Abstract
Reducing the levels of toxic protein aggregates has become a focus of therapy for disorders like Alzheimer's and Parkinson's diseases, as well as for the general deterioration of cells and tissues during aging. One approach has been an attempt to influence the production or activity of a class of reparative chaperones called heat shock proteins (HSPs), of which HSP70 is a promising candidate. Manipulation of HSP70 expression results in disposal of misfolded protein aggregates that accumulate in aging and disease models. Recently, HSP70 has been shown to bind specifically to an amino-terminal sequence of a human diffusible survival evasion peptide (DSEP), dermcidin. This sequence includes CHEC-9, an orally available anti-inflammatory and cell survival peptide. In the present study, we found that the CHEC-9 peptide also binds HSP70 in the cytosol of the cerebral cortex after oral delivery in normal rats. Western analysis of non–heat-denatured, unreduced samples suggested that peptide treatment increased the level of active HSP70 monomers from the pool of chaperone oligomers, a process that may be stimulated by potentiation of the chaperone's adenosine triphosphatase (ATPase). In these samples, a small but consistent gel shift was observed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a multifunctional protein whose aggregation is influenced by HSP70. CHEC-9 treatment of an in vitro model of α-synuclein aggregation also results in HSP70-dependent dissolution of these aggregates. HSP70 oligomer–monomer equilibrium and its potential to control protein aggregate disease warrant increased experimental attention, especially if a peptide fragment of an endogenous human protein can influence the process.
Introduction
Several of the heat shock proteins (HSPs) are now considered prime targets for therapy of age-related aggregate disorders.1–4 Experimental manipulation of the levels and activity of HSPs can increase disposal of the accumulated protein aggregates that characterize such diseases, and, at least in roundworms5 and fruit flies,6 will extend life span. If it were possible to manage specific HSP levels for patients in the earlier stages of disorders like Alzheimer's, Huntington's, and Parkinson's diseases, the expected result would be delayed neuron death and slowing of disease progression.
HSP70 has been particularly well studied in disease models because of its close association with ubiquitin–proteasome systems for protein quality control through intracellular protein repair, including the disassembly and the disposal of protein aggregates. HSP70 also influences several other aspects of the pathology of aggregate disorders, such as inflammation, oxidative stress, and cell death, all processes that accompany excessive protein aggregation.7–9 Recently, Stocki et al.10 reported specific binding of HSP70 to a bioactive sequence near the amino-terminus of a human diffusible survival evasion peptide (DSEP), dermcidin. The targeted sequence includes CHEC-9, the cyclized version of which is a broad-spectrum secreted phospholipase A2 inhibitor with multiple survival and anti-inflammatory activities in vitro and in vivo.11–14 Given the well-known peptide carrier functions of HSP70, and noting that the properties of HSP70 and the DSEP peptide(s) are similar, these authors suggested that the chaperone actually protected the peptide and prolonged its survival/anti-inflammatory activity.10 Whatever the case, the relationship of HSP70 to this portion of the DSEP protein is likely to be of interest because potentially therapeutic peptides have been derived from this region (Y-P30, CHEC-7, CHEC-9). In addition, in vivo effects of the CHECs can be demonstrated after oral delivery, making the peptide a convenient candidate for clinical applications. In this study, we documented peptide binding to HSP70 in the central nervous system (CNS) of rats and documented the effects of peptide treatment on both HSP70 itself and on client proteins whose aggregation state is regulated by HSP70.
Methods
Animals
The study used 41 Sprague–Dawley rats of both sexes, weighing 225–300 grams. For the data reported, we did not detect consistent differences that could be attributed to gender. The rats were housed at least two/cage and fed a standard diet of rat chow. They were handled daily for 1 week prior to treatment to minimize stress during handheld introduction of experimental compounds. All animal procedures were conducted under the auspices of a protocol approved by the Institutional Animal Care & Use Committee of Drexel University.
Peptide synthesis, cross-linking, and dosing
CHEC-9 was synthesized as a linear peptide (Celtek, Nashville, TN). It was internally cross-linked at a concentration of 273 μM overnight after dissolving in 20 mM Tris (pH 7.8) (for storage as aliquots of solution at −80°C) or in 5 mM NaH2PO4 (pH 7.6) for drying and storage as solid aliquots. Cysteine linkage was monitored by using Ellman's reagent, and internal linkages verified intermittently by mass spectroscopy.13 The rats were fed a 150-μL solution of dilute strawberry gelatin with or without CHEC-9. Dosage and sacrifice times were selected on the basis of pharmacodynamics demonstrated in previous studies12 and on the basis of pilot experiments in the present study (see Results). Three more rats were sacrificed without treatment to establish parameters for both detection and quantification of immunoreactivity on western blots and in immunoprecipitation experiments.
