Abstract
Significance: Inflammasomes are multiprotein complexes localized within the cytoplasm of the cell that are responsible for the maturation of proinflammatory cytokines such as interleukin-1β (IL-1β) and IL-18, and the activation of a highly inflammatory form of cell death, pyroptosis. In response to infection or cellular stress, inflammasomes are assembled, activated, and involved in host defense and pathophysiology of diseases. Clarification of the molecular mechanisms leading to the activation of this intracellular inflammatory machinery may provide new insights into the concept of inflammation as the root of and route to human diseases. Recent Advances: The activation of inflammasomes, specifically the most fully characterized inflammasome—the nucleotide-binding oligomerization domain (NOD)-like receptor containing pyrin domain 3 (NLRP3) inflammasome, is now emerging as a critical molecular mechanism for many degenerative diseases. Several models have been developed to describe how NLRP3 inflammasomes are activated, including K+ efflux, lysosome function, endoplasmic reticulum (ER) stress, intracellular calcium, ubiquitination, microRNAs, and, in particular, reactive oxygen species (ROS). Critical Issues: ROS may serve as a “kindling” or triggering factor to activate NLRP3 inflammasomes as well as “bonfire” or “effector” molecules, resulting in pathological processes. Increasing evidence seeks to understand how this spatiotemporal action of ROS occurs during NLRP3 inflammasome activation, which will be a major focus of this review. Future Directions: It is imperative to know how this dual action of ROS works during NLRP3 inflammation activation on different stimuli and what relevance such spatiotemporal redox regulation of NLRP3 inflammasomes has in cell or organ functions and possible human diseases. Antioxid. Redox Signal. 22, 1111–1129.
Introduction
Through pattern recognition receptors (PRRs), the human innate immune system identifies exogenous pathogen-associated molecular patterns (PAMPs) and endogenous danger signals or damage-associated molecular patterns (DAMPs) derived from injured tissue or cells, by which a host defense reaction or an inflammatory response is activated. Multiple families of PRRs exist and include C-type Lectin receptors, toll-like receptors (TLRs), and pentraxins that survey the extracellular milieu as well as the nucleotide-binding domain leucine-rich repeats (NLRs) and RIG-I-like receptors (RLRs) which detect intracellular signals. Although these multiple PRR families converge in the regulation of cytokine and chemokine transcription, the NLR family is more specifically responsible for maturation of pro-inflammatory cytokines interleukin-1β (IL-1β) or IL-18. Among the NLR family, the nucleotide-binding oligomerization domain (NOD)-like receptor containing pyrin domain 3 (NLRP3, also known as NALP3, CIAS1, cryopyrin, or PYPAF1) inflammasome, has been more fully characterized compared with others, and is now considered a key mediator in the activation of the innate immune system in response to a wide range of danger signals derived from disease and infection, including PAMPs and DAMPs (10, 78, 100, 106, 149). It has been shown that oligomerization of the NLRP3 protein, the adaptor molecule apoptosis-associated speck-like protein containing a CARD (caspase recruitment domain) (ASC), and the cysteine protease caspase-1 form this cytosolic multiprotein complex, causing the maturation of IL-1β and IL-18. NLRP3 inflammasomes instigate the innate immune response in different cells, and its activation is particularly critical for the sterile inflammatory reaction to DAMPs during chronic degenerative diseases (81, 122).
More recently, NLRP3 inflammasome activation has also been reported to directly trigger other cell injury responses through noninflammatory actions (147). These noninflammatory actions of NLRP3 inflammasome activation may include mechanisms related to pyroptosis, glycolysis, lipid metabolism, and cell survival (65). These actions, concurrent with the initiation of the innate immune response, may result in dysfunction or loss of structural integrity of cells or tissues during chronic diseases such as atherosclerosis, Alzheimer's diseases, diabetes mellitus, metabolic disorders, and gout (82). In particular, the role of inflammasomes in the pathogenesis of metabolic syndrome is considered rather complicated and the notion that inflammasome activation contributes only to the inflammatory progression of disease may be oversimplified (49), especially when caspase-1-mediated cleavage of glycolytic enzymes has been shown to also regulate adipocyte metabolism and energy pathways (118). With 121 substrates identified for caspase-1, a recent review by Denes et al. describes the extensive potential of inflammasome and caspase-1 activation far beyond the canonical inflammatory responses and into other pathways related to cell death, cytoskeletal arrangement, and metabolism, undoubtedly contributing to altered cell function (31).
Over the last 5 years, our laboratory has extensively studied the role of NLRP3 inflammasomes in the development of atherosclerosis during hyperlipidemia and in the progression of glomerular sclerosis during hyperhomocysteinemia (hHcys). We have elucidated the redox mechanisms regulating its activation, which involve “kindling” reactive oxygen species (ROS) serving as a trigger to its local activation in cells of glomeruli or in arterial endothelial cells. NLRP3 inflammasome activation and subsequent infiltration of inflammatory cells, such as T-cells or macrophages, in these tissues exacerbate the production of ROS, causing severe local oxidative stress or “bonfire” ROS, ultimately developing into tissue fibrosis or sclerosis (4, 18). In addition, we found that activation of the inflammasome produced a number of actions independent of inflammation which also contribute to the development of atherosclerosis or glomerular sclerosis, including acute injury of endothelial function (readers are directed to an article in this forum by Zhang et al.), enhanced capability of lipid deposition within macrophages (69), and reduced nephrin synthesis in podocytes (150). Our findings along with an analysis of the literature will be integrated throughout this forum review as we discuss the background of NLRP3 inflammasomes, the possible mechanisms of its activation, the specific origins and roles of ROS as a trigger or effector, and the relevance of these findings to human disease. We hope this forum review will provide a comprehensive picture of the NLRP3 inflammasome and its redox regulation under physiological and pathological conditions.
Different Types of Inflammasomes
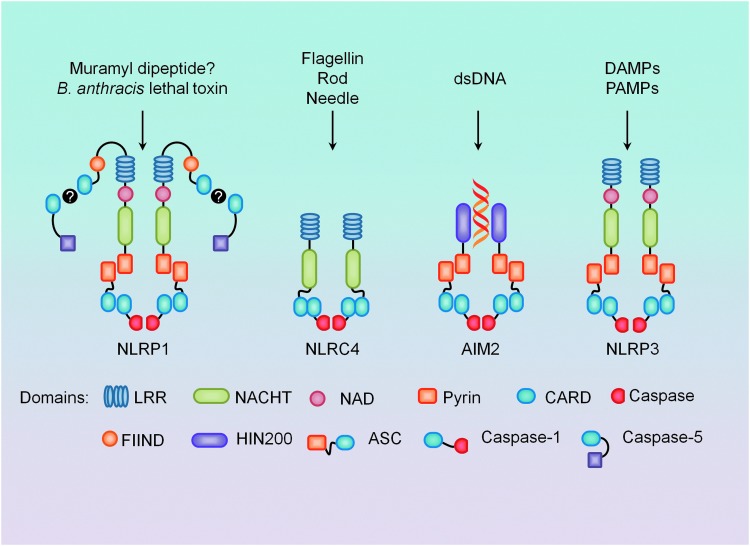
The pro-inflammatory cytokine IL-1β, a powerful inflammatory mediator, is one of the most studied cytokines related to the innate immune response (32). Jurg Tschopp and his group identified the inflammasome as the molecular platform required for the activation of caspase-1, previously known as IL-converting enzyme, which is responsible for the maturation of IL-1β from its precursor form (79). Various types of inflammasomes are centered on different members of the NLR family, and although 23 NLR genes have been identified to date, only a few form oligomeric complexes result in post-translational activation of caspases (13). Although caspases are generally thought to be pro-apoptotic, there is a subclass of inflammatory caspases that is responsible for the maturation of inactive cytokine precursors such as IL-1β and IL-18 (32). The major caspase-processing inflammasomes currently found throughout the literature include the NLRP1, NLRC4, AIM2 (absent in melanoma 2), and NLRP3 inflammasomes (Fig. 1).
FIG. 1.
Four major types of inflammasomes and their known stimulators. Nucleotide-binding oligomerization domain (NOD)-like receptor containing pyrin domain 3 (NLRP1), proposed to be activated by bacterial cell wall component muramyl dipeptide (MDP) and Bacillus anthracis lethal toxin, can directly cause caspase-5 processing, but the presence of adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) results in more robust activation. NLRC4 activation is mostly associated with gram-negative bacteria components and can also directly process caspase-1 through its caspase recruitment domain (CARD). Double-stranded DNA (dsDNA) binds preferentially to the HIN200 domain of AIM2 (absent in melanoma 2), and requires ASC for caspase-1 processing. NLRP3 also requires ASC and caspase-1, is activated in response to both exogenous and endogenous danger signals, and is mostly recognized for its role in sterile inflammation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The NLRP1 inflammasome was the first discovered and characterized NLR, and was initially related to direct caspase-5 as well as to caspase-1 processing in the presence of ASC (79). Extensive studies in macrophages and in reconstituted systems have demonstrated that NLRP1 is sensitive to both bacterial cell wall component muramyl dipeptide (MDP) and Bacillus anthracis lethal toxin (19, 38). However, more recent work in murine macrophages has shown lethal toxin, but not MDP, to be NLRP1 dependent (63). The NLRC4 inflammasome, also recognized as IL-1β-converting enzyme protease-activating factor (IPAF), is most associated with caspase-1 activation and IL-1β production in response to various gram-negative bacteria. It is hypothesized that NLRC4 activates caspase-1 on sensing the presence of bacteria-specific and conserved proteins: flagellin, rod, and needle (62, 88, 153). In an agonist-dependent manner, it is reported that NLR family apoptosis inhibitory proteins (NAIPs), homologs of NLRC4, actually bind to the stimuli and associate to NLRC4 to activate caspase-1 processing (62, 64, 71). AIM2 inflammasomes contain a HIN200 domain, which preferentially binds to cytosolic double-stranded DNA, and a pyrin domain for ASC recruitment, leading to the proteolytic cleavage of caspase-1 through CARD-CARD interactions (111).
NLRP3 inflammasomes
The most characterized member of the NLR family is the NLRP3 inflammasome. Before being recognized as a caspase-activating molecular platform, mutations in NLRP3 were first identified in patients with familial cold urticaria (FCU) and Muckle–Wells syndrome (MWS) (5). Agostini et al. later demonstrated that the spontaneous secretion of IL-1β in patients with MWS derived from oligomeric complexes comprised NLRP3, ASC, and caspase-1, which was termed the inflammasome (6). Since then, the NLRP3 inflammasome has been shown to respond to a very diverse range of activators, including those of microbial origin, endogenous danger signals, and exogenous nonmicrobial stimuli; of particular interest is the fact that the NLRP3 inflammasome is considered a general sensor of DAMPs, which may be important in sterile inflammation observed in many human diseases. This led to great interest in the scientific community to identify activators of the NLRP3 inflammasomes and an attempt to understand how such a broad range of stimuli can activate the same molecular platform (24).
