Abstract
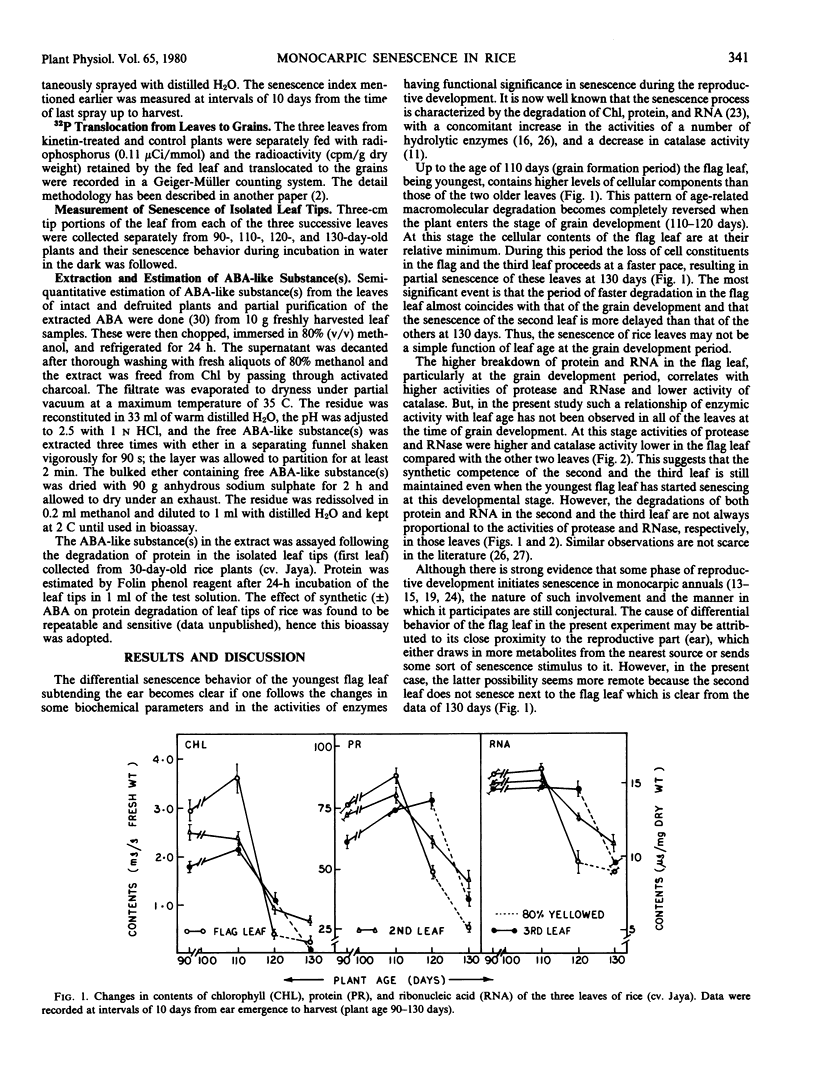
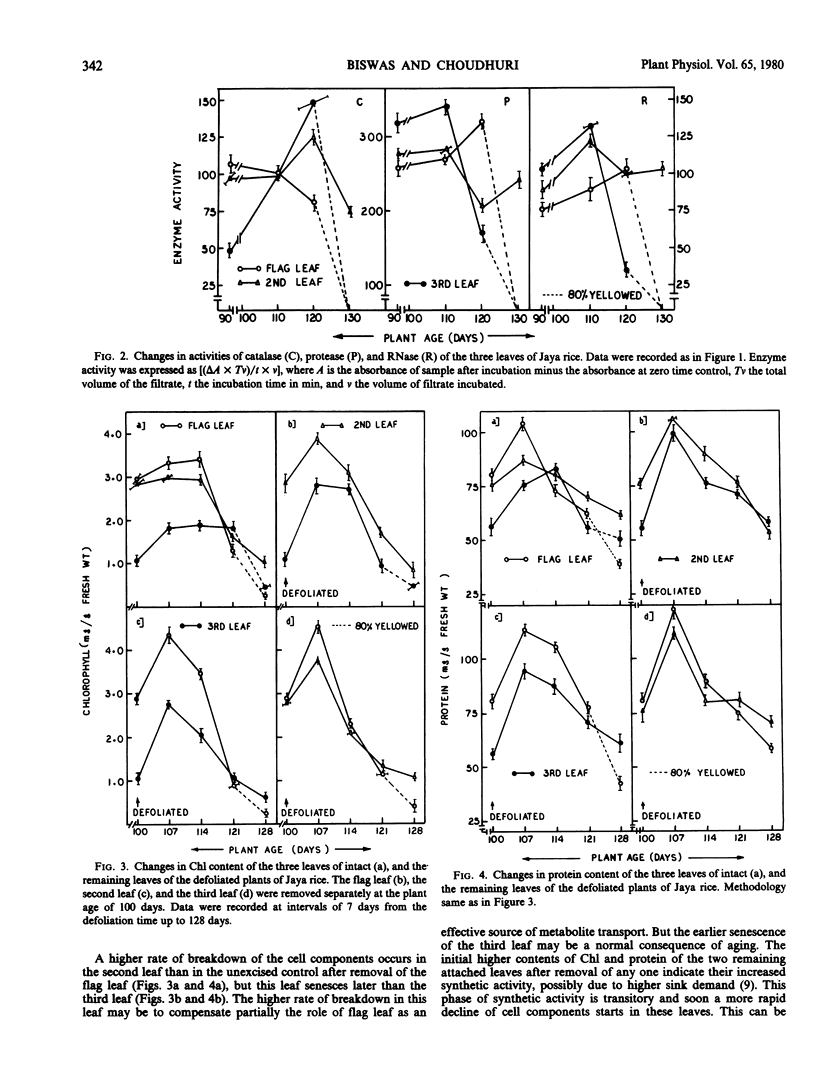
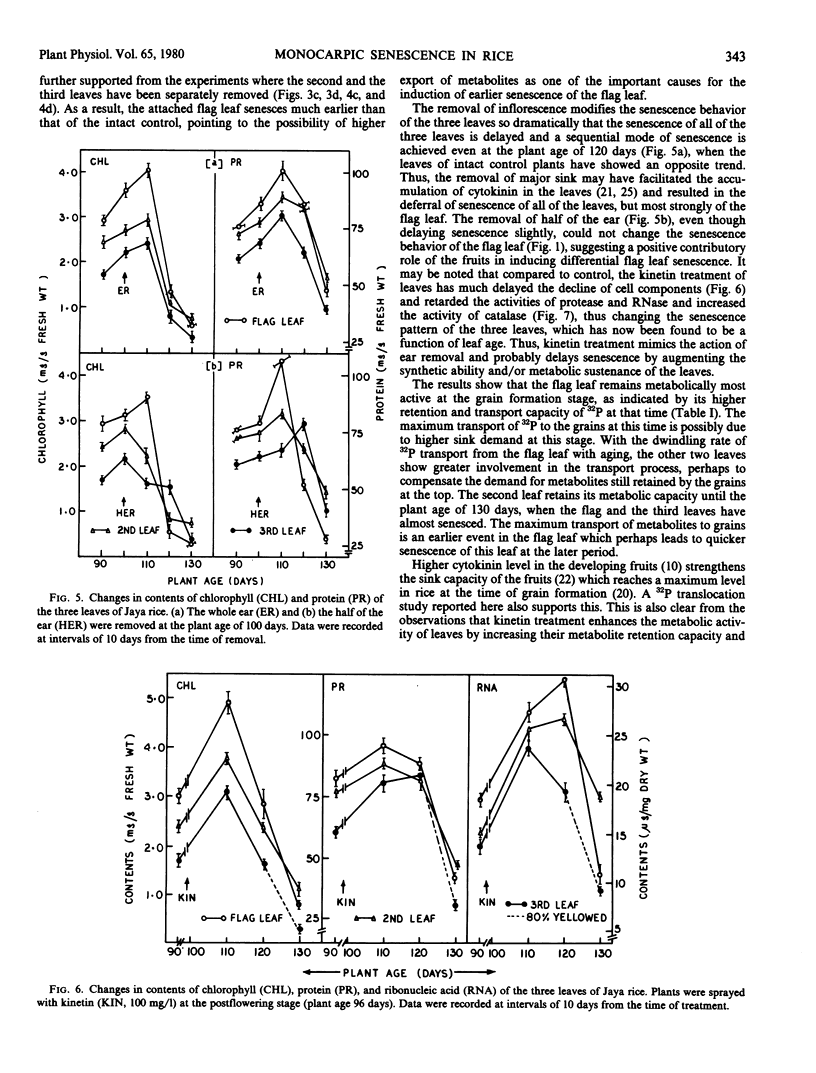
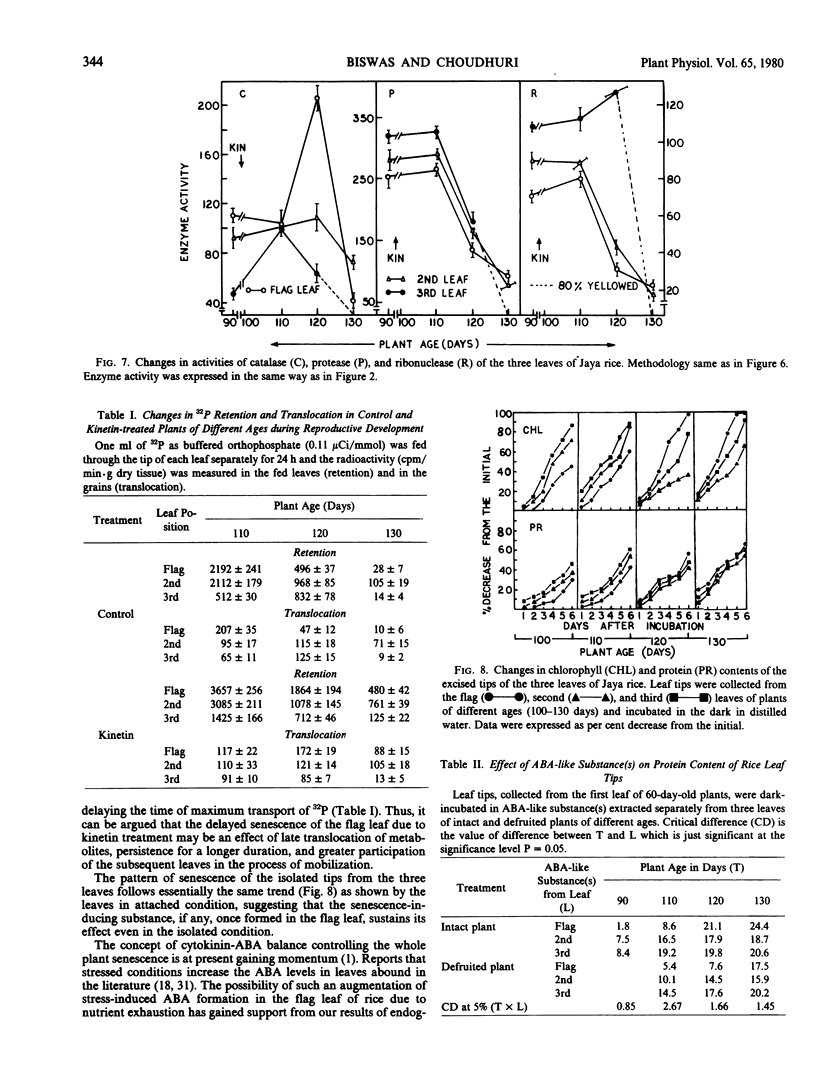
During grain formation stage (90 to 110 days), the youngest flag leaf of rice (Oryza sativa L. cv. Jaya) remained metabolically most active (as indicated by cellular constituents and enzyme activities) and the third leaf the least active. At the grain development stage (110 to 120 days) the above pattern of age-related senescence of the flag leaf completely changed and it senesced at a faster rate than the second leaf which remained metabolically active even up to grain maturation time (120 to 130 days), when both the flag and the third leaf partially senesced. Removal of any leaf temporarily arrested senescence of the remaining attached leaves, that of flag leaf did not hasten senescence of the second leaf, while that of either the second or the third accelerated senescence of the flag. Removal of the inflorescence after emergence or foliar treatment of intact plant with kinetin equally delayed senescence and produced an age-related, sequential mode of senescence or leaves. Both translocation and retention