Tissue and plasma preparation
The animals were sacrificed by decapitation with or without prior saline perfusion under isoflurane anesthesia. Cytosol preparations were from the frontal cerebral cortex. Dissections included the entire mediolateral expanse of tissue from the frontal pole (at the junction with the olfactory bulb) exactly 5 mm caudally. The cortical tissue, including white matter and above, was further dissected and immediately homogenized in 25 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 2 mM EDTA with protease inhibitor cocktail (Complete Mini, Roche). Cytosol samples were obtained from the supernatant (S2) following ultracentrifugation (100,000×g, 1 hr) after an initial nuclear spin (500×g, 20 min) of the homogenate (S1). Plasma was prepared from trunk blood as in previous studies.11,12
Sodium dodecyl sulfate polyacrylamide gel electrophoresis, western blotting, and immunoprecipitation
Gel electrophoresis and western blotting using chemiluminescent detection were as described in prior studies.14 Protein loading was equivalent, and loading controls were used in all cases. The migration of putative control protein glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in non-denatured, un-reduced gels was influenced by peptide treatment so it was used only with chemically reduced samples. Loading controls for unreduced non–heat-denatured gels prepared from tissue were residual Coomassie Blue–stained bands recorded after protein transfer, or actin immunostaining for the in vitro experiments. Immunostaining for immunoglobulin G (IgG) light chain served as a loading control for plasma samples. Measurements of the control bands that were used for quantification of the blots showed no statistical differences between experimental groups and varied by no more than 10%. Heat denaturation and chemical reduction as noted for some experiments were accomplished by inclusion of 5% β-mercaptoethanol in the sample buffer and boiling the samples for 5 min prior to loading. After transfer, the blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody, and the membranes were processed for enhanced chemoluminescence (ECL) immunodetection. Pairs of peptide-treated and control-treated samples were run side-by-side on the gels and processed together. The area and density of all immunopositive bands were measured using ImageJ. Monomeric HSP70 levels were determined by adding the adjusted whole-band densities of the two to three HSP70 immunopositive species between 70–78 kD in non-denatured, unreduced gels. Gels containing serial dilutions of total protein loaded for both normal rat cortex cytosol and SY5Y lysates were used to verify linearity of the chemiluminescent signal under the conditions used.
Immunochemicals were from commercial sources. Primary antibody (Ab) against HSP70 was Thermo monoclonal antibody (mAb) M3-008. (Note: HSP70s are a family of proteins encoded by more than a dozen different genes. The family includes both constitutive [Hsc70] and induced isoforms sometimes referred to as HSP72. We did not differentiate between these; immune reagents reveal from one to three bands near 70K, which we referred to collectively as HSP70.) Other antibodies were against α-synuclein (Cell Signaling, mAb 2647S), CHEC-9 (Neo Group, Inc., affinity-pure polyAb), GAPDH (Sigma, G9545, affinity-pure polyAb), and actin (A3853, Sigma-Aldrich). Anti-IgG light chain and all secondary antibodies were from Jackson Immunolabs. Immunoprecipitation of cytosolic samples with the CHEC-9 antibody was accomplished after incubation of the antibody with protein A/G sepharose beads (Rockland Immunochemicals, Inc.) following the manufacturer's protocol. The beads were boiled in sodium dodecyl sulfate (SDS) sample buffer with reducing agent to remove bound proteins prior to western blotting.
In vitro studies
Cultures of the human SY5Y cell line (American Type Culture Collection, Manassas, VA) were prepared as described previously.14 The cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and antibiotic/antimycotic (Gibco) at 37°C in a saturated humidity atmosphere containing 95% air and 5% CO2. Established cultures were exposed to 0.04 μM rotenone (Sigma), followed in 10 min by CHEC-9 or vehicle. Adherent cells were collected after 24 hr. In some experiments, VER-155008 (Sigma) was added 2 hr prior to rotenone exposure to inhibit HSP70.15 Lysis buffer was the same as homogenizing buffer (see above) with the addition of 0.2% Tween 20.
ATPase assay
HSP70/40 adenosine triphosphatase (ATPase) activity was measured using the reaction conditions designed specifically for HSP40/70 ATPase activity.16 Both chaperones are used to demonstrate baseline activity. Pi was detected fluorometrically.17 HSP70 and HSP40 (ENZO NSP-555, SPP-405) were prepared in assay buffer (0.017% Triton X-100, 100 mM Tris-HCl, 20 mM KCl, and 6 mM MgCl2, pH 7.4) to achieve a final HSP40 and HSP70 concentration of 1.0 and 1.1 μM, respectively, in a 150-μL total reaction volume. An aliquot of 14 μL of this mixture was added into each well of a 96-well plate. To this solution, 116 μL of the Pi fluorometric indicator solution (N[7]-methylguanosine, nucleoside phosphorylase) was added (final concentrations 45 μM and 0.1 unit, respectively). CHEC-9 at the indicated concentrations or an equivalent volume of vehicle was added in buffer containing 0.1 M Tris-HCl (pH 7.5), 4 mM CaCl2. The entire mixture was incubated for 20 min at 37°C, after which 20 μL of adenosine triphosphate (ATP) (final concentration 1 mM) was added to start the reaction. The average decrease in fluorescence over 5 min was recorded in a Perkin Elmer Wallac 1420 microplate reader. The measurements from at least five experiments per reaction condition were collected and compared to a dipostassium phosphate (K2PO4) standard curve with the same indicator solutions. Specific reagents were omitted in various controls.
Results
CHEC dosing and survival time
Traditional pharmacokinetic studies were not possible due to CHEC binding of HSP70 and perhaps other plasma proteins, as well as the apparent necessity of this binding for biological activity. Therefore, we relied on pharmacodynamic parameters as in previous studies. Specifically, previous dose–response experiments12 showed that plasma inhibition of secreted phospholipase A2 activity and changes in specific plasma lipids were maximal at 3 hr with an oral CHEC dose of 1.0 mg/kg. For the present study, we also used the observation that the oral peptide at this dose elevated plasma HSP70 levels when comparing CHEC-treated and vehicle-treated control rats (Fig. 1D). Therefore, a pilot study was conducted, sacrificing CHEC- and control-treated rats in pairs at each of 30 min and 1, 1.5, 2.5, and 5 hr (two to three pairs/time point). We found consistent increases in plasma HSP immunoreactivity at 150 min post-ingestion in these and subsequent studies of five more pairs of animals (seven out of eight rat pairs total showed this increase). Therefore, the data derived from samples of frontal cortex cytosol were from rats treated according to this regimen.