With regard to exogenous NLRP3 inflammasome activators, many studies have provided evidence of NLRP3 inflammasome activation in response to a whole host of foreign danger signals and microbes, including the influenza virus, adenoviruses, Staphylococcus aureus, Escherichia coli, Neisseria gonorrhoe, and Candida albicans (36, 55, 59, 76, 95, 125). Although the ability of these microbial toxins to form membrane pores is linked to their ability to activate NLRP3, it still remains unknown whether a single or a combination of PAMPs is directly responsible for its activation. Another group of NLRP3 stimulators are the nonmicrobial phagocytosed materials, where monosodium urate (MSU) crystal accumulation associated with gout was one of the first inflammatory diseases linked to the activation of NLRP3 (80). In a similar manner, silica, asbestos, and aluminum salts have also been shown to trigger caspase-1 cleavage and IL-1β production via NLRP3 activation (33, 53).
However, the activation of NLRP3 inflammasomes to a very wide range of endogenous danger signals or DAMPs is what sets NLRP3 apart from other inflammasomes. It is proposed that the formation and activation of this inflammasome may be a critical pathogenic mechanism mediating many degenerative diseases such as atherosclerosis, Alzheimer's disease, glomerular sclerosis, lung fibrosis, and liver cirrhosis (30, 50, 69, 150). For example, excessive levels of ATP were described as one of the first endogenous DAMPs to induce NLRP3 inflammasome formation and activation, a mechanism involving high concentrations of extracellular ATP binding to the purinergic P2X7 receptor (104). The aggregation of endogenous peptides such as amyloid-β are also sensed by NLRP3 (44), leading to the production of pro-inflammatory cytokines, which explains the elevation of IL-1β detected in the brains of patients with Alzheimer's disease (43). Cholesterol crystals are known to cause phagolysosomal damage, and have been shown to lead to the early activation of NLRP3 and the promotion of a pro-atherosclerotic phenotype (35); while an endogenous danger signal of trauma, hyaluronan, also triggers chemokine release in affected tissues through NLRP3 (146). Damage to pancreatic islet cells by hyperglycemia caused NLRP3 inflammasome activation, glucose intolerance, and insulin resistance in a murine model of diabetes (154). Our recent studies demonstrated that elevated levels of homocysteine (Hcys), a thiol-containing amino acid derived from methionine, stimulate NLRP3 inflammasome formation and activation, leading to podocyte cell injury and, eventually, glomerular sclerosis in the kidney (150). Inhibition of the inflammasome via ASC short hairpin RNA (shRNA) or the caspase-1 inhibitor, WEHD (peptide sequence Ac-Tyr-Val-Ala-Asp-CHO), prevented Hcys-induced detrimental effects on glomerular structure and function, signifying a critical role for NLRP3 in the pathogenesis of end-stage renal disease (ESRD) related to hHcys (150). In the coronary arterial wall, we also showed that endothelial NLRP3 inflammasomes were activated on stimulation with the adipokine visfatin, which may be an initiating factor resulting in vascular inflammation and injury leading to atherosclerosis (143). Taken together, NLRP3 inflammasomes have contributed to a paradigm shift in how we understand the pathogenesis of different human degenerative diseases and develop new therapeutic strategies for its treatment.
NLRP3 Inflammasome Activation Models
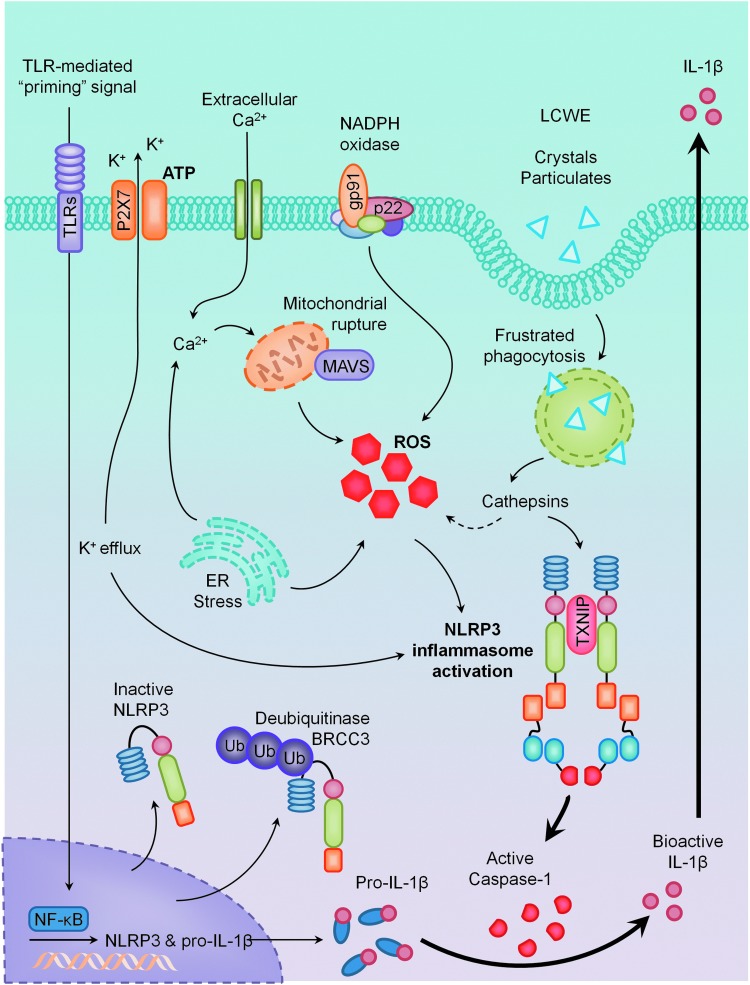
The activation of NLRP3 inflammasomes has been implicated in a growing number of diverse pathological conditions, ranging from bacterial infections to cardiovascular dysfunction and metabolic syndrome (17, 35, 48, 102). Despite rapid and extensive efforts in identifying various agents that stimulate the NLRP3 inflammasome, the underlying mechanisms by which these diverse danger signals activate the same molecular machinery remain poorly understood. This section will discuss some current models hypothesized to facilitate NLRP3 inflammasome activation, which is summarized in Figure 2.
FIG. 2.
Models of NLRP3 inflammasome activation. Considered to be a two-step mechanism, the primary signal comes from the activation of toll-like receptors (TLRs) and is responsible for the upregulation of NLRP3 and pro-interleukin-1β (IL-1β) in an NF-kappaB (NF-κB)-dependent manner. Secondary signals come from multiple pathways: K+ efflux via P2X7 receptor activation, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, NADPH oxidase, frustrated phagocytosis, and lysosomal rupture pathways, all of which appear to converge in the production of reactive oxygen species (ROS). Together, these primary and secondary signals activate the NLRP3 inflammasome, resulting in proteolytic cleavage of caspase-1 and the maturation of IL-1β. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
ATP and K+ efflux
ATP, an endogenous DAMP, was one of the first described NLRP3 inflammasome activators, where high extracellular concentrations resulted in the depletion of cytosolic K+, leading to NLRP3- and ASC-dependent caspase-1 activation and IL-1β secretion (76). K+ efflux was the first model described that linked all known NLRP3 activators at the time, including ATP, nigericin, MSU crystals, and pore-forming toxins (93, 107). K+ efflux and low intracellular K+ concentration caused by activation of the P2X7 purinergic receptor in response to ATP is now considered an important signaling pathway to activate NLRP3 inflammasomes (76, 121). ATP has also been demonstrated to cause transient pore formation through pannexin-1, enabling NLRP3 stimulators to cross the plasma membrane and directly promote inflammasome assembly and activation (104).
Lysosome destabilization and frustrated phagocytosis
Another activating mechanism of NLRP3 inflammasomes is related to the actions of lysosomal enzymes in response to phagosomal stimulations that are too large to cross the membrane and instead are taken up into the cell via phagocytosis. Martinon et al. were the first to demonstrate that insoluble uric acid, which accumulates at the joints of patients with hyperuricemia to form MSU crystals, activated NLRP3 inflammasomes, causing IL-1β maturation, producing the inflammatory phenotype typically seen in patients suffering from gout (80). Dostert et al. furthered this study, finding that incomplete phagocytosis of crystals leads to lysosome swelling and destabilization, causing lysosomal rupture and the release of cathepsin B, a lysosomal enzyme shown to activate NLRP3 inflammasomes (33). Much evidence supports a crucial role for lysosome stability in downstream caspase-1 activation and IL-1β release in response to MSU crystals, as inhibition of cathepsin B greatly abrogated these effects (53). These mechanisms extended not only to other endogenous crystalline structures such as cholesterol crystals (35) but also to environmentally derived crystals such as asbestos, silica, and aluminum salts typically used in vaccine adjuvants (33, 53). However, the mechanism of how cathepsin release results in NLRP3 activation still remains unknown.
Reactive oxygen species
Although NLRP3 was originally hypothesized to be a cytosolic receptor, with such a broad range of stimuli demonstrated to cause its activation, it seems highly improbable that NLRP3 acts as a receptor to directly bind to all of these diverse stimuli. ROS, produced by many known activators of NLRP3 inflammasomes, are shown to be a critical mechanism triggering NLRP3 inflammasome formation and activation in response to many exogenous stimuli as well as endogenously produced or secreted molecules from damaged cells such as DAMPs (127). The hypothesis of ROS as an NLRP3-activating trigger arose when inhibition of NADPH oxidase-derived ROS prevented ATP-induced caspase-1 activation and IL-1β production in alveolar macrophages (28). Further substantiating this hypothesis, knockdown of the p22phox subunit of NADPH oxidase significantly suppressed IL-1β release in THP1 cells in response to asbestos and MSU challenge (33). Interestingly, incomplete phagocytosis of crystalline particulates by phagocytic cells such as macrophages is a source of ROS as well as reactive nitrogen species (41). The crystal structure of NLRP3 contains a highly conserved disulfide bond connecting the PYD domain and the nucleotide-binding site domain, which is highly sensitive to altered redox states (9). The presence of this unexpected disulfide bond between Cys-8 and Cys-108 spans across six species, including humans, monkeys, and mice, and the strict conservation of this bond is indicative of a crucial redox role for NLRP3. The production of ROS offers a link as to how many different stimuli, including frustrated phagocytosis, can activate the same molecular platform.
Endoplasmic reticulum stress and unfolded protein response
With the NLRP3 inflammasome being more widely accepted as a general sensor for alterations in cellular homeostasis, it is logical that endoplasmic reticulum (ER) stress, generated in response to the accumulation of misfolded proteins and an indicator of metabolic disturbances, could also be a trigger to activate NLRP3 inflammasomes. Induction of ER stress promoted IL-1β secretion in human macrophages, suggesting that the generation of ER stress alone was sufficient to activate NLRP3 inflammasomes (87). Interestingly, this activation occurred independently of the unfolded protein response (UPR), but required the production of ROS as well as K+ efflux (87). Contradictorily, the UPR and the IRE1α and PERK pathways are necessary for IL-1β secretion in pancreatic β cells, where thioredoxin-interacting protein (TXNIP) acts as the link between ER stress, NLRP3 inflammasome activation, and inflammation related to diabetes (66, 101). Genetic deletion of TXNIP suppressed IL-1β release from islet cells and prevented ER stress-induced β cell death, verifying its role as a key mediator in NLRP3 activation and the detrimental effects associated with uncontrolled ER stress. Currently, the effect of ER stress and the role of UPR appear to be cell type specific, and require further studies to clearly understand its role in inflammasome activation.
