Abstract
CD34+ cell dose provides a measure of hematopoietic tissue that predicts the rate of engraftment upon transplant. It is positively correlated with multiple measures of hematopoietic recovery, including platelet engraftment. Here we identify a subpopulation of CD34+ cells that coexpress a surface antigen—MA6, which is more positively correlated with platelet engraftment in a clinical setting than CD34+ alone. The specific identity and function of MA6 remain to be determined, however, it is expressed by primitive megakaryocyte (MK) progenitors, but is lost with differentiation and is not expressed by platelets. Commitment of CD34+MA6+ cells to the MK lineage was confirmed by in vitro assays and their significance in hematopoietic transplantation explored by flow cytometric analysis of cryopreserved samples of granulocyte colony stimulating factor-mobilized peripheral blood progenitor cell (PBPC) products along with a retrospective analysis of platelet engraftment data. Platelet engraftment by day 21 was predicted by receipt of ≥6×106 CD34+ cells/kg or ≥0.3×106 CD34+MA6+ cells/kg. Subsequent analysis of cord blood (CB) CD34+ cells revealed <0.2% coexpressed MA6+, compared to 8% of PBPC CD34+ cells. This low proportion of CD34+MA6+ cells may be responsible, at least in part, for the delayed platelet engraftment associated with CB transplantation. However, platelet engraftment is markedly improved in recipients of ex vivo-expanded CB. This may be a consequence of an increased proportion of CD34+MA6+ cells present in the ex vivo-expanded product and also suggests that optimizing ex vivo culture conditions to generate CD34+MA6+ cells might further improve platelet engraftment in CB recipients.
Introduction
While the generation of platelets from megakaryocytes (MKs) in the bone marrow (BM) has been shown to be supported by a hierarchy of progenitors that are ultimately derived from CD34+ hematopoietic cells, measures of a hematopoietic graft that are strongly predictive of platelet engraftment following hematopoietic transplantation remain to be thoroughly identified. While clinical data suggest that the time to platelet engraftment is correlated with the dose of CD34+ hematopoietic progenitor cells transplanted [1,2], other measures of hematopoietic progenitors contained within the transplanted tissue have also shown some correlation [3–8]. Lineage-committed MK progenitors can be characterized by in vitro colony-forming assays and their generation of burst- and colony-forming units (BFU-MK and CFU-MK, respectively) [9–12]. However, these in vitro measures of MK progenitors are of limited value. Although they may provide a more definitive assessment of MK progenitor numbers, they are time-consuming, do not allow real-time evaluation and, since the colonies are the product of the proliferation of MK progenitors, they do not allow the analysis of the MK progenitor itself. However, MK progenitors can also be characterized by their expression of a range of cell surface markers, including CD41a and CD61 [13–15]. Such assessments can be performed rapidly and provide the potential for real-time assessment of MK progenitor cell numbers, prospective isolation, additional analyses, and therapeutic application. Nevertheless, flow cytometric data can be compromised by the presence of false-positive events arising as a consequence of the binding of CD41a+61+ platelets to non-MK cells. As a consequence and in an attempt to identify a cell surface marker that might better define early commitment of hematopoietic progenitors to the MK lineage, mice were immunized with a mixture of human CD34+41+ and CD34−41+ cells generated by the ex vivo expansion of peripheral blood progenitor cells (PBPC) [16]. Resulting hybridomas were screened for antibodies reactive against the MK cell line, Meg01. The antibody product of hybridoma MA6 was identified for further investigation. In this study, the reactivity of the MA6 hybridoma supernatant is characterized (the hybridoma MA6 and the MA6 antigen discussed here are not related to the rat monoclonal anti-integrin alpha 6 antibody, also designated “MA6”). We report that the MA6 IgM antibody generated by Horsfall et al. [16], identifies a unique, stage-specific cell surface molecule acquired by primitive MK progenitors, but is lost with differentiation to more mature MK progenitors and is absent on platelets. These data prompted the current investigation of whether the dose of CD34+MA6+ cells contained in granulocyte colony stimulating factor (G-CSF)-mobilized PBPC products would correlate with time to platelet engraftment in cohorts of patients in the autologous and allogeneic stem cell transplant setting. In addition, we sought to investigate whether CD34+MA6+ cells might be a subpopulation to investigate in the setting of umbilical cord blood (CB) transplantation, where delayed platelet engraftment remains a major obstacle.