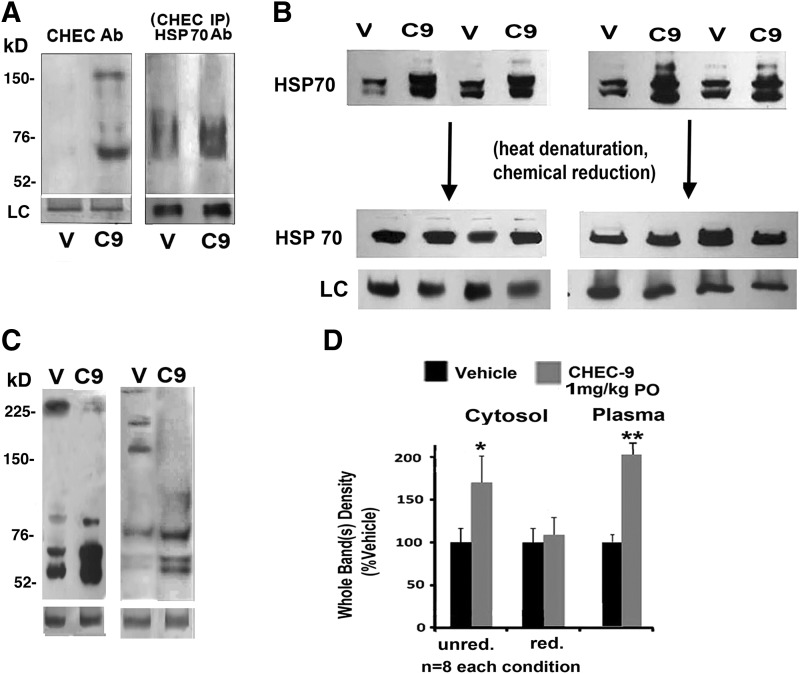
FIG. 1.
Binding and effects of oral CHEC-9 in the frontal cerebral cortex of rats. (A) CHEC immunostaining in frontal cortex cytosol 150 min after 1 mg/kg CHEC-9 per os. Gels were run under non-reducing conditions. (Left panel) Immunopositive bands appeared only in CHEC-9–treated rats. Loading controls were partially transferred Coomassie Blue–stained gel bands (see Methods). (Right panel) Immunoprecipitation of cytosolic samples with CHEC-9 reactive antibody also comparing peptide- and vehicle-treated rats. Elution of the affinity matrix required boiling and chemical reduction of the samples prior to electrophoresis. These blots, immunostained for HSP70, showed bands also in the 70-kD region, prominent in CHEC-9–treated rats. Loading controls were glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are representative of five CHEC—vehicle pairs. (B) The response of cytosolic HSP70 to CHEC treatment. Multiple examples of paired CHEC-9– and vehicle-treated rats (C9 and V) run on 12.5% polyacrylamide gels. Individual pairs, shown before and after boiling and disulfide reduction, are aligned vertically. LC-loading control is GAPDH for reduced samples. (C) Two examples of cytosolic samples run on 7% gels in which higher-molecular-weight species containing HSP70 were recovered in vehicle-treated rats. (D) Quantification of the results. HSP70 immunostaining in the cytosol of rats treated with CHEC-9 was significantly increased in gels without heat denaturation and chemical reduction (unred.). After inclusion of these steps (red.), the differences were no longer significant. Plasma samples also show differences in HSP70 levels, in these cases independent of pretreatment (see text). n=8 for each condition. (**) p<0.01.
Peptide binding and effects on HSP70 in vivo
The affinity of the CHEC sequence for HSP70, documented previously in vitro,10 was examined in the cytosolic fraction of the frontal cortex of rats after oral delivery of the peptide. Blotted proteins reacted with the affinity-purified CHEC-9 antibody revealed a prominent band between 70 and 78 kD (Fig. 1A). There were more variably appearing bands at higher molecular weights. All of these bands were absent in control-treated rats. After immunoprecipitation of the cytosolic samples with the same peptide antibody, we recovered HSP70 immunopositive bands in this same region, suggesting that the peptide was bound to HSP70 in the frontal cortex cytosol (Fig. 1A).
There were also significant effects of CHEC-9 treatment on HSP70 levels, effects that differed depending on whether or not the samples were boiled and pretreated with β-mercaptoethanol, a disulfide reducing agent. Non-thermally denatured, un-reduced samples showed up to three HSP70 monomer bands, as has been described by others working with native polyacrylamide gels.18 These bands were consistently increased in the peptide-treated rats (Fig. 1B). Reduced, denatured samples, on the other hand, produced single bands that were not different quantitatively between peptide-treated and control-treated rats (Fig. 1D). When the same non-denatured samples were run on lower-percentage gels, higher-molecular-weight aggregates appeared at variable positions in vehicle-treated samples (Fig. 1C). Some of these were distinct bands and some were higher-molecular-weight species accumulated at the top of the gels. It was also assumed, on the basis of the potential size of HSP complexes reported by others under native conditions, that some complexes did not enter the gels at all.19 As noted above, plasma samples from these same-peptide-treated rats also showed increased HSP monomers (Fig. 1D), but in this case the increase was found with and without heat denaturation and chemical reduction (see Discussion).