Ca2+ signaling
The first implication of intracellular Ca2+ ([Ca2+]i) signaling in NLRP3 inflammasome activation came from a study by Brough et al. that demonstrated reduced IL-1β production by Ca2+ chelator BAPTA-AM in ATP-treated murine macrophages (20). More recently, it was discovered that ATP induced Ca2+ mobilization from both intracellular and extracellular stores, and that both inhibition of intracellular ER Ca2+ stores by thapsigargin and incubation with Ca2+-free media to inhibit extracellular Ca2+ entry were able to prevent ATP-induced caspase-1 activation and IL-1β secretion (94). The same was required of nigericin, MSU, and alum-induced NLRP3 inflammasome activation in macrophages. Excessive Ca2+ can lead to an increase in mitochondrial ROS (mtROS) production, collapse of the mitochondrial membrane potential, and eventual mitochondria rupture (34). Thus, Murakami et al. further hypothesized that mitochondrial damage and dysfunction caused by sustained levels of high [Ca2+]i may be the link to NLRP3 inflammasome activation (94). Further substantiated by Triantafilou et al., the complement membrane attack complex instigates NLRP3 activation via increased [Ca2+]i, which accumulates in the mitochondrial matrix, leading to loss of membrane potential (126). Importantly, blocking mitochondrial Ca2+ uniporter prevented not only accrual of mitochondrial Ca2+ but also IL-1β production.
Deubiquitination
In some cells such as neutrophils or macrophages, there is evidence that NLRP3 inflammasome activation is a two-step mechanism requiring a primary TLR-mediated signal which activates NF-kappaB (NF-κB) to drive transcription of NLRP3 and pro-IL-1β; while a second triggering signal results in pro-IL-1β caspase-1-dependent cleavage (13). Priming, typically by lipopolysaccharide (LPS), has been shown to take approximately 2 h to upregulate NLRP3 expression (16). However, recent reports have suggested a rapid, nontranscriptional priming mechanism that occurs within minutes. Through coimmunoprecipitation studies in mouse and human macrophages, it was demonstrated that there exists a basal level of ubiquitinated NLRP3 which on LPS or ATP treatment is rapidly deubiquitinated (60). This process was found to be essential for NLRP3 inflammasome activation, as inhibition of deubiquitinases by small-molecule inhibitors completely blocked IL-1β production (73). Breast cancer 1 or 2 protein (BRCA1/BRCA2)-containing complex subunit 3, BRCC3 is an NLRP3-specific deubiquitinase enzyme that has recently been identified as the critical regulator of NLRP3 ubiquitination and its subsequent activation (110).
MicroRNAs
Specific to myeloid cells, a genome-wide microRNA (miRNA) screen identified miRNA-223 as a strong candidate specifically binding to the 3′ untranslated region of NLRP3 and negatively regulating NLRP3 expression (15). During differentiation of monocytes to macrophages, miR-233 inversely correlated with NLRP3 expression where miR-233 mRNA expression was higher in monocytes and NLRP3 protein expression was higher in macrophages (45). Coinciding with a clear involvement in NLRP3 inflammasome regulation, it is interesting that mice deficient in miR-233 have previously been shown to exhibit serious sterile inflammation similar to that of NLR-derived autoimmune diseases (58). In addition, in a less direct fashion, activation of IRE1α through ER stress pathways caused rapid reduction of another miRNA, miR-17. miR-17 negatively regulates TXNIP mRNA stability; therefore, the lack of miR-17 increased stability of TXNIP mRNA and promoted NLRP3 inflammasome activation (66).
Redox Activation of NLRP3 Inflammasomes
Due to the leucine-rich repeats found in the C-terminus of NLRP3, it was originally hypothesized to act as a cytosolic receptor and directly bind to a ligand. However, this hypothesis was neglected after the discovery of many diverse stimuli which were found to activate NLRP3, and it became much more accepted that NLRP3 senses molecular intermediates generated by these broad activators. The generation of ROS was one of the first intermediates discovered to be common to ATP, MSU, asbestos, and silica-induced NLRP3 activation (33). Since then, there have been many conflicting reports regarding the role of ROS in this process, creating much controversy in understanding the regulation of NLRP3 inflammasome activation. Evidence describing the role of ROS as an activator or regulator of NLRP3 inflammasomes on different stimuli is given next.
ROS as a requirement for NLRP3 “priming”
Particularly in phagocytes such as macrophages, it has been shown that NLRP3 inflammasome activation requires two signals—a “priming” transcriptional step that involves TLR/NF-κB signaling, followed by post-translational regulation responsible for the oligomerization of the inflammasome components and the secretion of IL-1β (16, 75). This TLR-dependent signal is necessary for the activation of the transcription factor NF-κB and the downstream induction of pro-IL-1β expression, while NLRP3 inflammasomes control its proteolytic maturation. Specific activation of TLR2, TLR3, TLR4, and TLR7 induced NLRP3 priming, which occurred in an adaptor protein MyD88-dependent manner (16). Furthermore, specific blockade of NF-κB activity by inhibitor Bay11-7082 revealed its necessity for NLRP3 priming and enhancement of expression. This TLR and NF-κB-dependent priming was required for NLRP3 inflammasome activation in response to pore-forming toxin nigericin, ATP, as well as crystalline stimuli such as MSU. This two-step mechanism that first requires activation of PRRs in response to danger signals followed by an activating stimulus may be an evolutionarily conserved process to help prevent uncontrolled NLRP3 activation and excessive IL-1β release. However, in other cell types such as endothelial cells and podocytes, a two-step signal may not be needed, where constitutive or basal expression of pro-IL-1 and other associated molecules may be adequate to form and activate NLRP3 inflammasomes, albeit to a lesser extent than those in phagocytes, but sufficient enough to produce pathological changes in chronic degenerative diseases (98). In this regard, NLRP3 inflammasome activation may hinge on the strict requirement of NLRP3 priming by a proinflammatory signal, a step that is blocked by ROS inhibitors. Studies in mouse macrophages demonstrated that TLR4 with Myd88 can rapidly and nontranscriptionally prime NLRP3 through its deubiquitination. This process is dependent on mtROS production and can be inhibited by antioxidants. Pharmacological inhibition of NLRP3 deubiquitination completely blocked NLRP3 activation in both mouse and human cells, indicating that deubiquitination of NLRP3 is required for its activation. It has been suggested that NLRP3 is activated by a two-step deubiquitination mechanism initiated by TLR signaling and mtROS (60).
NADPH oxidase-derived ROS
Due to the role of ROS being limited specifically to the priming step, there are studies supporting the dispensability of ROS in the activation of NLRP3 inflammasomes. Macrophages isolated from gp91phox−/− mice or patients with chronic granulomatous disease (CGD) and deficient NADPH oxidase displayed normal inflammasome function when challenged with NLRP3 agonists (53, 128). Caspase-1 activation and IL-1β secretion in response to cyclic stretch was undisturbed in murine alveolar macrophages, even in those isolated from gp91phox−/− mice, demonstrating the dispensability of NADPH oxidase to NLRP3 activation in this particular mechanism (142). This leads to arguments of whether ROS are truly involved in the activation of NLRP3 inflammasomes, but instead are derived from TLRs and contribute to the priming mechanism (14). Wu et al. demonstrated that mechanical ventilation causes IL-1β release in the lung; however, there was no inhibitory effect on released IL-1β in cyclic-stretched mouse alveolar macrophages isolated from gp91phox−/− mice (142). Instead, it was concluded that ROS derived from mitochondria were necessary for NLRP3 inflammasome activation induced by cyclic stretch. Furthermore, some studies showed that peripheral blood mononuclear cells (PBMCs) isolated from patients with CGD exhibited normal secretion of IL-1β and even exacerbated caspase-1 activation when compared with healthy controls (86, 128, 129). Interestingly, human neutrophils with mutations in the gp91phox subunit of NADPH oxidase, also isolated from patients with CGD, displayed impaired IL-1β release but displayed no difference in the ability to activate caspase-1 when compared with neutrophils from control patients (42). This suggested that NADPH oxidase, specifically gp91phox, and the production of ROS are dispensable for NLRP3 inflammasome activation, but crucial for IL-1β secretion, specifically in neutrophils but perhaps not in PBMCs. In addition, an emerging role for antioxidants as opposed to ROS has been implicated in mediating NLRP3 activation. It has been shown that superoxide dismutase 1 (SOD1) regulates caspase-1 activation, as macrophages isolated from SOD1-deficient mice displayed constitutively higher O2•− levels but inhibited caspase-1 activation (85). Similarly, the oxidant stress-responsive transcription factor Nrf2, which is responsible for induction of major antioxidant enzymes, is necessary for cholesterol crystal-induced NLRP3 activation (39).
Despite the evidence of ROS being dispensable and limited to the priming of NLRP3 inflammasomes, there are considerable reports supporting the contrary, showing the elevation of ROS, and in particular NADPH oxidase-derived ROS, to be critical for NLRP3 inflammasome activation. Some of the very early inflammasome studies reported the importance of NADPH oxidase-derived ROS in activating NLRP3 in response to ATP, asbestos, and silica (28, 33, 51). The first study of this kind demonstrated inhibited caspase-1 activation and IL-1β release in monocyte THP-1 cells in response to asbestos, MSU, and silica on specific knockdown of NADPH oxidase subunit p22phox or use of general ROS scavengers such as N-acetylcysteine and antioxidant ammonium pyrrolidine dithiocarbamate (33). Furthermore, ATP induced NADPH oxidase complex aggregation in these same cells, and diphenylene iodonium (DPI) treatment inhibited both ATP- and nigerin-induced IL-1β and caspase-1 processing (51). Through the use of either NADPH oxidase inhibitors or general ROS scavengers, additional studies have demonstrated that IL-1β production in response to even more diverse stimuli could be prevented (23, 72, 105). This has ever since been extended to more recent reports, where NADPH oxidase inhibition via DPI or more specifically through gp91phox subunit blockade can prevent free fatty acid, TNFα, and atheroprone oscillatory flow-induced NLRP3 inflammasome activation (7, 140, 145). A central role for NADPH oxidase and gp91phox was further established in ATP-induced NLRP3 inflammasome activation in LPS-primed murine macrophages, where general NADPH oxidase inhibitors DPI and apocynin as well as specific gp91phox siRNA inhibited caspase-1 activation and IL-1β secretion (70).
