greatly constrain the capacity to change ATP allocation under environmental stress. The continuous increase in metabolic rate per individual during growth may allow an accommodation to increased energy demand of major energy-consuming biochemical processes under acidification. A lack of difference in the metabolic rates of larvae under control and acidification treatments belies the substantial differences in ATP allocation. Such cellular changes were not revealed by measurements of oxygen consumption on whole organisms, because changes in ATP allocation were accommodated without an increase in metabolic rate (Fig. 1B). These findings demonstrate that a lack of increase in metabolic rate in response to acidification should not be interpreted to mean that an environmental perturbation has no impact on an organism.
Remarkably, it appears that sea urchin larvae can allocate on average 84% of their total ATP to just two processes without any immediate, negative impact on growth. The physiological limits in the ability of larvae to respond to ocean acidification without increasing metabolic rates may occur at higher pCO2. Such a suggestion is consistent with the findings of an increase in metabolic rates in feeding larvae of S. purpuratus in the presence of pCO2 levels higher than the 800 µatm tested in the current study (11, 16). Future studies of biological responses that focus on differential allocation of ATP could help identify and predict the limits of physiological resilience to compounding interactions of ocean acidification, temperature, food availability, and other consequences of global change.
Tradeoffs of ATP allocation—a basis for physiological resilience.
Ocean acidification experiments often show limited impact at the level of the whole organism. Our results demonstrate that major compensatory responses occur at the cellular level, even when whole-organism responses are minimal. A long-standing question in metabolic regulation is at what point sublethal stress becomes lethal because of energy limitation (21, 23). Our findings of changes in ATP allocation within a tightly constrained energy budget have important implications for organismal resilience. Under changing environments, in which ATP demand may exceed metabolic capacity, individuals with maintenance costs less sensitive to environmental stressors are more likely to survive. Genetically determined variations in the ability to allocate ATP likely will influence the resilience of individuals and the adaptive potential of populations in a changing environment (5). Physiologically, it is important to define the hierarchy of ATP-consuming processes that support the maintenance of essential functions under benign and stressful conditions. The ability to measure the associated metabolic costs, tradeoffs, and limits to ATP allocation to support critical cellular functions will help provide a mechanistic understanding of resilience potential to global change.
Materials and Methods
General Approach and Rationale.
We used an integrative biological approach to study the biochemical and physiological responses to seawater acidification in developing sea urchins. Details of each specific method are given in SI Materials and Methods. In brief, in vivo measurements of metabolic rates, protein synthesis rates, and ion transport rates of Na+,K+-ATPase were conducted. These were complemented with measurements of larval size, in vitro analyses of protein content, total Na+,K+-ATPase enzyme activity, and Na+,K+-ATPase gene expression. To enable the calculation of absolute rates of protein synthesis, individuals at selected ages from control and acidification treatments were analyzed for the mole-percent amino acid composition and for the calculation of the average molecular mass of whole-body total protein (Table S2). A series of experiments was undertaken to determine the energy cost of protein synthesis. The integration of this suite of measurements was used to determine the major biochemical processes responsible for differential energy allocation in response to ocean acidification.
Sea Urchin Culturing and CO2 Treatments.
Adult sea urchins (S. purpuratus) were induced to spawn by intracoelomic injection of 0.5 M KCl. Gametes from males and females were pooled and allowed to fertilize in filtered (pore size, 0.2 µm) seawater at 15 °C (SI Materials and Methods). Eggs from individual females were pretested for fertilization and were used for experiments only if more than 95% fertilization success was observed. For each treatment, newly fertilized eggs were placed in 200-L cylindrical culture vessels equipped with motorized stirring paddles providing slow vertical movement. Cultures were stocked at an initial density of 20 eggs/mL. Control-group cultures were aerated continuously with ambient, atmospheric air. Seawater acidification treatments were aerated with a premixed, compressed air source supplemented with CO2 (Gilmore Air) to yield an average pCO2 of 800.6 ± 9.07 μatm based on daily measurements from each culture vessel (Table S1). The pCO2 levels used reflect present-day and near-future IPCC projections (∼800 µatm) (1). A complete water change was performed for all culture vessels every 2 d. Water was replaced with fresh seawater that had been pre-equilibrated to the appropriate temperature and CO2 treatment level. At the onset of exogenous feeding, 4-d-old larvae from both the control and acidification treatments were stocked in 20-L culture vessels at a starting concentration of 10 larvae/mL into fed and unfed treatments; larvae numbers decreased to ∼2/mL because of sampling during the course of experiments. Larvae were kept in suspension with rotary motorized stirrers. Fed treatments (ad libitum) were supplied with the algae Rhodomonas lens at 30,000 cells/mL, and food was replenished daily. Seawater acidification experiments were replicated with gametes obtained from different sets of adults (i.e., different larval cohorts; see SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Drs. D. Hedgecock and C. Frieder for helpful comments on the manuscript and C. Capron, R. Sawyer, T. Hild, and B. Lentz for technical assistance. This work was supported by National Science Foundation Grant Emerging Frontiers 1220587.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1416967112/-/DCSupplemental.