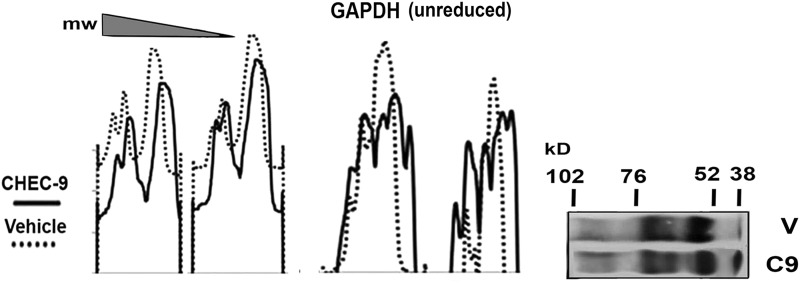
GAPDH gel shift
Immunostaining for GAPDH was originally planned as a gel loading control for the western blots of the cytosol samples. In chemically reduced, heat-denatured samples, both the HSP70 and GAPDH bands appeared as expected, allowing the latter to be used successfully to control the experiments (Fig. 1B, bottom panels). Without these steps, several GAPDH aggregate bands appeared. The bands were shifted in the gels prepared from peptide-treated rats, suggesting lower-molecular-weight species now populated the samples. This gel shift was observed by both inspection and by density plots of the gels (Fig. 2). To rule out some unknown mechanism where CHEC operates systemically as a nonspecific reducing agent in the cortex, CHEC-treated samples were also immunostained for other related molecules, including HSP40 and HSP90. We did not observe any reduction of higher-molecular-weight complexes of these other proteins after peptide treatment (data not shown).
FIG. 2.
CHEC-9 treatment produces glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gel shift in unreduced non-denatured cytosolic samples. Multiple examples of GAPDH immunopositive bands appear near the bottom of gels in the treated rats (from a total of eight pairs of animals). The shift toward smaller-molecular-weight species is apparent in densitometric scans of the gels and in the gel lanes (at right).
In vitro studies of α-synuclein disaggregation
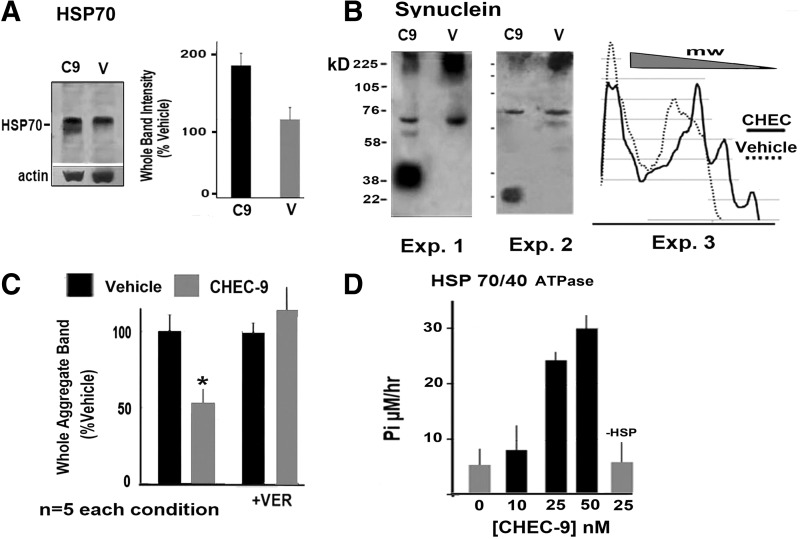
To further determine the functional relevance of the effects of CHEC treatment on HSP70 monomer levels, we tested the peptide in an established in vitro model of α-synuclein aggregation, a model generally used to simulate the aggregate pathology of Parkinson's and Lewy body diseases.20 Exposure of human SY5Y neural cells to rotenone (a mitochondrial complex 1 inhibitor) resulted in the accumulation of α-synuclein aggregates in lysates of the cells, as has been described.20 In the rotenone-treated cultures, also treated with CHEC-9 (50 nM, 10 min after rotenone exposure), we found increased HSP70 immunostaining in unreduced, non–heat-denatured gels, as was expected from the in vivo experiments (Fig. 3A). In these cases, the monomeric isomers of HSP70 were much less distinct than observed for rat cortex. There were striking differences in the migration of α-synuclein bands, with a clear shift to lower-molecular-weight aggregates after peptide treatment. These differences were seen by direct observation of the western blots and in density plots of the blots (three of the five different experimental pairs are shown; Fig. 3B), and after quantification of the largest aggregate bands appearing on the gels (Fig. 3C). The variability in the extent of synuclein disaggregation, i.e., in the different observed sizes of the reduced bands appearing after peptide treatment, would be expected if a chaperone-related mechanism were operative, since such a mechanism may produce intermediates of various sizes.21 The involvement of HSP70 was tested directly by pretreatment of the cultures with VER-155008, a specific HSP70 inhibitor that targets the amino-terminal ATPase (nucleotide binding) portion of the HSP70 molecule. Measurement of the largest aggregate bands from these lysates suggested that CHEC-9's disaggregation activities fell to control levels after HSP70 inhibition (Fig 3C).
FIG. 3.