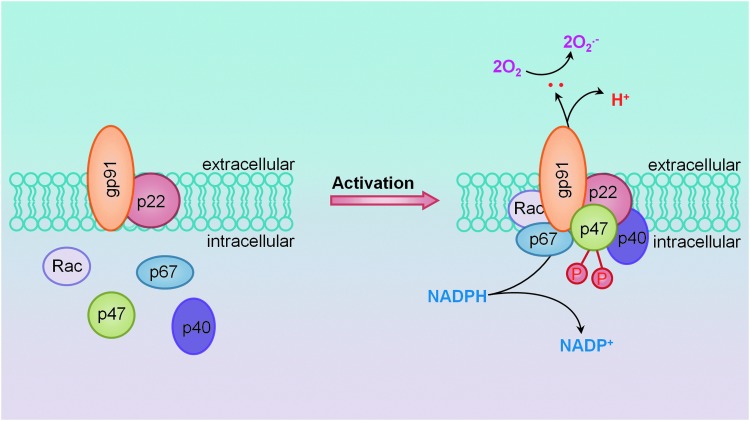
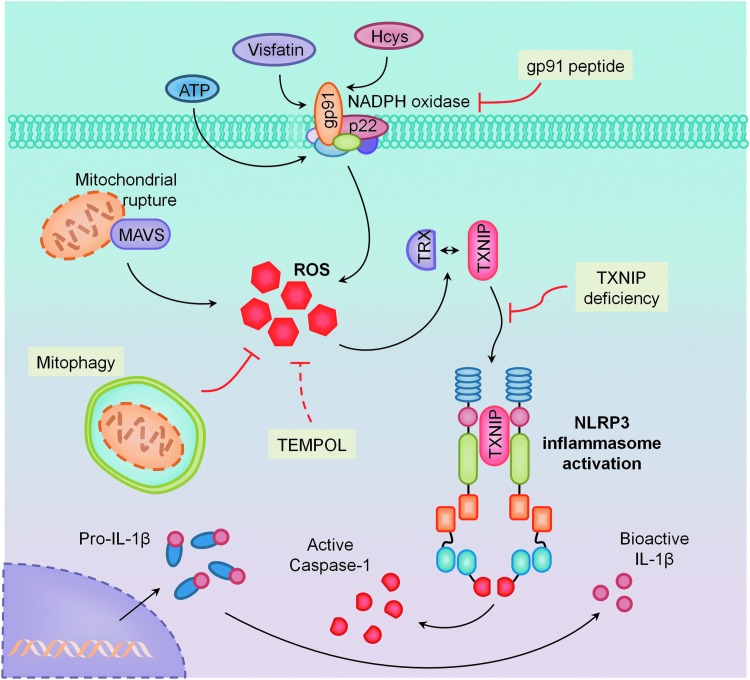
Studies from our laboratory have demonstrated a major contribution of NADPH oxidase-derived ROS, specifically gp91phox, in the development of hHcys-induced glomerular damage (151). As mentioned earlier, we have established the important involvement of NLRP3 inflammasome activation in the pathogenesis of hHcys-induced glomerular sclerosis, where locally silencing the ASC gene in the kidney significantly reduced NLRP3 inflammasome formation and IL-1β production in glomeruli of mice with hHcys. Pathologically, hHcys-associated albuminuria, foot process effacement of podocytes, loss of podocyte slit diaphragm molecules, and late-stage glomerulosclerosis were significantly improved by local ASC gene silencing or by caspase-1 inhibition (150). In agreement with the aforementioned studies revealing the important contribution of ROS, we too have attributed this activation of NLRP3 inflammasomes to NADPH oxidase and the production of O2•− (4). Substantiating the importance of NADPH oxidase redox signaling, we found that overexpression of the guanine nucleotide exchange factor Vav2 and consequent activation of NADPH oxidase, independent of elevated Hcys, were also able to produce NLRP3 inflammasome-activating effects, suggesting that NADPH oxidase activation alone was sufficient to initiate the cascade, leading to NLRP3 inflammasome activation and downstream glomerular injury (1). In addition, we found that ATP can activate NLRP3 inflammasomes in renal tubular cells, resulting in the production of inflammasome-derived IL-1β, which acts directly on tubular cells to enhance sodium reabsorption and reduce medullary blood flow. All of these effects were inhibited by SOD mimetic 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL), suggesting an important contribution of ROS to ATP-induced NLRP3 inflammasome activation (4). In vascular endothelial cells, the formation and activation of NLRP3 inflammasomes by the adipokine visfatin served as an important initiating mechanism to turn on the endothelial inflammatory response, leading to arterial inflammation and endothelial dysfunction in mice during early-stage obesity (143); this action of visfatin may also be associated with the activation of NADPH oxidase (144). In our laboratory, we have defined a novel mechanism mediating NADPH oxidase activation in response to many different stimuli, termed lipid raft (LR) redox signalosomes. These LR signalosomes use membrane rafts as a platform to conduct redox signaling, and are centered on the enzymatic NADPH oxidase subunits clustering and activating to produce O2•− (57). NADPH oxidase-derived ROS can act downstream to conduct transmembrane or intracellular signaling, leading to the redox regulation of cell and organ function (67). As shown in Figure 3, we have reported that stimuli such as Hcys, visfatin, or ATP act on the cell membrane, stimulate acid sphingomyelase to produce and form ceramide-enriched LR platforms, and increase NADPH oxidase-dependent O2•− production from cells (4, 18, 57, 143, 150). As mentioned earlier, we have reported these stimuli to also activate the NLRP3 inflammasome and in this regard, this redox signaling platform may provide the O2•− necessary to trigger NLRP3 inflammasome activation.
FIG. 3.
Membrane NADPH oxidase assembly and activation through lipid raft (LR)-mediated clustering to form redox signaling platforms. Under rest condition, all subunits of NADPH oxidase are separated and the enzyme may not be active. When vascular or kidney cells are stimulated by inflammatory stimuli such as Hcys or visfatin, the formation of LR platforms occurs. In such platforms, NADPH oxidase subunits such as gp91phox and p47phox and other proteins become aggregated, clustered, or recruited, resulting in a rapid assembling of NADPH oxidase into an enzyme complex, producing O2•− and ROS that conduct transmembrane or intracellular signaling. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
This controversy regarding the precise role of ROS may be explained through the biphasic redox response and the homeostatic balance between pro- and antioxidant systems. First described by Tassi et al., an initial increase in oxidant stress followed by a delayed antioxidant response are two necessary steps required for IL-1β processing and secretion in human monocytes stimulated by PAMPs such as LPS, MDP, S. aureus, and zymosan (124). The basal redox state of different myeloid cells also contributes greatly to potential IL-1β production (22). Primary human monocytes displayed low levels of ROS and antioxidants that resulted in efficient IL-1β secretion on TLR stimulation. However, THP-1 cells or cultured macrophages exhibited an upregulation of antioxidant systems that buffered and suppressed TLR-triggered IL-1β processing, suggesting the importance of the redox state in understanding the specific effects of ROS on NLRP3 inflammasome activation.
Mitochondrial ROS
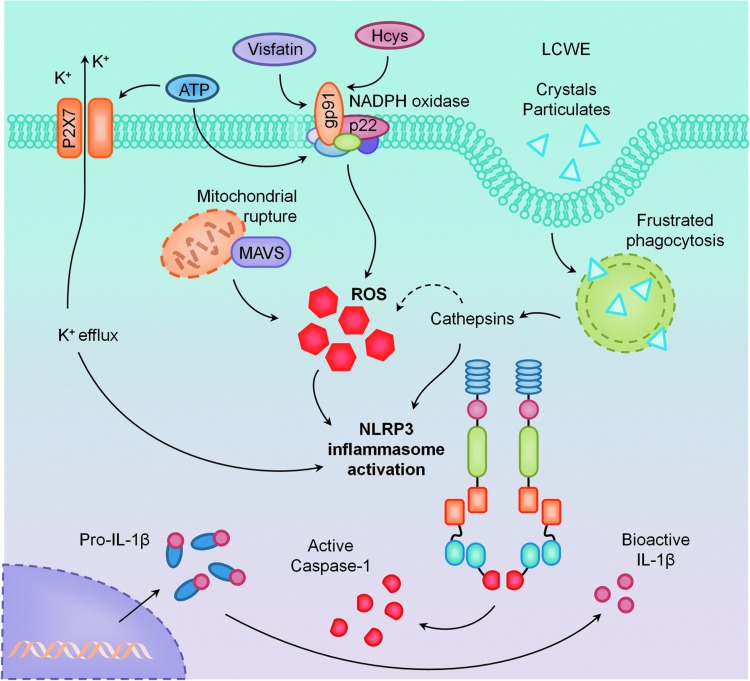
Alternatively, there is an emerging role for mtROS in the mechanism of NLRP3 inflammasome activation (21, 155). Mitochondrial complex I inhibitor rotenone or complex III inhibitor antimycin robustly increased mtROS production and, as a result, increased NLRP3 activation (21, 155). Additional evidence suggested that NLRP3 localized to the cytosol and ER during rest, but relocalized to the mitochondria on stimulation. This recruitment of NLRP3 to the mitochondria was mediated by mitochondrial anti-viral signaling protein (MAVS) and was required for IL-1β production in response to nigericin stimulation (120). In addition to mtROS, mitochondrial DNA (mtDNA) has also been reported to directly cause NLRP3 inflammasome activation (116). In an NF-κB-dependent manner, a second inflammasome-activating signal resulted in mitochondrial dysfunction, apoptosis, and release of mtDNA into the cytosol, which bound to and caused activation of NLRP3. As previously mentioned, Wu et al. revealed that specific inhibition of mtROS by mitochondrial antioxidant SS-13, and not inhibition of NADPH oxidase, prevented cyclic stretch-induced IL-1β in alveolar macrophages (142). The roles of ROS from membrane NADPH oxidase and mitochondria in activation of NLRP3 inflammasomes are summarized in Figure 4.
FIG. 4.
Primary activating pathways of NLRP3 inflammasomes. It was demonstrated that in the kidney and vasculature, NLRP3 inflammasomes are activated by NADPH oxidase-derived ROS through LR clustering. Some stimuli such as ATP also alter K+ efflux or lysosome stability to activate NLRP3 inflammasomes. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Other sources and species of ROS
Apart from NADPH oxidase and mitochondria, there are many enzymatic systems that contribute to the production of ROS, including xanthine/xanthine oxidase (X/XO), lipoxygenases (LOXs), cyclooxygenases (COXs), and cytochrome P450s, and, thus, we cannot discount the role of these other sources. Although many of these systems remain to be explored in NLRP3 inflammasome activation, there exists some evidence suggesting the involvement of COXs, X/XO, and LOXs, as well as more specific species of reactive oxygen such as peroxynitrite (ONOO−) and hydrogen peroxide (H2O2). In response to P2X7 receptor stimulation by ATP, which is known to increase the generation of ROS, COX-2-derived prostaglandin E2 (PGE2) release was necessary for IL-1β release (12). In response to bacteria, PGE2 is a central component in an inflammasome-mediated “eicosanoid storm” that contributes to the pathogenesis associated with bacterial infection (132). Moreover, leukotriene B4 derived from 5-lipoxygenase (5-LOX) is involved in NLRP3 inflammasome activation and tissue inflammation related to gout (8). 5-LOX, although not necessary for the recruitment of neutrophils in response to MSU, is required for IL-1β production. In addition, uric acid as another well-established activator of NLRP3 is usually generated via xanthine oxidase accompanying the generation of O2•−. In human THP-1 cells, activation of TLR7/8 by ligand R848 resulted in increased ROS production, caspase-1, and IL-1β processing, all of which were abrogated by xanthine oxidase inhibitor allopurinol (97). This source of O2•− may also be involved in NLRP3 inflammasome activation.