References
- 1.Clarke L, et al. In: Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Edenhofer O, et al., editors. Cambridge Univ Press; Cambridge, New York: 2014. [Google Scholar]
- 2.Feely RA, et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305(5682):362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- 3.Hönisch B, et al. The geological record of ocean acidification. Science. 2012;335(6072):1058–1063. doi: 10.1126/science.1208277. [DOI] [PubMed] [Google Scholar]
- 4.Somero GN. The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J Exp Biol. 2010;213(6):912–920. doi: 10.1242/jeb.037473. [DOI] [PubMed] [Google Scholar]
- 5.Applebaum SL, Pan TCF, Hedgecock D, Manahan DT. Separating the nature and nurture of the allocation of energy in response to global change. Integr Comp Biol. 2014;54(2):284–295. doi: 10.1093/icb/icu062. [DOI] [PubMed] [Google Scholar]
- 6.Hamdoun A, Epel D. Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA. 2007;104(6):1745–1750. doi: 10.1073/pnas.0610108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: Vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol. 2011;49:1–42. [Google Scholar]
- 8.Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Annu Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- 9.Dupont S, Ortega-Martínez O, Thorndyke M. Impact of near-future ocean acidification on echinoderms. Ecotoxicology. 2010;19(3):449–462. doi: 10.1007/s10646-010-0463-6. [DOI] [PubMed] [Google Scholar]
- 10.Kroeker KJ, et al. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob Change Biol. 2013;19(6):1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stumpp M, Wren J, Melzner F, Thorndyke MC, Dupont ST. CO2 induced seawater acidification impacts sea urchin larval development I: Elevated metabolic rates decrease scope for growth and induce developmental delay. Comp Biochem Physiol A Mol Integr Physiol. 2011;160(3):331–340. doi: 10.1016/j.cbpa.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Stumpp M, et al. Acidified seawater impacts sea urchin larvae pH regulatory systems relevant for calcification. Proc Natl Acad Sci USA. 2012;109(44):18192–18197. doi: 10.1073/pnas.1209174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stumpp M, et al. Digestion in sea urchin larvae impaired under ocean acidification. Nat Clim Chang. 2013;3(12):1044–1049. [Google Scholar]
- 14.Waldbusser GG, et al. Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat Clim Chang. 2015;5(3):273–280. [Google Scholar]
- 15.Kurihara H, Shirayama Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar Ecol Prog Ser. 2004;274:161–169. [Google Scholar]
- 16.Dorey N, Lançon P, Thorndyke M, Dupont S. Assessing physiological tipping point of sea urchin larvae exposed to a broad range of pH. Glob Change Biol. 2013;19(11):3355–3367. doi: 10.1111/gcb.12276. [DOI] [PubMed] [Google Scholar]
- 17.Pespeni MH, et al. Evolutionary change during experimental ocean acidification. Proc Natl Acad Sci USA. 2013;110(17):6937–6942. doi: 10.1073/pnas.1220673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pace DA, Manahan DT. Fixed metabolic costs for highly variable rates of protein synthesis in sea urchin embryos and larvae. J Exp Biol. 2006;209(1):158–170. doi: 10.1242/jeb.01962. [DOI] [PubMed] [Google Scholar]
- 19.Gnaiger E. In: Polarographic Oxygen Sensors: Aquatic and Physiological Applications. Gnaiger E, Forstner H, editors. Springer; New York: 1983. pp. 337–345. [Google Scholar]
- 20.Shilling FM, Manahan DT. Energetics of early development for the sea urchins Strongylocentrotus purpuratus and Lytechinus pictus and the crustacean Artemia sp. Mar Biol. 1990;106(1):119–127. [Google Scholar]
- 21.Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar Environ Res. 2012;79:1–15. doi: 10.1016/j.marenvres.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Matson PG, Yu PC, Sewell MA, Hofmann GE. Development under elevated pCO2 conditions does not affect lipid utilization and protein content in early life-history stages of the purple sea urchin, Strongylocentrotus purpuratus. Biol Bull. 2012;223(3):312–327. doi: 10.1086/BBLv223n3p312. [DOI] [PubMed] [Google Scholar]
- 23.Hochachka PW, Somero GN. 2002. Biochemical Adaptation: Mechanism and Process in Physiological Evolution (Oxford Univ Press, New York, NY), 466 pp.