In vitro and solution assays following treatment with the CHEC peptide. Heat shock protein (HSP) and α-synuclein immunostaining were measured after CHEC-9 treatment of rotenone-exposed SY5Y cells. Immunoblots are from SY5Y cell lysates run without heat denaturation and chemical reduction after the cultures were treated with 0.04 μM rotenone followed by CHEC-9 or vehicle for 24 hr. (A) Quantification of HSP70 immunoreactive bands in lysates. The data are expressed as percent vehicle. (B) Immunostaining of α-synuclein aggregates. Three of the experiments are shown, with the western blots and with a densitometric scan. The high-molecular-weight aggregates were diminished and prominent reduced bands appeared in peptide-treated samples. In other cases, the α-synuclein bands were simply spread to include lower-molecular-weight oligomers or smaller α-synuclein complexes, species that were not detected in un-reduced non–heat-denatured vehicle controls after rotenone treatment. (C) Quantification of the results comparing the size of the largest aggregated band in CHEC-9 and vehicle-treated pairs. Significant differences in the sizes of these large aggregates were evident in peptide-treated lysates. Pretreating the cultures for 2 hr with HSP70 inhibitor VER-155008 eliminated the CHEC—vehicle differences. (D) Graph showing HSP70/40 adenosine triphosphatase (ATPase) activities in solution. Chaperone ATPase activity was potentiated significantly after addition of CHEC-9 to the reaction mixture. As noted in text, the HSP40/70 combination was required to demonstrate baseline activity. Significance levels are as in Fig. 1. The data in Fig. 3 were from five CHEC–vehicle pairs in all experimental conditions.
Synuclein aggregates also appeared without rotenone treatment, but inconsistently, presumably related to the level of endogenous oxidative stress of the cells.22 We did not include these no-rotenone conditions in our measurements because of these inconsistencies in the in vitro model and questionable relevance to in vivo mechanisms. Rotenone consistently exaggerated synuclein aggregation in all cases, overcoming the variable baseline and allowing the peptide's disaggregation effects to be readily quantified.
HSP70 ATPase activity
An investigation of peptide effects on HSP70's ATPase activity was prompted by the results with VER-155008, which inhibits HSP70 at the nucleotide binding site, and by the dependence of HSP70 monomerization on ATP.19,23 These experiments showed that HSP70/HSP40 ATPase was potentiated by CHEC-9 in solution (Fig. 3D; a starting mixture of HSPs 40 and 70 was required to demonstrate baseline activity). All controls (omitting various reagents) were negative, except there was a non-significant trend for the highest concentration of CHEC (50 nM) to hydrolyze ATP in the absence of HSP40/70.
Discussion
Peptides of DSEP/dermcidin
The CHEC-9 peptide fragment and its parent polypeptides have been consistently identified as “survival-promoting.” 24–30 The active species include full-length DSEP when expressed and exogenously applied synthetic amino-terminal peptides, including Y-P30, CHEC-9, and CHEC-7. DSEP has also been identified as dermcidin, and the carboxy-terminal portion of the molecule appears to be anti-microbial. A glycosylated peptide that includes a sequence similar to Y-P30 may constitute a cachexic factor, also known as proteolysis-inducing factor (PIF).31,32 There are also species restrictions in that the coding for DSEP/dermcidin may be limited to primates, although biological activity of the peptides appears to extend to rats. CHEC-9 and CHEC-7 are the smallest biologically active fragments of DSEP, and synthetic versions of these peptides inhibit several isoforms of secreted phospholipase A2, consistent with their survival and anti-inflammatory activities.11,12 The enzyme inhibition has been verified in vitro and in vivo for rats and ex vivo for human samples, but is greatly attenuated in mouse (C57BL/6, unpublished observations), a fact that limits the models available for testing of the peptide. The DSEP/dermcidin protein and its peptide derivatives therefore have a rich and diverse biological history.
HSP70 binding
The report of specific HSP70 binding to a peptide that includes CHEC-9 was suggested to contribute to the peptide's pro-survival activities.10 HSP70 itself is actually involved in both cell survival and inflammation, as well as serving a “triage” function by channeling misfolded proteins for either repair or proteasomal destruction.33 We also investigated HSP70 interactions and found that that HSP70 binding occurs in the cytosol of the CNS after systemic introduction of the peptide in rats. Peptide immunopositive bands appeared exclusively in the treated rats and HSP70 was recovered after immunoprecipitation of the same cytosolic fractions of cerebral cortex with a peptide antibody. There were also consistent effects of CHEC-9 treatment on HSP70 levels in the cytosol, effects that differed depending on whether or not the samples were boiled and pretreated with β-mercaptoethanol, a disulfide reducing agent (Fig. 1B, D). Heating and chemical reduction precluded oligomerization and disrupted differences in tertiary structure responsible for the unique gel migration properties of the different HSP70 monomers.18,34 As a result, the quantitative differences in HSP monomers between peptide- and control-treated rats disappeared. These observations strongly suggested that native HSP70 monomers were increased by reduction of HSP70 oligomers and complexes after systemic peptide treatment. HSP70 not only oligomerizes with itself but also binds a number of species, notably large aggregates in need of repair and co-chaperone complexes that are required for processing of misfolded proteins.19 Some of these complexes were in fact observed in vehicle-treated samples when run on low-percentage gels, although their appearance was variable, as might be expected, depending on the requirements for proteostasis during the treatment period. The larger HSP70 complexes may have provided for reduced antibody accessibility or were unable to enter the gel at all when run under non-denaturing conditions.19
The data showed that CHEC targets these HSP70 complexes in the cytosol, the likely site of the chaperone's activities related to protein repair or disposal. HSP70 is also secreted from a variety of cell types.35 Therefore, it was not surprising that plasma HSP70, which is one manifestation of the secreted chaperone, appeared in excess, regardless of how the samples are prepared, assuming the increase was generated intracellularly prior to secretion. Furthermore, the fact that there was a different response of cytosolic and plasma HSP70 to peptide treatment argued against some technical artifact introduced after recovery of the samples.