In addition to O2•−, an important role for ONOO− and H2O2 in NLRP3 activation has also been reported. ONOO− scavenger 5,10,15,20-Tetrakis(4-sulfonatophenyl)porphyrinato iron (III) chloride (FeTPPS) reduced nigericin-induced caspase-1 activation and IL-1β secretion in human monocytes (51). Jurg Tschopp's group demonstrated that direct treatment of THP-1 cells with H2O2 resulted in mature IL-1β release (154). Studies from our laboratory have also attempted to dissect the specific contribution of various species of ROS to the activation of NLRP3 inflammasomes. Through specific scavenging studies using SOD mimetic TEMPOL and H2O2 decomposer catalase, we further demonstrated the importance not only of O2•− but H2O2 as well (3). TEMPOL and catalase administration attenuated in vitro Hcys-induced NLRP3 inflammasome formation, caspase-1 activity, and IL-1β production, as well as protected from proteinuria and damaged glomerular morphology related to hHcys in vivo. These greatly varying effects between the involvement of certain species and sources of ROS demonstrate the profound complexity of the redox system and suggest that the mechanisms contributing to NLRP3 activation may perhaps be a cell-selective and stimuli-specific phenomenon (4).
Mediators of ROS action to activate NLRP3 inflammasomes
While there is a plethora of evidence supporting ROS activation of NLRP3 inflammasomes, the exact mechanisms by which NLRP3 senses these changes in oxidative stress are still largely unknown. In this regard, two distinct proteins have been demonstrated to associate with NLRP3–TXNIP and MAVS. The canonical work done by Zhou et al. provided strong evidence of TXNIP as a binding partner to NLRP3, where association between these two proteins was necessary for downstream inflammasome activation in pancreatic islet cells in response to high glucose (154). TXNIP, the negative regulator of the antioxidant thioredoxin (TRX), may time dependently dissociate from TRX to bind with NLRP3, leading to inflammasome formation and activation. Since then, multiple studies have confirmed the requirement of TXNIP to NLRP3 inflammasome activation (37, 89, 136). We have also demonstrated the involvement of TXNIP in Hcys-induced NLRP3 activation, where Hcys treatment was able to stimulate TXNIP association with NLRP3 in podocytes (2). Inhibition of TXNIP by local kidney shRNA transfection or through calcium channel blocker verapamil, demonstrated to be a potent TXNIP inhibitor (25), prevented TXNIP-NLRP3 binding in hyperhomocysteinemic mice and subsequent inflammasome activation and hHcys-induced glomerular injury. However, TXNIP involvement may also be specific to these nonphagocytic cells, as the converse has been shown in bone marrow-derived macrophages isolated from TXNIP-deficient mice where there was no difference in IL-1β secretion compared with wild-type macrophages (83). This evidence suggests that in a cell-type specific manner, TXNIP may act as a sensor to the changing levels of these ROS signaling molecules.
As previously mentioned, MAVS is a mitochondrial adaptor protein shown by Subramanian et al. to mediate the relocalization and association of NLRP3 to mitochondria (120). However, shown in the same study, this effect appeared to be specific to ATP and nigericin stimulation, as NLRP3 inflammasome activation appeared normal in MAVS-deficient macrophages on crystalline stimulations such as alum and silica. More recently, through coimmunoprecipitation studies, MAVS also associates with NLRP3 to facilitate its oligomerization with ASC and caspase-1 in response to Sendai virus challenge (103). Interestingly, the mitophagic process may help scavenge ROS, thereby blocking activation of inflammasomes (74). The actions of TXNIP and MAVS as possible mediators of ROS derived from NADPH oxidase or mitochondria to activate NLRP3 inflammasomes are summarized in Figure 5.
FIG. 5.
Mediators of ROS action to activate NLRP3 inflammasomes. Two distinct proteins have been demonstrated to be associated with NLRP3–thioredoxin-interacting protein (TXNIP) and mitochondrial anti-viral signaling protein (MAVS). TXNIP as a binding partner to NLRP3 time dependently dissociates from thioredoxin (TRX) and then binds with NLRP3, leading to inflammasome formation and activation. MAVS also associates with NLRP3 to facilitate its oligomerization with ASC and caspase-1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
NLRP3 Inflammasomes in Oxidative Injury and Chronic Degenerative Diseases
“Kindling” and “Bonfire” ROS
As discussed earlier, ROS can serve as a redox signaling molecule to activate or regulate NLRP3 inflammasome activation. This action is similar to the findings in many other cellular processes that use ROS as a messenger to mediate or regulate cell–cell communication and intracellular signal transduction (11, 117, 135). It is well known that under physiological or pathological conditions, ROS can be produced as a signaling messenger to maintain cell and organ function or can be increasingly generated and released in response to various stimuli. Meanwhile, these active molecules are also constantly scavenged by endogenous antioxidant systems mainly composed of enzyme-mediated pathways such as SOD, catalase, glutathione peroxidase, glutathione-S-transferase, TRX/TRX reductase, and other peroxidases. In addition, the direct reactions between ROS and different molecules such as nitric oxide, thiols, vitamin E, β-carotene, ceruloplasmin, ferritin, transferin, hemoglobin, and ascorbate may also have antioxidant action (11, 117, 135). Being tightly regulated under normal conditions, intracellular and extracellular ROS are maintained at very low levels, which play physiological signaling roles (less than 1% of produced ROS) (41, 61, 77, 108, 138). With regard to NLRP3 inflammasomes, early ROS production may be a signaling mechanism to activate the formation and activation of this intracellular machinery. We have demonstrated that increased O2•− derived from NADPH oxidase precedes the assembly of NLRP3 inflammasomes due to its action on TXNIP binding (2). This NADPH oxidase-produced O2•− or ROS may serve mainly as “kindling” signaling molecules, but may not yet have large injurious effects on cell function or activities.
When NLRP3 inflammasomes are activated on different stimuli such as hHcys, hypercholesterolemia, or DAMPs, the local inflammatory response occurs, and on recruitment and activation of inflammatory cells such as macrophages and T-cells, “bonfire” O2•− and cytokines are produced, resulting in chronic sterile inflammation and leading to tissue injury and sclerosis (4, 69, 150). If the generation of ROS is largely in excess of its scavenging during the local inflammatory response, intracellular and extracellular oxidative stress occurs; damage of DNAs, proteins, lipids, and glycols is inevitable, and, eventually, leads to the progression of various pathophysiological processes and respective diseases (11, 41, 61). Therefore, ROS derived from inflammasome activation and downstream immune cell recruitment may be important pathogenic factors in many chronic degenerative diseases. However, IL-1β or other inflammasome products may also directly act on cells such as podocytes or endothelial cells to decrease functional protein expression and NO availability, further contributing to local tissue fibrosis (4, 69, 143, 150).
NLRP3 inflammasome activation in chronic degenerative diseases
It has been reported that mutations in NLRP3 are associated with autoinflammatory disorders such as MWS, FCU, Familial Mediterranean fever, and neonatal-onset multisystem inflammatory disease (5, 96). Typical symptoms include fever after exposure to cold, skin lesions and rashes, neutrophilia, painful arthritis, sensorineural deafness, conjunctivitis, and Amyloid A amyloidosis (91). Those with mutations in the NACHT domain of NLRP3 have constitutively activated NLRP3 complexes and display exaggerated IL-1β secretion, especially in response to LPS when compared with healthy controls (6). MWS patients respond remarkably well to IL-1β antagonist, anakinra, implicating a crucial role of IL-1β in the pathogenic phenotype observed in these patients (46). In addition to IL-1β, NLRP3 inflammasome activation releases IL-18 and DAMP high mobility group box-1 (HMGB1). Release of HMGB1 is dependent on NLRP3 inflammasome processing, with its release attenuated in NLRP3−/− as well as ASC−/− macrophages during bacterial infection (141). However, although HMGB1 alone was not sufficient to activate NLRP3 inflammasomes or caspase-1 cleavage, HMGB1 treatment of THP-1 macrophages led to an upregulation in the synthesis of pro-IL-1β and pro-IL-18 (47), thus creating a vicious cycle of inflammasome activation potentiating both the synthesis and secretion of these powerful cytokines. Aberrant IL-1β secretion, HMGB1 release, and NLRP3 inflammasome activation has extended to many traditionally considered noninflammatory disorders, including diabetes, obesity, silicosis, liver toxicity, and kidney diseases (33, 80, 99, 133, 137, 139). Here, we will briefly focus on the role of NLRP3 in disorders associated with metabolic abnormality, including hHcys, hypercholesterolemia, and obesity.
Redox activation of NLRP3 inflammasomes in chronic kidney disease
It has been reported that NLRP3 mRNA levels inversely correlate with renal function in patients with chronic kidney disease (CKD), and IL-1β and IL-18 levels are increased in both animal models and patients with CKD (84, 131). In mice, NLRP3 gene knockout has been shown to protect from both renal ischemic acute tubular necrosis and the progression of CKD in the unilateral ureteral obstruction model (56, 131). In addition, glomerular IL-1β mRNA is enhanced after the first day of the mouse model of streptozotocin-induced diabetic glomerulosclerosis (114). These studies strongly support a crucial role for NLRP3 inflammasome involvement in the progression of ischemic kidney injury and CKD.
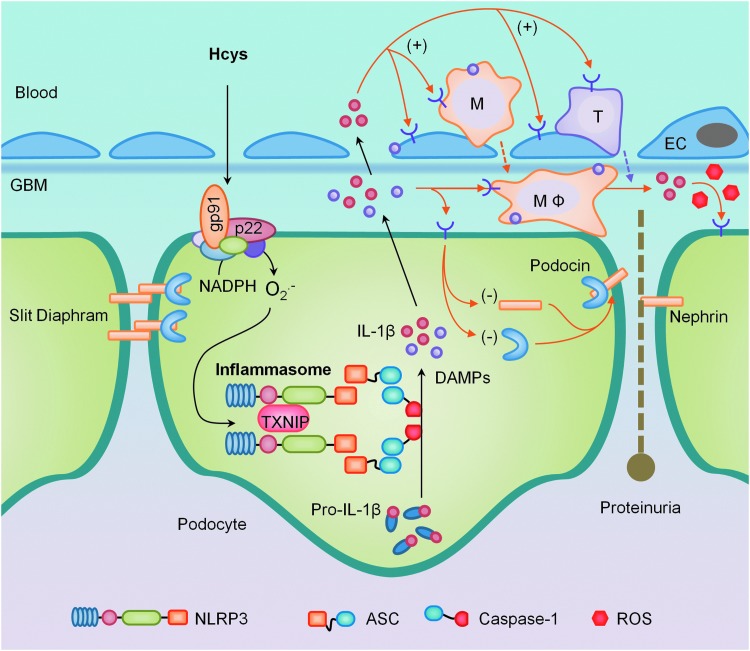
Efforts in our laboratory have focused on understanding the molecular pathogenesis of glomerular injury related to elevated Hcys, which, if left unattended, has the potential to progress to CKD and, eventually, ESRD (148). These damaging and sclerotic effects of hHcys have been associated with an inflammatory response mediated by the upregulation of proinflammatory molecules such as MCP-1, NF-κB, and IL-8, and adhesion molecules VCAM-1 and E-selectin (29, 134) and in the recruitment of lymphocytes (109). With podocytes being a major site of IL-1β synthesis (98), hHcys-induced activation of NLRP3 inflammasomes may explain how hHcys initiates the innate immune system in glomeruli (150). As summarized in Figure 6, when plasma Hcys levels increase, NADPH oxidase in podocytes or glomerular endothelial cells is activated via LR clustering to produce O2•−, which results in the formation of NLRP3 inflammasomes, activation of caspase-1, and the proteolytic cleavage of IL-1β and IL-18 into their biologically active form, which produce other DAMPs. These factors may recruit inflammatory cells such as macrophages and T-cells in glomeruli, contributing to the “bonfire” O2•− and cytokine production associated with chronic sterile glomerular inflammation and leading to tissue injury and sclerosis. IL-1β or other inflammasome products may also directly act on podocytes to decrease expression of podocyte-specific proteins such as nephrin and podocin, resulting in slit diaphragm derangement and proteinuria. In addition, excessive production of active caspase-1 may directly damage podocytes through pyroptosis, leading to foot process effacement and developing into glomerular sclerosis and ESRD.