- 24.Cole BJ, Hamdoun A, Epel D. Cost, effectiveness and environmental relevance of multidrug transporters in sea urchin embryos. J Exp Biol. 2013;216(20):3896–3905. doi: 10.1242/jeb.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77(3):731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 26.Berg WE, Mertes DH. Rates of synthesis and degradation of protein in the sea urchin embryo. Exp Cell Res. 1970;60(2):218–224. doi: 10.1016/0014-4827(70)90508-2. [DOI] [PubMed] [Google Scholar]
- 27.Schubert U, et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404(6779):770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 28.Pace DA, Maxson R, Manahan DT. Ribosomal analysis of rapid rates of protein synthesis in the Antarctic sea urchin Sterechinus neumayeri. Biol Bull. 2010;218(1):48–60. doi: 10.1086/BBLv218n1p48. [DOI] [PubMed] [Google Scholar]
- 29.Sherman MY, Qian SB. Less is more: Improving proteostasis by translation slow down. Trends Biochem Sci. 2013;38(12):585–591. doi: 10.1016/j.tibs.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Escusa-Toret S, Vonk WIM, Frydman J. Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat Cell Biol. 2013;15(10):1231–1243. doi: 10.1038/ncb2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pace DA, Manahan DT. Efficiencies and costs of larval growth in different food environments (asteroidea: Asterina miniata) J Exp Mar Biol Ecol. 2007;353(1):89–106. [Google Scholar]
- 32.Pace DA, Manahan DT. Cost of protein synthesis and energy allocation during development of antarctic sea urchin embryos and larvae. Biol Bull. 2007;212(2):115–129. doi: 10.2307/25066589. [DOI] [PubMed] [Google Scholar]
- 33.Marsh AG, Maxson RE, Jr, Manahan DT. High macromolecular synthesis with low metabolic cost in Antarctic sea urchin embryos. Science. 2001;291(5510):1950–1952. doi: 10.1126/science.1056341. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins AJS, Widdows J, Bayne BL. The relevance of whole-body protein metabolism to measured costs of maintenance and growth in Mytilus edulis. Physiol Zool. 1989;62(3):745–763. [Google Scholar]
- 35.Fuery CJ, Withers PC, Guppy M. Protein synthesis in the liver of Bufo marinus: Cost and contribution to oxygen consumption. Comp Biochem Physiol A Mol Integr Physiol. 1998;119(2):459–467. doi: 10.1016/s1095-6433(97)00452-2. [DOI] [PubMed] [Google Scholar]
- 36.Storch D, Pörtner HO. The protein synthesis machinery operates at the same expense in eurythermal and cold stenothermal pectinids. Physiol Biochem Zool. 2003;76(1):28–40. doi: 10.1086/367945. [DOI] [PubMed] [Google Scholar]
- 37.Hwang PP. Ion uptake and acid secretion in zebrafish (Danio rerio) J Exp Biol. 2009;212(11):1745–1752. doi: 10.1242/jeb.026054. [DOI] [PubMed] [Google Scholar]
- 38.Melzner F, et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeosciences. 2009;6(10):2313–2331. [Google Scholar]
- 39.Wright SH, Manahan DT. Integumental nutrient uptake by aquatic organisms. Annu Rev Physiol. 1989;51(1):585–600. doi: 10.1146/annurev.ph.51.030189.003101. [DOI] [PubMed] [Google Scholar]
- 40.Schwanhäusser B, et al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 41.Marsh AG, Leong PKK, Manahan DT. Gene expression and enzyme activities of the sodium pump during sea urchin development: Implications for indices of physiological state. Biol Bull. 2000;199(2):100–107. doi: 10.2307/1542869. [DOI] [PubMed] [Google Scholar]
- 42.Feder ME, Walser JC. The biological limitations of transcriptomics in elucidating stress and stress responses. J Evol Biol. 2005;18(4):901–910. doi: 10.1111/j.1420-9101.2005.00921.x. [DOI] [PubMed] [Google Scholar]
- 43.Suarez RK, Moyes CD. Metabolism in the age of ‘omes’. J Exp Biol. 2012;215(14):2351–2357. doi: 10.1242/jeb.059725. [DOI] [PubMed] [Google Scholar]
- 44.Yang TH, Somero GN. Activity of lactate dehydrogenase but not its concentration of messenger RNA increases with body size in barred sand bass, Paralabrax nebulifer (Teleostei) Biol Bull. 1996;191(2):155–158. doi: 10.2307/1542918. [DOI] [PubMed] [Google Scholar]
- 45.Hand SC. In: Handbook of Thermal Analysis and Calorimetry. Kemp RB, editor. Vol 4. Elsevier Science; Amsterdam: 1999. pp. 469–510. [Google Scholar]
- 46.Leong PKK, Manahan DT. Metabolic importance of Na+/K+-ATPase activity during sea urchin development. J Exp Biol. 1997;200(22):2881–2892. doi: 10.1242/jeb.200.22.2881. [DOI] [PubMed] [Google Scholar]
- 47.Thomsen J, Casties I, Pansch C, Körtzinger A, Melzner F. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: Laboratory and field experiments. Glob Change Biol. 2013;19(4):1017–1027. doi: 10.1111/gcb.12109. [DOI] [PubMed] [Google Scholar]
- 48.Shilling FM, Manahan DT. Energy metabolism and amino acid transport during early development of Antarctic and temperate echinoderms. Biol Bull. 1994;187(3):398–407. doi: 10.2307/1542296. [DOI] [PubMed] [Google Scholar]
- 49.Moran AL, Manahan DT. Physiological recovery from prolonged ‘starvation’ in larvae of the Pacific oyster Crassostrea gigas. J Exp Mar Biol Ecol. 2004;306(1):17–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.