Therefore, we concluded from these results that peptide binding to HSP70 serves to mobilize the monomeric forms of the molecule. The significance of this result may lie in the well-established fact that monomers of HSP70 are required for protein targeting and subsequent repair, disaggregation, and disposal activities (see below). Importantly, this is not the first demonstration of the ability of a synthetic peptide to increase the supply of active chaperone monomers. A similar effect, demonstrated with similar techniques, has been reported after peptide treatment of binding immunoglobulin protein (BiP).36 In the present case, the target was the CNS and the CHEC peptide was orally available, factors that should further increase interest in this mechanism of chaperone activation.
Glyceraldehyde-3-phosphate dehydrogenase
GAPDH is commonly viewed as a housekeeping protein and often used as a loading control in polyacrylamide SDS gels run under reducing conditions. However, GAPDH and its aggregates have an important role in oxidative stress-induced brain damage and progressive neurodegenerative disorders.37 GAPDH aggregation can also be regulated by HSP70. For example, elevated Hsp70 protects neurons by removing homotypic and heterotypic (GAPDH+polyQ) aggregates in a model of Huntington's disease.4 In the present study, several of these aggregates appeared on the gels in the unreduced, non–heat-denatured samples. These were affected by peptide treatment, as demonstrated by a shift of aggregates to lower molecular weights. In other words, the peptide-induced increases in disaggregase activity of HSP70 may have affected larger GAPDH aggregates, producing the observed shift in molecular weights.
In vitro disaggregase activity and CHEC-9 targeting of HSP70 ATPase
The predominance of aggregated versus non-aggregated forms of HSP70 depends on environmental stress, levels of ATP, and the presence of denatured proteins.18,19,23,38 Therefore, regulation of monomer–oligomer equilibrium of HSP70 is potentially important for regulation of its function, as is the case for many other cellular proteins. The present study suggested that the reduction of α-synuclein aggregates after CHEC-9 treatment depended on these HSP70 activities because the disaggregase activity was eliminated with the HSP inhibitor VER-150008. Because VER-150008 targets the amino-terminal ATPase (nucleotide-binding) portion of HSP70, the result was also consistent with peptide targeting of the ATPase site on HSP70. The enzyme assays showed potentiation of HSP70/40 ATPase activity by CHEC-9 and therefore were also consistent with the targeting of HSP70 ATPase. The results of Blond-Elguindi et al., demonstrating peptide stimulated production of monomers of the BiP chaperone, was also via a BiP ATPase.36
Additional support for an enzymatic mechanism underlying CHEC-9 activity was found by inspection of the peptide's amino acid sequence: Cys-His-Glut-Ala-Ser-Ala-Ala-Gln-Cys. This familiar motif consisted of a “catalytic” histidine followed by a metal-binding acidic residue (glutamic or aspartic acid) and flanked by stabilizing cystines. These are all conserved features of the active site of several phosphatases and phospholipases. The sequence was therefore consistent with a putative enzyme inhibitor, mimetic, or possibly both. Inhibition of secreted phospholipase A2 by the CHEC peptide has been demonstrated.11,12 This inhibition was assumed to be basis for the anti-inflammatory and neuroprotective properties of the peptide. Interestingly, a longer DSEP peptide that contains the CHEC sequence Y-P30 showed phosphatase activity when reacted with the experimental phosphatase substrate p-nitrophenylphosphate (pNPP).30 This history, the fact that VER-155008 blocks HSP70 at the nucleotide binding site, the dependence of HSP70 monomerization on ATP,19,23 and finally, the peptide's potentiation of HSP70 ATPase activity in solution, all led to the suggestion that CHEC acts at the ATPase site of HSP70 rather than the expected carboxy-terminal substrate/peptide binding site (see, however, ref. 10). Alternatively, CHEC binding at the carboxy-terminal site may have occurred because binding in this domain also regulates ATPase activity indirectly via allosteric mechanisms involving an inter-domain linker.34
Regulation of HSP70 disaggregase activity
The disaggregase functions of HSP70 are complex. The relationship of ATPase activity to these functions has been well studied but with mixed results. Experiments that targeted cells with experimentally-induced protein aggregates characteristic of neurodegenerative diseases (α-synuclein [Parkinson's], β-amyloid [Alzheimer's], polyglutamine expansion [Huntington's], tau [Alzheimer's, tauopathies]) demonstrated that manipulation of HSP70 ATPase activity indeed effected the chaperone's pro-survival and disaggregase activities.2,39–41 However, both targeted inhibition and exaggeration of the ATPase increased (or decreased) cell survival or aggregate protein disposal mechanisms (alone or together), depending on the model. The inconsistencies are perhaps explained by the fact that there are at least two ATP-driven processes associated with HSP70's targeting of misfolded proteins—one related to HSP70 monomer production and a second related to the catch and release of client proteins or peptides in need of repair, disposal, or other processing. These different ATPase-dependent steps are currently indistinguishable.23 Adding to this complexity is the fact that other HSPs and helper molecules participate in these functions. In human cytosol, for example, HSP110 is a significant collaborator with HSP70/40/nucleotide exchange factor machinery for disaggregation activities.42,43 We found no evidence that the CHEC-9 peptide was directly involved in this or any other specific protein repair or disposal machinery. Rather the results suggested that peptide simply augmented this machinery by mobilizing and increasing the supply of monomeric HSP70.