FIG. 6.
Implications of NLRP3 inflammasomes in podocyte injury and ultimate glomerular sclerosis. In response to pathological stimuli, NADPH oxidase in podocytes or glomerular endothelial cells is activated via LR clustering to produce O2•−, which results in the formation of NLRP3 inflammasomes to produce IL-1β, IL-18, and other molecules such as damage-associated molecular patterns (DAMPs). These factors may recruit and activate inflammatory cells such as macrophages (MΦ) and T-cells in glomeruli, where “bonfire” O2•− and cytokines are produced to initiate typical chronic sterile glomerular inflammation, leading to tissue injury and sclerosis. Coinciding with other direct actions of IL-1β or other inflammasome products, this sterile inflammatory response leads to podocyte loss and foot process effacement, progressing into glomerular sclerosis and end-stage renal disease (ESRD). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Interplay of NLRP3 inflammasomes and ROS in cardiovascular diseases
Recent studies have demonstrated that the activation of NLRP3 inflammasome pathways is linked to the pathogenesis of cardiovascular diseases such as atherosclerosis, ischemic injury, cardiomyopathy, myocardial infarction, and Kawasaki disease. While the primary roles for NLRP3 inflammasomes are mostly related to the inflammatory responses due to the production of IL-1β and IL-18 or DAMPs such as HMGB1 (68, 123), emerging evidence has also revealed that inflammasome activation may also exert noncanonical effects, which are distinct from inflammasome-secreted cytokines that induce the activation and recruitment of inflammatory cells. As mentioned earlier, these mechanisms may potentially include other downstream targets of caspase-1 independent of IL-1β or IL-18, such as pyroptosis, inhibition of glycolysis, noninflammatory cell death, and activation of lipid biogenesis pathways (49, 65, 90, 115). In addition, recently elucidated pathways involving IL-1α and the innate immunity complement system during inflammasome activation may also contribute to such noncanonical actions (40, 113). These noninflammatory pathways may be importantly implicated in the development of atherosclerosis, glomerular disease, and other chronic degenerative diseases, and solely targeting inflammatory pathways may not eliminate the root of these inflammasome-related diseases. This is exemplified by the recent failure of clinical trials that only targeted inflammatory pathways such as phospholipase A2 and COX-2 to treat cardiovascular diseases (27, 92, 112).
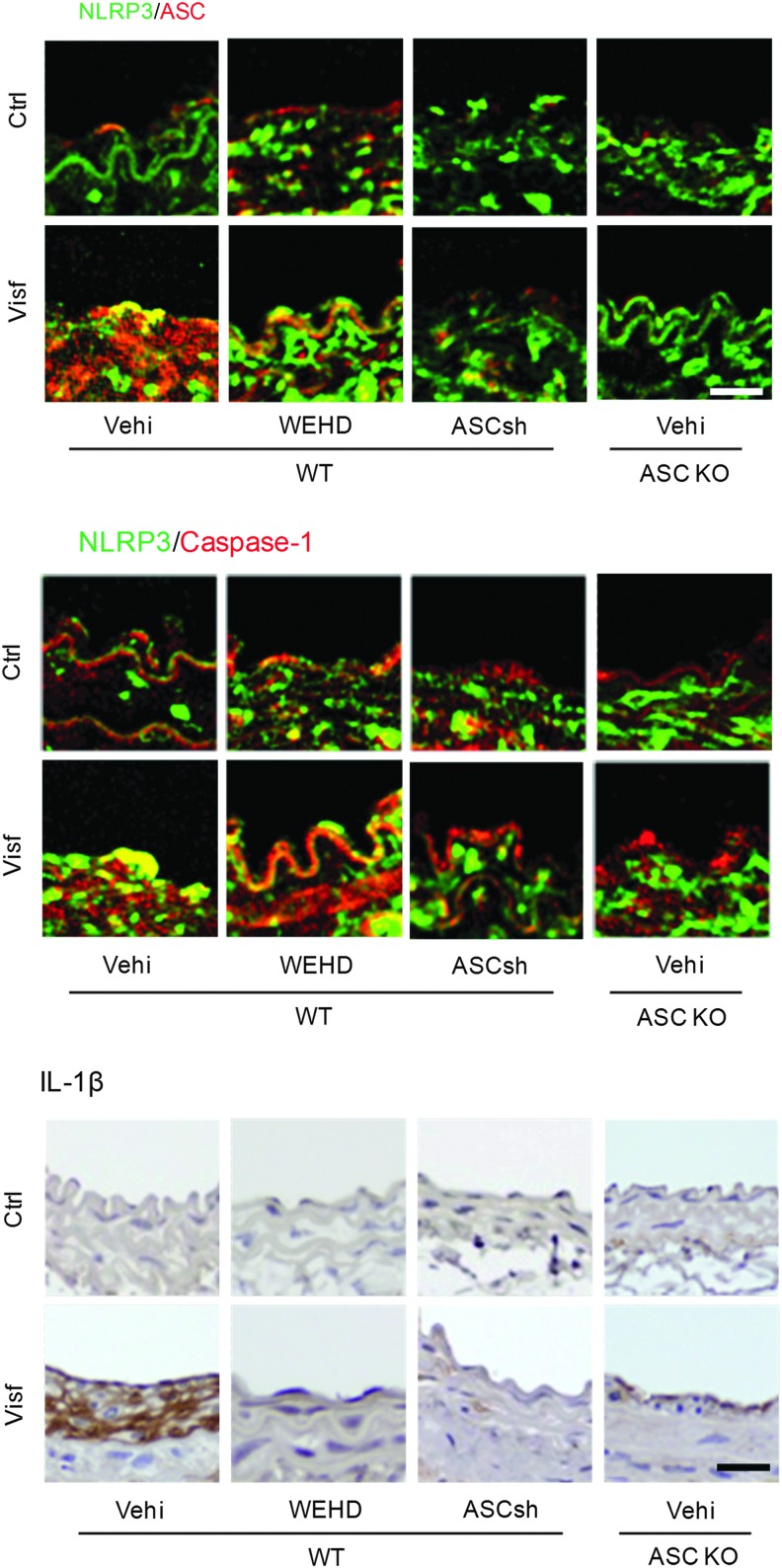
In a recent study, we demonstrated that activation of NLRP3 inflammasomes impaired the ability of macrophages to properly handle lipid metabolism or transport and enhanced their migration capacity (69). The formation and activation of NLRP3 inflammasomes by nonatherogenic danger factors, ATP or MSU crystals resulted in abnormal lysosomal cholesterol deposition, impaired postlysosomal trafficking of glycosphingolipids, and increased macrophage migration ability (69). These novel actions of inflammasomes in regulating macrophage functions may occur even before vascular inflammation contributes to atherosclerosis. Since endothelial dysfunction develops at the very early stages of vascular disease in response to risk factors such as hypertension, dyslipidemia, obesity, diabetes mellitus, or hHcys, we tested the redox activation of NLRP3 inflammasomes and its implication in endothelial injury. In particular, we revealed that the adipokine visfatin induced the formation and activation of NLRP3 inflammasomes in endothelial cells in vitro, which was dependent on membrane raft redox signaling platform-derived ROS and consequent TXNIP-NLRP3 interaction (143). As shown in Figure 7, activation of endothelial NLRP3 inflammasomes was markedly observed in the intima of partially ligated carotid arteries, which locally induced vascular injury and inflammation. IL-1β production in the intima was almost completely blocked by caspase-1 inhibitor and ASC gene knockout or silencing. Visfatin markedly decreased the expression of tight and adhesion junction proteins and increased vascular permeability in the coronary arterial endothelium of mice fed a 6 week high-fat diet (HFD) (26). These visfatin-induced changes in endothelial cells depend on HMGB1/RAGE signaling, which contributes to the vascular permeability leading to the onset of metabolic vasculopathy that, ultimately, results in atherosclerosis.
FIG. 7.
The formation and activation of NLRP3 inflammasome in the endothelium of carotid arteries during ligation injury. The formation and activation of endothelial NLRP3 inflammasomes were observed in the intima of carotid arteries during ligation that induces vascular injury or inflammation locally. IL-1β production in the intima could almost be completely blocked by caspase-1 inhibitor and ASC gene silencing or knocking out. Scale bar=10 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In addition, some in vivo studies in our laboratory demonstrated that increased plasma cholesterol (hypercholesterolemia) impaired endothelial dysfunction as assayed by endothelium-dependent vasodilator response in mouse coronary arteries, which were also associated with endothelial NLRP3 inflammasome activation and HMGB1 signaling (152). It is plausible that in addition to the classical inflammatory injury linked to the activation and recruitment of inflammatory cells such as macrophages and T-cells in the arterial wall leading to atherogenesis, the initial injurious response of coronary arteries due to the activation of endothelial inflammasomes by endogenous danger signals may be related to very early endothelial dysfunction. These data imply that endothelial inflammasome activation may represent a novel early event that leads to endothelial dysfunction and injury, and, if targeted, may prevent initiation or exacerbation of atherosclerosis during obesity or hypercholesterolemia.
ROS activation of NLRP3 inflammasomes in obesity
Accumulating evidence demonstrated that NLRP3 inflammasomes are implicated in the development of obesity and associated pathologies. Knockout of the NLRP3 inflammasome (NLRP3−/−, ASC−/−, and caspase-1−/−) significantly protected mice from HFD-induced obesity, increased adiposity, insulin resistance, glucose intolerance, and inflammation (52, 119, 130). The expression of the NLRP3 inflammasome subunits in adipose tissue correlates directly with body weight in mouse models and obese individuals with type 2 diabetes mellitus (130). In our recent studies, mice lacking the ASC gene demonstrated significant attenuation of HFD-induced obesity compared with ASC+/+ mice, and were also protected from obesity-associated glomerular and podocyte injury (18). The mechanism of HFD-induced inflammasome activation may be due to the high production of the fatty acid metabolites ceramide and palmitate, as saturated fatty acids have been shown to induce inflammasome activation through a mechanism that involves defective autophagy and the accumulation of mtROS (140). Vandanmagsar et al. showed that adding ceramide to adipose tissue explants led to NLRP3-dependent IL-1β production, suggesting that ceramide acts a danger signal to stimulate the NLRP3 inflammasome (130). In general, obesity is associated with perturbations of major cellular homeostatic pathways, such as ER stress, mitochondrial dysfunction, and autophagy deficiency, that are linked to ROS accumulation and oxidative stress (54). Thus, multiple mechanisms may contribute to oxidative stress pathways to activate the NLRP3 inflammasome in obese animals. Further studies are required to explore whether abnormal activation of NLRP3 inflammasomes occurs in adipose tissue of obese individuals to reveal new therapeutic targets for interventions in the prevention or treatment of obesity and related pathologies.