of 32P by the flag leaf were maximum at the time of grain formation and that by the second leaf was maintained even up to grain maturation time. The induction of senescence of the flag leaf was preceded by a plentiful transport of 32P to the grains. Kinetin treatment decreased the transport of 32P, prolonged its duration, and almost equally involved all of the leaves in this process. The pattern of senescence of isolated leaf tips was similar to that of attached leaves. The level of endogenous abscisic acid-like substance(s) maintained a close linearity with the senescence behavior of the leaves of intact and defruited plants during aging, and the rise in abscisic acid in the flag leaf was also preceded by higher 32P transport to the grains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fick G. N., Qualset C. O. Genetic control of endosperm amylase activity and gibberellic Acid responses in standard-height and short-statured wheats. Proc Natl Acad Sci U S A. 1975 Mar;72(3):892–895. doi: 10.1073/pnas.72.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., de Zacks R., Kende H. Cytokinin formation in pea seeds. Naturwissenschaften. 1974 Apr;61(4):170–170. doi: 10.1007/BF00602596. [DOI] [PubMed] [Google Scholar]
- Kar M., Mishra D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976 Feb;57(2):315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C., Niedergang-Kamien E., Janick J. Experimental Modification of Plant Senescence. Plant Physiol. 1959 Sep;34(5):570–573. doi: 10.1104/pp.34.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindoo S. J., Noodén L. D. Studies on the behavior of the senescence signal in anoka soybeans. Plant Physiol. 1977 Jun;59(6):1136–1140. doi: 10.1104/pp.59.6.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi Y., Richmond A. E. Abscisic Acid in relation to mineral deprivation. Plant Physiol. 1972 Dec;50(6):667–670. doi: 10.1104/pp.50.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair T. R., de Wit C. T. Photosynthate and nitrogen requirements for seed production by various crops. Science. 1975 Aug 15;189(4202):565–567. doi: 10.1126/science.189.4202.565. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I., Ware G. The effect of kinetin on nucleic acids and nucleases of excised barley leaves. Plant Physiol. 1965 Jan;40(1):62–64. doi: 10.1104/pp.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]