Conclusions
The present results highlight the potential of regulating HSP70 oligomer–monomer equilibrium as a worthwhile approach to age-related aggregate disease and perhaps to the aging process itself. Further studies are required to determine if the principal targets of the CHEC peptide, HSP70 and sPLA2, as well as the distinct but related functional effects that have been associated with peptide activity at these targets, represent independent or interdependent and synergistic processes. The anti-inflammatory effects of the peptide may be particularly important in this respect if they can be shown to operate alongside the disaggregase activity. In the best case, therefore, peptide structure and dosing regimens might be optimized to simultaneously target all three of the most serious pathological changes in aging tissues—inflammation, cell death, and the accumulation of protein waste.
Acknowledgments
This work was supported by Pennsylvania Department of Health, Commonwealth Universal Research Enhancement Program and by NS16487 from National Institute of Neurological Disorders and Stroke (NINDS). The authors thank Dolly Testa for technical assistance.
Author Disclosure Statement
CHEC-9, the peptide used in these studies, has been patent protected by Drexel University, and three of the authors: T. Cunningham, J. Greenstein, and L. Yao. No competing financial interests exist for the remaining authors.
References
- 1.Kalia SK, Kalia LV, McLean PJ. Molecular chaperones as rational drug targets for Parkinson's disease therapeutics. CNS Neurol Disord Drug Targets 2010:9:741–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jinwal UK, Koren J, O'Leary JC, Jones JR, Abisambra JF, Dickey CA. Hsp70 ATPase modulators as therapeutics for Alzheimer's and other neurodegenerative diseases. Mol Cell Pharmacol 2010;2,:43–46 [PMC free article] [PubMed] [Google Scholar]
- 3.Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 reduces α-synuclein aggregation and toxicity. J Biol Chem 2004:279:25497–25502 [DOI] [PubMed] [Google Scholar]
- 4.Lazarev VF, Sverchinskyi DV, Ippolitova MV, Stepanova AV, Guzhova IV, Margulis BA. Factors Affecting aggregate formation in cell models of Huntington's disease and amyotrophic lateral sclerosis. Acta Naturae 2013;5:81–89 [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu A-L, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003;300:1142–1145 [DOI] [PubMed] [Google Scholar]
- 6.Sarup P, Sorensen P, Loeschcke V. The long-term effects of a life-prolonging heat treatment on the Drosophila melanogaster transcriptome suggest that heat shock proteins extend lifespan. Exp Gerontol 2014;50:34–39 [DOI] [PubMed] [Google Scholar]
- 7.Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann NY Acad Sci 2005;1053:74–83 [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA. The 70 kDa heat shock protein protects against experimental traumatic brain injury. Neurobiol Dis 2013;58:289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson's disease. Parkinsonism Relat Disord 2004;10:S3–S7 [DOI] [PubMed] [Google Scholar]
- 10.Stocki P, Wang XN, Morris NJ, Dickinson AM. HSP70 natively and specifically associates with an N-terminal dermcidin-derived peptide that contains an HLA-A*03 antigenic epitope. J Biol Chem 2011;286:12803–12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham TJ, Maciejewski J, Yao L. Inhibition of secreted phospholipase A2 by neuron survival and anti-inflammatory peptide CHEC-9. J Neuroinflamm 2006;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham T, Lucena A, Greenstein J, Yao L. Uncompetitive phospholipase a2 inhibition by CHEC sequences including oral treatment of experimental autoimmune myeloencephalitis. The Open Enzyme Inhibition Journal 2009;2:1–17 [Google Scholar]
- 13.Cunningham TJ, Souayah N, Jameson B, Mitchell J, Yao L. Systemic treatment of cerebral cortex lesions in rats with a new secreted phospholipase A2 inhibitor. J Neurotrauma 2004;21:1683–1691 [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Yao L, Cunningham TJ. Secreted phospholipase A2 involvement in neurodegeneration: Differential testing of prosurvival and anti-inflammatory effects of enzyme inhibition. PloS One 2012;7:e39257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massey AJ, Williamson DS, Browne H, Murray JB, Dokurno P, Shaw T, Macias AT, Daniels Z, Geoffroy S, Dopson M, Lavan P, Matassova N, Francis GL, Graham CJ, Parsons R, Wang Y, Padfield A, Comer M, Drysdale MJ, Wood M. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother Pharmacol 2010;66:535–545 [DOI] [PubMed] [Google Scholar]
- 16.Chang L, Bertelsen EB, Wisen S, Larsen EM, Zuiderweg ER, Gestwicki JE. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal Biochem 2008;372:167–176 [DOI] [PubMed] [Google Scholar]
- 17.Banik U, Roy S. A continuous fluorimetric assay for ATPase activity. Biochem J 1990;266:611–614 [PMC free article] [PubMed] [Google Scholar]
- 18.Benaroudj N, Fouchaq B, Ladjimi MM. The COOH-terminal peptide binding domain is essential for self-association of the molecular chaperone HSC70. J Biol Chem 1997;272: 8744–8751 [DOI] [PubMed] [Google Scholar]
- 19.Angelidis CE, Lazaridis I, Pagoulatos GN. Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem/FEBS 1999;259:505–512 [DOI] [PubMed] [Google Scholar]