Conclusions
Here, we reviewed the major mediating and modulatory mechanisms thought to regulate NLRP3 inflammasome activation, including the involvement of K+, lysosomes, ER stress, Ca2+, ubiquitination, miRNAs, mitochondria, and ROS. All these studies have provided innovative insights into ways to prevent aberrant inflammasome signaling and IL-1β release with the ultimate goal of preventing the pathogenesis of a very broad range of chronic degenerative diseases. In our attempt to more fully and specifically understand the role of ROS in NLRP3 inflammasome pathologies, we reveal much more complex and interlaced mechanisms, where perhaps anti-inflammatory therapies would not be sufficient enough in treating the root of the disease. A majority of these therapies target the later “bonfire” ROS stages of disease, a consequence of immune cell activation and infiltration during the inflammatory response. However, the ultimate goal is to target early “kindling” ROS events, such as NLRP3 inflammasome activation, that would not only prevent instigation of the innate immune response but, as we have shown, also prevent direct injurious effects to host cells. Despite the rapid burst of literature regarding NLRP3 inflammasomes in just the previous 5 years and despite the strong evidence for the involvement of ROS, deeper investigation is of utmost importance to understand how these various activating pathways interact to mediate the activity of this cytoplasmic protein complex, especially the complexity of redox perturbations and its role in NLRP3 inflammasome activation.
Abbreviations Used
- AIM2
absent in melanoma 2
- ASC
apoptosis-associated speck-like protein containing a CARD
- [Ca2+]i
intracellular Ca2+
- CARD
caspase recruitment domain
- CGD
chronic granulomatous disease
- CKD
chronic kidney disease
- COXs
cyclooxygenases
- DAMPs
damage-associated molecular patterns
- DPI
diphenylene iodonium
- dsDNA
double-stranded DNA
- ER
endoplasmic reticulum
- ESRD
end-stage renal disease
- FCU
familial cold urticaria
- H2O2
hydrogen peroxide
- Hcys
homocysteine
- HFD
high-fat diet
- hHcys
hyperhomocysteinemia
- HMGB1
high mobility group box-1
- IL-1β
interleukin-1β
- IPAF
interleukin-1β-converting enzyme protease-activating factor
- LPS
lipopolysaccharide
- LOXs
lipoxygenases
- LR
lipid raft
- MAVS
mitochondrial anti-viral signaling protein
- MDP
muramyl dipeptide
- miRNAs
microRNAs
- MSU
monosodium urate
- mtDNA
mitochondrial DNA
- mtROS
mitochondrial ROS
- MWS
Muckle–Wells syndrome
- NAIPs
NLR family apoptosis inhibitory proteins
- NF-κB
NF-kappaB
- NLRP3
NOD-like receptor containing pyrin domain 3
- NLRs
nucleotide-binding domain leucine-rich repeats
- ONOO−
peroxynitrite
- PAMPs
pathogen-associated molecular patterns
- PBMCs
peripheral blood mononuclear cells
- PGE2
prostaglandin E2
- PRRs
pattern recognition receptors
- RLRs
RIG-I-like receptors
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- SOD
superoxide dismutase
- TEMPOL
4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl
- TLRs
toll-like receptors
- TRX
thioredoxin
- TXNIP
thioredoxin-interacting protein
- UPR
unfolded protein response
- X/XO
xanthine/xanthine oxidase
Acknowledgments
Many studies cited in this review from our laboratory were supported by grants HL57244, HL075316, DK54927, and HL091464 (to P.L.) and 1F31AG043289-01 (to J.M.A.) from the National Institutes of Health.
References
- 1.Abais JM, Xia M, Boini KM, and Li PL. Contribution of guanine nucleotide exchange factor Vav2 to homocysteine-induced NLRP3 inflammasome activation in mouse podocytes. FASEB J 28: 1063.6, 2014 [Google Scholar]
- 2.Abais JM, Xia M, Li G, Chen Y, Conley SM, Gehr TW, Boini KM, and Li PL. Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein during hyperhomocysteinemia. J Biol Chem 289: 27159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abais JM, Xia M, Li G, Gehr TW, Boini KM, and Li PL. Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic Biol Med 67: 211–220, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abais JM, Zhang C, Xia M, Liu Q, Gehr TW, Boini KM, and Li PL. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid Redox Signal 18: 1537–1548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, Lachmann HJ, Bybee A, Gaudet R, Woo P, Feighery C, Cotter FE, Thome M, Hitman GA, Tschopp J, and McDermott MF. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum 46: 2445–2452, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, and Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319–325, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Alvarez S, Munoz-Fernandez MA. TNF-Alpha may mediate inflammasome activation in the absence of bacterial infection in more than one way. PLoS One 8: e71477, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaral FA, Costa VV, Tavares LD, Sachs D, Coelho FM, Fagundes CT, Soriani FM, Silveira TN, Cunha LD, Zamboni DS, Quesniaux V, Peres RS, Cunha TM, Cunha FQ, Ryffel B, Souza DG, and Teixeira MM. NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum 64: 474–484, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Bae JY. and Park HH. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem 286: 39528–39536, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker PJ, Butter LM, Kors L, Teske GJ, Aten J, Sutterwala FS, Florquin S, and Leemans JC. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int 85: 1112–1122, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Baran CP, Zeigler MM, Tridandapani S, and Marsh CB. The role of ROS and RNS in regulating life and death of blood monocytes. Curr Pharm Des 10: 855–866, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Barbera-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, and Pelegrin P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1beta release. FASEB J 26: 2951–2962, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, and Hornung V. Inflammasomes: current understanding and open questions. Cell Mol Life Sci 68: 765–783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, and Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol 187: 613–617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, and Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol 189: 4175–4181, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, and Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benko S, Philpott DJ, and Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine 43: 368–373, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Boini KM, Xia M, Abais JM, Li G, Pitzer AL, Gehr TW, Zhang Y, and Li PL. Activation of inflammasomes in podocyte injury of mice on the high fat diet: effects of ASC gene deletion and silencing. Biochim Biophys Acta 1843: 836–845, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyden ED. and Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38: 240–244, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, and Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol 170: 3029–3036, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, and Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208: 519–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, and Rubartelli A. The rate of interleukin-1beta secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. J Biol Chem 286: 27069–27080, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, and Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A 105: 9035–9040, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassel SL. and Sutterwala FS. Sterile inflammatory responses mediated by the NLRP3 inflammasome. Eur J Immunol 40: 607–611, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Cha-Molstad H, Szabo A, and Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab 296: E1133–E1139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Pitzer AL, Li X, Xu M, Zhang Y, and Li PL. High mobility group box protein 1 as a novel vascular permeability factor derived from endothelial inflammasome activation during obesity. FASEB J 28: 6885, 2014 [Google Scholar]
- 27.Coomes E, Chan ES, and Reiss AB. Methotrexate in atherogenesis and cholesterol metabolism. Cholesterol 2011: 503028, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, and Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282: 2871–2879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai J. and Wang X. Immunoregulatory effects of homocysteine on cardiovascular diseases. Sheng Li Xue Bao 59: 585–592, 2007 [PubMed] [Google Scholar]
- 30.De Nardo D, De Nardo CM, and Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol 184: 42–54, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denes A, Lopez-Castejon G, and Brough D. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis 3: e338, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27: 519–550, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, and Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol 529 Pt 1: 57–68, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, and Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, and Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol 182: 6460–6469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Azab MF, Baldowski BR, Mysona BA, Shanab AY, Mohamed IN, Abdelsaid MA, Matragoon S, Bollinger KE, Saul A, and El-Remessy AB. Deletion of thioredoxin-interacting protein preserves retinal neuronal function by preventing inflammation and vascular injury. Br J Pharmacol 171: 1299–1313, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, and Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25: 713–724, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, and Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol 41: 2040–2051, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, and Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol 14: 1045–1053, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Fubini B. and Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med 34: 1507–1516, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Gabelloni ML, Sabbione F, Jancic C, Bass JF, Keitelman I, Iula L, Oleastro M, Geffner JR, and Trevani AS. NADPH oxidase derived reactive oxygen species are involved in human neutrophil IL-1beta secretion but not in inflammasome activation. Eur J Immunol 43: 3324–3335, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, and Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A 86: 7611–7615, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, and Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9: 857–865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O'Neill LA, and Masters SL. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol 189: 3795–3799, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Hawkins PN, Lachmann HJ, Aganna E, and McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum 50: 607–612, 2004 [DOI] [PubMed] [Google Scholar]
- 47.He Q, You H, Li XM, Liu TH, Wang P, and Wang BE. HMGB1 promotes the synthesis of pro-IL-1beta and pro-IL-18 by activation of p38 MAPK and NF-kappaB through receptors for advanced glycation end-products in macrophages. Asian Pac J Cancer Prev 13: 1365–1370, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, and Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482: 179–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henao-Mejia J, Elinav E, Strowig T, and Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol 13: 321–324, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, and Golenbock DT. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493: 674–678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hewinson J, Moore SF, Glover C, Watts AG, and MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor-induced IL-1 beta processing in human monocytes. J Immunol 180: 8410–8420, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Horng T. and Hotamisligil GS. Linking the inflammasome to obesity-related disease. Nat Med 17: 164–165, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, and Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichinohe T, Lee HK, Ogura Y, Flavell R, and Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206: 79–87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, and Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 106: 20388–20393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin S, Zhou F, Katirai F, and Li PL. Lipid raft redox signaling: molecular mechanisms in health and disease. Antioxid Redox Signal 15: 1043–1083, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, and Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451: 1125–1129, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, and Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol 183: 3578–3581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, and Alnemri ES. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem 287: 36617–36622, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan N. and Swartz H. Measurements in vivo of parameters pertinent to ROS/RNS using EPR spectroscopy. Mol Cell Biochem 234–235: 341–357, 2002 [PubMed] [Google Scholar]
- 62.Kofoed EM. and Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, Lommatzsch SE, Huang MT, Ting JP, and Koller BH. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J Immunol 189: 2006–2016, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lage SL, Longo C, Branco LM, da Costa TB, Buzzo Cde L, and Bortoluci KR. Emerging Concepts about NAIP/NLRC4 Inflammasomes. Front Immunol 5: 309, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol 11: 213–220, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M, Heintz N, Greengard P, Hui S, Tang Q, Trusina A, Oakes SA, and Papa FR. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 16: 250–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li PL, Zhang Y, and Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid Redox Signal 9: 1457–1470, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Li X, Deroide N, and Mallat Z. The role of the inflammasome in cardiovascular diseases. J Mol Med (Berl) 92: 307–319, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Li X, Zhang Y, Xia M, Gulbins E, Boini KM, and Li PL. Activation of Nlrp3 inflammasomes enhances macrophage lipid-deposition and migration: implication of a novel role of inflammasome in atherogenesis. PLoS One 9: e87552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liao PC, Chao LK, Chou JC, Dong WC, Lin CN, Lin CY, Chen A, Ka SM, Ho CL, and Hua KF. Lipopolysaccharide/adenosine triphosphate-mediated signal transduction in the regulation of NLRP3 protein expression and caspase-1-mediated interleukin-1beta secretion. Inflamm Res 62: 89–96, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, Sauer JD, Dunipace EA, Warren SE, Miao EA, and Vance RE. Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun 79: 1606–1614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindauer M, Wong J, and Magun B. Ricin toxin activates the NALP3 inflammasome. Toxins (Basel) 2: 1500–1514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopez-Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E, and Brough D. Deubiquitinases regulate the activity of caspase-1 and interleukin-1beta secretion via assembly of the inflammasome. J Biol Chem 288: 2721–2733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lupfer C, Thomas PG, Anand PK, Vogel P, Milasta S, Martinez J, Huang G, Green M, Kundu M, Chi H, Xavier RJ, Green DR, Lamkanfi M, Dinarello CA, Doherty PC, and Kanneganti TD. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol 14: 480–488, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mariathasan S. ASC, Ipaf and Cryopyrin/Nalp3: bona fide intracellular adapters of the caspase-1 inflammasome. Microbes Infect 9: 664–671, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, and Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol 40: 616–619, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Martinon F, Agostini L, Meylan E, and Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol 14: 1929–1934, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Martinon F, Burns K, and Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Martinon F, Petrilli V, Mayor A, Tardivel A, and Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Martinon F. and Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol 26: 447–454, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Masters SL. Specific inflammasomes in complex diseases. Clin Immunol 147: 223–228, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, and O'Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol 11: 897–904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsumoto K. and Kanmatsuse K. Elevated interleukin-18 levels in the urine of nephrotic patients. Nephron 88: 334–339, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Meissner F, Molawi K, and Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol 9: 866–872, 2008 [DOI] [PubMed] [Google Scholar]
- 86.Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, and Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 116: 1570–1573, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, and Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis 3: e261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, and Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A 107: 3076–3080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohamed IN, Hafez SS, Fairaq A, Ergul A, Imig JD, and El-Remessy AB. Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet. Diabetologia 57: 413–423, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monack DM, Detweiler CS, and Falkow S. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol 3: 825–837, 2001 [DOI] [PubMed] [Google Scholar]
- 91.Muckle TJ, Wellsm Urticaria, deafness, and amyloidosis: a new heredo-familial syndrome. Q J Med 31: 235–248, 1962 [PubMed] [Google Scholar]
- 92.Mullard A. GSK's darapladib failures dim hopes for anti-inflammatory heart drugs. Nat Rev Drug Discov 13: 481–482, 2014 [DOI] [PubMed] [Google Scholar]
- 93.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, and Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, and Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109: 11282–11287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, and Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452: 103–107, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Neven B, Callebaut I, Prieur AM, Feldmann J, Bodemer C, Lepore L, Derfalvi B, Benjaponpitak S, Vesely R, Sauvain MJ, Oertle S, Allen R, Morgan G, Borkhardt A, Hill C, Gardner-Medwin J, Fischer A, and de Saint Basile G. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood 103: 2809–2815, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Nicholas SA, Bubnov VV, Yasinska IM, and Sumbayev VV. Involvement of xanthine oxidase and hypoxia-inducible factor 1 in Toll-like receptor 7/8-mediated activation of caspase 1 and interleukin-1beta. Cell Mol Life Sci 68: 151–158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Niemir ZI, Stein H, Dworacki G, Mundel P, Koehl N, Koch B, Autschbach F, Andrassy K, Ritz E, Waldherr R, and Otto HF. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int 52: 393–403, 1997 [DOI] [PubMed] [Google Scholar]
- 99.Nishi Y, Satoh M, Nagasu H, Kadoya H, Ihoriya C, Kidokoro K, Sasaki T, and Kashihara N. Selective estrogen receptor modulation attenuates proteinuria-induced renal tubular damage by modulating mitochondrial oxidative status. Kidney Int 83: 662–673, 2013 [DOI] [PubMed] [Google Scholar]
- 100.O'Connor W, Jr., Harton JA, Zhu X, Linhoff MW, and Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol 171: 6329–6333, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Oslowski CM, Hara T, O'Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, and Urano F. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab 16: 265–273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Overley-Adamson B, Artlett CM, Stephens C, Sassi-Gaha S, Weis RD, and Thacker JD. Targeting the unfolded protein response, XBP1, and the NLRP3 inflammasome in fibrosis and cancer. Cancer Biol Ther 15, 420–462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park S, Juliana C, Hong S, Datta P, Hwang I, Fernandes-Alnemri T, Yu JW, and Alnemri ES. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J Immunol 191: 4358–4366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelegrin P. and Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pelegrin P. and Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 28: 2114–2127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petrilli V. and Martinon F. The inflammasome, autoinflammatory diseases, and gout. Joint Bone Spine 74: 571–576, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, and Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14: 1583–1589, 2007 [DOI] [PubMed] [Google Scholar]
- 108.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, and Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 244: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Postea O, Koenen RR, Hristov M, Weber C, and Ludwig A. Homocysteine up-regulates vascular transmembrane chemokine CXCL16 and induces CXCR6+ lymphocyte recruitment in vitro and in vivo. J Cell Mol Med 12: 1700–1709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Py BF, Kim MS, Vakifahmetoglu-Norberg H, and Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49: 331–338, 2013 [DOI] [PubMed] [Google Scholar]
- 111.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, and Stacey KJ. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323: 1057–1060, 2009 [DOI] [PubMed] [Google Scholar]
- 112.Rosenson RS. and Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J 33: 2899–2909, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, Lappegard KT, Brekke OL, Lambris JD, Damas JK, Latz E, Mollnes TE, and Espevik T. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol 192: 2837–2845, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sassy-Prigent C, Heudes D, Mandet C, Belair MF, Michel O, Perdereau B, Bariety J, and Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 49: 466–475, 2000 [DOI] [PubMed] [Google Scholar]
- 115.Shao W, Yeretssian G, Doiron K, Hussain SN, and Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem 282: 36321–36329, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, and Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Silva JP, Proenca F, and Coutinho OP. Protective role of new nitrogen compounds on ROS/RNS-mediated damage to PC12 cells. Free Radic Res 42: 57–69, 2008 [DOI] [PubMed] [Google Scholar]
- 118.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, and Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab 12: 593–605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, and Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 108: 15324–15329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, and Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153: 348–361, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Surprenant A, Rassendren F, Kawashima E, North RA, and Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272: 735–738, 1996 [DOI] [PubMed] [Google Scholar]
- 122.Sutterwala FS, Ogura Y, and Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol 82: 259–264, 2007 [DOI] [PubMed] [Google Scholar]
- 123.Takahashi M. Role of the inflammasome in myocardial infarction. Trends Cardiovasc Med 21: 37–41, 2011 [DOI] [PubMed] [Google Scholar]
- 124.Tassi S, Carta S, Vene R, Delfino L, Ciriolo MR, and Rubartelli A. Pathogen-induced interleukin-1beta processing and secretion is regulated by a biphasic redox response. J Immunol 183: 1456–1462, 2009 [DOI] [PubMed] [Google Scholar]
- 125.Thomas PG, Dash P, Aldridge JR, Jr., Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, and Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30: 566–575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Triantafilou K, Hughes TR, Triantafilou M, and Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci 126: 2903–2913, 2013 [DOI] [PubMed] [Google Scholar]
- 127.Tschopp J. and Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10: 210–215, 2010 [DOI] [PubMed] [Google Scholar]
- 128.van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, and van den Berg TK. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood 115: 5398–5400, 2010 [DOI] [PubMed] [Google Scholar]
- 129.van de Veerdonk FL, Smeekens SP, Joosten LA, Kullberg BJ, Dinarello CA, van der Meer JW, and Netea MG. Reactive oxygen species-independent activation of the IL-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A 107: 3030–3033, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, and Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17: 179–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, and Muruve DA. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21: 1732–1744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.von Moltke J, Trinidad NJ, Moayeri M, Kintzer AF, Wang SB, van Rooijen N, Brown CR, Krantz BA, Leppla SH, Gronert K, and Vance RE. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490: 107–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang C, Pan Y, Zhang QY, Wang FM, and Kong LD. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One 7: e38285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang G, Woo CW, Sung FL, Siow YL, and O K. Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arterioscler Thromb Vasc Biol 22: 1777–1783, 2002 [DOI] [PubMed] [Google Scholar]
- 135.Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang YJ, Wu WN, Dong LD, and Chen JG. Protection by tetrahydroxystilbene glucoside against cerebral ischemia: involvement of JNK, SIRT1, and NF-kappaB pathways and inhibition of intracellular ROS/RNS generation. Free Radic Biol Med 47: 229–240, 2009 [DOI] [PubMed] [Google Scholar]
- 136.Wang W, Wang C, Ding XQ, Pan Y, Gu TT, Wang MX, Liu YL, Wang FM, Wang SJ, and Kong LD. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br J Pharmacol 169: 1352–1371, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang W, Wang X, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, and Muruve DA. Inflammasome-independent NLRP3 augments TGF-beta signaling in kidney epithelium. J Immunol 190: 1239–1249, 2013 [DOI] [PubMed] [Google Scholar]
- 138.Wang YX. and Zheng YM. ROS-dependent signaling mechanisms for hypoxic Ca(2+) responses in pulmonary artery myocytes. Antioxid Redox Signal 12: 611–623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J, and French LE. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 127: 1956–1963, 2007 [DOI] [PubMed] [Google Scholar]
- 140.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, and Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12: 408–415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Willingham SB, Allen IC, Bergstralh DT, Brickey WJ, Huang MT, Taxman DJ, Duncan JA, and Ting JP. NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1 activation, necrosis, and HMGB1 release via inflammasome-dependent and -independent pathways. J Immunol 183: 2008–2015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, and Hu G. Activation of NLRP3 inflammasome in alveolar macrophages contributes to mechanical stretch-induced lung inflammation and injury. J Immunol 190: 3590–3599, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia M, Boini KM, Abais JM, Xu M, Zhang Y, and Li PL. Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. Am J Pathol 184: 1617–1628, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xia M, Zhang C, Boini KM, Thacker AM, and Li PL. Membrane raft-lysosome redox signalling platforms in coronary endothelial dysfunction induced by adipokine visfatin. Cardiovasc Res 89: 401–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, and Shyy JY. Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility. Circulation 128: 632–642, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, Grant EP, Coyle AJ, Misaghi A, Hoffman HM, and Gallo RL. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem 284: 12762–12771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, Shi W, Li F, Xin T, Pang Q, and Yi F. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab 34: 660–667, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yi F. and Li PL. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol 28: 254–264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, and Yang XF. Inflammasomes: sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed) 18: 638–649, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang C, Boini KM, Xia M, Abais JM, Li X, Liu Q, and Li PL. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension 60: 154–162, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang C, Hu JJ, Xia M, Boini KM, Brimson CA, Laperle LA, and Li PL. Protection of podocytes from hyperhomocysteinemia-induced injury by deletion of the gp91phox gene. Free Radic Biol Med 48: 1109–1117, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Y, Li X, Pitzer AL, and Li PL. Coronary endothelial dysfunction induced by Nlrp3 inflammasome activation during hypercholesterolemia: beyond inflammation. FASEB J 28: 85113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, and Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600, 2011 [DOI] [PubMed] [Google Scholar]
- 154.Zhou R, Tardivel A, Thorens B, Choi I, and Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140, 2010 [DOI] [PubMed] [Google Scholar]
- 155.Zhou R, Yazdi AS, Menu P, and Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]