- 20.Watabe M, Nakaki T. Rotenone induces apoptosis via activation of bad in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Therapeut 2004;311:948–953 [DOI] [PubMed] [Google Scholar]
- 21.Sharma SK, De Los Rios P, Goloubinoff P. Probing the different chaperone activities of the bacterial HSP70-HSP40 system using a thermolabile luciferase substrate. Proteins 2011;79:1991–1998 [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Hsu LJ, Xia Y, Takeda A, Sisk A, Sundsmo M, Masliah E. Oxidative stress induces amyloid‐like aggregate formation of NACP/α‐synuclein in vitro. NeuroReport 1999;10:717–721 [DOI] [PubMed] [Google Scholar]
- 23.Benaroudj N, Triniolles F, Ladjimi MM. Effect of nucleotides, peptides, and unfolded proteins on the self-association of the molecular chaperone HSC70. J Biol Chem 1996;271:18471–18476 [DOI] [PubMed] [Google Scholar]
- 24.Landgraf P, Sieg F, Wahle P, Meyer G, Kreutz MR, Pape HC. A maternal blood-borne factor promotes survival of the developing thalamus. FASEB J 2005;19:225–227 [DOI] [PubMed] [Google Scholar]
- 25.Lowrie AG, Wigmore SJ, Wright DJ, Waddell ID, Ross JA. Dermcidin expression in hepatic cells improves survival without N-glycosylation, but requires asparagine residues. Br J Cancer 2006;94:1663–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter D, Weremowicz S, Chin K, Seth P, Keshaviah A, Lahti-Domenici J, Bae YK, Monitto CL, Merlos-Suarez A, Chan J, Hulette CM, Richardson A, Morton CC, Marks J, Duyao M, Hruban R, Gabrielson E, Gelman R, Polyak K. A neural survival factor is a candidate oncogene in breast cancer. Proc Natl Acad Sci USA 2003;100:10931–10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schittek B. The multiple facets of dermcidin in cell survival and host defense. J Innate Immun 2012;4:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macharadze T, Landgraf P, Pape HC, Wahle P, Kreutz MR. Y-P30 confers neuroprotection after optic nerve crush in adult rats. Neuroreport 2011;22:544–547 [DOI] [PubMed] [Google Scholar]
- 29.Cunningham TJ, Jing H, Akerblom I, Morgan R, Fisher TS, Neveu M. Identification of the human cDNA for new survival/evasion peptide (DSEP): Studies in vitro and in vivo of overexpression by neural cells. Exp Neurol 2002;177:32–39 [DOI] [PubMed] [Google Scholar]
- 30.Cunningham TJ, Hodge L, Speicher D, Reim D, Tyler-Polsz C, Levitt P, Eagleson K, Kennedy S, Wang Y. Identification of a survival-promoting peptide in medium conditioned by oxidatively stressed cell lines of nervous system origin. J Neurosci 1998;18:7047–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todorov P, Cariuk P, McDevitt T, Coles B, Fearon K, Tisdale M. Characterization of a cancer cachectic factor. Nature 1996;379:739–742 [DOI] [PubMed] [Google Scholar]
- 32.Todorov PT, Deacon M, Tisdale MJ. Structural analysis of a tumor-produced sulfated glycoprotein capable of initiating muscle protein degradation. J Biol Chem 1997;272:12279–12288 [DOI] [PubMed] [Google Scholar]
- 33.Lanneau D, Wettstein G, Bonniaud P, Garrido C. Heat shock proteins: Cell protection through protein triage. TheScientificWorldJournal 2010; 10:1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aprile FA, Dhulesia A, Stengel F, Roodveldt C, Benesch JL, Tortora P, Robinson CV, Salvatella X, Dobson CM, Cremades N. Hsp70 oligomerization is mediated by an interaction between the interdomain linker and the substrate-binding domain. PloS One 2013;8:e67961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods (San Diego, CA) 2007;43:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blond-Elguindi S, Fourie AM, Sambrook JF, Gething MJ. Peptide-dependent stimulation of the ATPase activity of the molecular chaperone BiP is the result of conversion of oligomers to active monomers. J Biol Chem 1993;268:12730–12735 [PubMed] [Google Scholar]
- 37.Nakajima H, Amano W, Kubo T, Fukuhara A, Ihara H, Azuma YT, Tajima H, Inui T, Sawa A, Takeuchi T. Glyceraldehyde-3-phosphate dehydrogenase aggregate formation participates in oxidative stress-induced cell death. J Biol Chem 2009;284:34331–34341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao B, Eisenberg E, Greene L. Effect of constitutive 70-kDa heat shock protein polymerization on its interaction with protein substrate. J Biol Chem 1996;271:16792–16797 [DOI] [PubMed] [Google Scholar]
- 39.Klucken J, Shin Y, Hyman BT, McLean PJ. A single amino acid substitution differentiates Hsp70-dependent effects on α-synuclein degradation and toxicity. Biochem Biophys Res Commun 2004;325:367–373 [DOI] [PubMed] [Google Scholar]
- 40.Sharda PR, Bonham CA, Mucaki EJ, Butt Z, Vacratsis PO. The dual-specificity phosphatase hYVH1 interacts with Hsp70 and prevents heat-shock-induced cell death. Biochem J 2009;418:391–401 [DOI] [PubMed] [Google Scholar]
- 41.Wang AM, Morishima Y, Clapp KM, Peng HM, Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Inhibition of hsp70 by methylene blue affects signaling protein function and ubiquitination and modulates polyglutamine protein degradation. J Biol Chem 2010;285:15714–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattoo RU, Sharma SK, Priya S, Finka A, Goloubinoff P. Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J Biol Chem 2013;288:21399–21411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shorter J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PloS One 2011;6:e26319. [DOI] [PMC free article] [PubMed] [Google Scholar]