Materials and Methods
MA6 antibody generation
An antibody discovery program was initiated by Paul Simmons to identify cell surface markers for late-stage MK progenitor cells. After immunization of mice with a mixture of CD34+41+ and CD34−41+ cells generated by ex vivo expansion of PBPC in a combination of interleukin (IL)-3, IL-11, stem cell factor, and thrombopoietin, the resulting hybridomas were screened for antibodies reactive with the MK cell line Meg01 and nonreactive with a mixed pool of myeloid and lymphoid leukemia cell lines (HL-60, U937; Jurkat and Daudi). Screening of antibodies that met these criteria on BM cells and PBPC led to the identification of a number of reactive antibodies, one of which, MA6, was selected for further investigation [16].
Patient cohorts
Cryopreserved aliquots of G-CSF-mobilized products from three different cohorts of transplant patients were analyzed. Cohort 1: thirty autotransplant patients with high-risk breast cancer treated at centers in Australia (auto-Australia), Cohort 2: sixty-four autologous transplant patients with lymphoma or myeloma treated at the MD Anderson Cancer Center (auto-MDACC), and Cohort 3: thirty-five allogeneic PBPC transplant patients with acute leukemia treated at the MDACC (allo-MDACC). This retrospective review of platelet engraftment and the preclinical stem cell donations for research were all performed under MD Anderson Cancer Center Institutional Review Board-approved protocols.
Engraftment study design
Samples of cryopreserved PBPC products were thawed and analyzed by flow cytometry for the expression of CD34 and MA6. The transplanted dose of each subset was calculated by multiplying the percent of the subpopulation of interest by the number of total nucleated cells (TNC) infused per kilogram.
Flow cytometric analysis
Flow cytometric analysis of the PBPC samples (and in selected experiments, CB samples) was performed using a directly fluorochrome-conjugated anti-CD34 antibody (BD Biosciences). The hybridoma supernatant containing the MA6 antibody was generated and binding was revealed using a fluorochrome-conjugated rat anti-mouse IgM secondary antibody (BD Biosciences). Data were acquired using a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using CELLQuestPro software (Becton Dickinson).
Clonogenic assays
In specific experiments, the MegaCult™-C and medium (StemCell Technologies) and recommended staining procedure were used as per the manufacturer's instructions to identify MK progenitors. CFU-MK and BFU-MK colonies were revealed by expression of glycoprotein llb/llla (CD41).
Statistical analyses of CD34+ and CD34+MA6+ cell doses infused and assessment of correlation with time to platelet engraftment
Descriptive statistical analyses were used to summarize CD34+ and CD34+MA6+ cell doses (cells/kg) and the type of transplant (auto, allo). Platelet engraftment was defined as the first of 7 consecutive days post-transplant with >20,000 platelet/μL blood and without platelet transfusion. Cox proportional hazards regression [17] was performed to model time to platelet engraftment as a function of CD34+ and CD34+MA6+ cell dose. The product-limit estimator of Kaplan and Meier [18] was used to illustrate the relationship between CD34+ and CD34+MA6+ cell doses and time to platelet engraftment. Quartiles of the distributions of CD34+ and CD34+MA6+ cell doses were selected a priori as cut points for categorizing cell doses as low, medium, and high. Quartiles that were not significantly different with respect to time to platelet engraftment were grouped together to create a new category.
Ex vivo CB expansion
CB units were obtained with donor informed consent under MD Anderson IRB-approved protocols. CB mononuclear cells (MNC) were cocultured with mesenchymal stem cells for 7 days in a medium supplemented with 10% fetal bovine serum and containing 100 ng/mL each of SCF, Flt-3L, TPO (all CellGenix), and G-CSF (Neupogen), as previously described [19]. On day 7, nonadherent cells were moved to liquid culture with the MK-inducing factors TPO (100 ng/mL), SCF (100 ng/mL), IL-3 (10 ng/mL; all Cellgenix), and IL-11 (100 ng/mL; R&D Systems) for an additional 7 days for a total culture period of 14 days. Pre- and postculture CB cells were evaluated morphologically following Giemsa staining of cytospins and by flow cytometry for CD34 and MA6 expression.
Results
MA6 staining of BM MNC
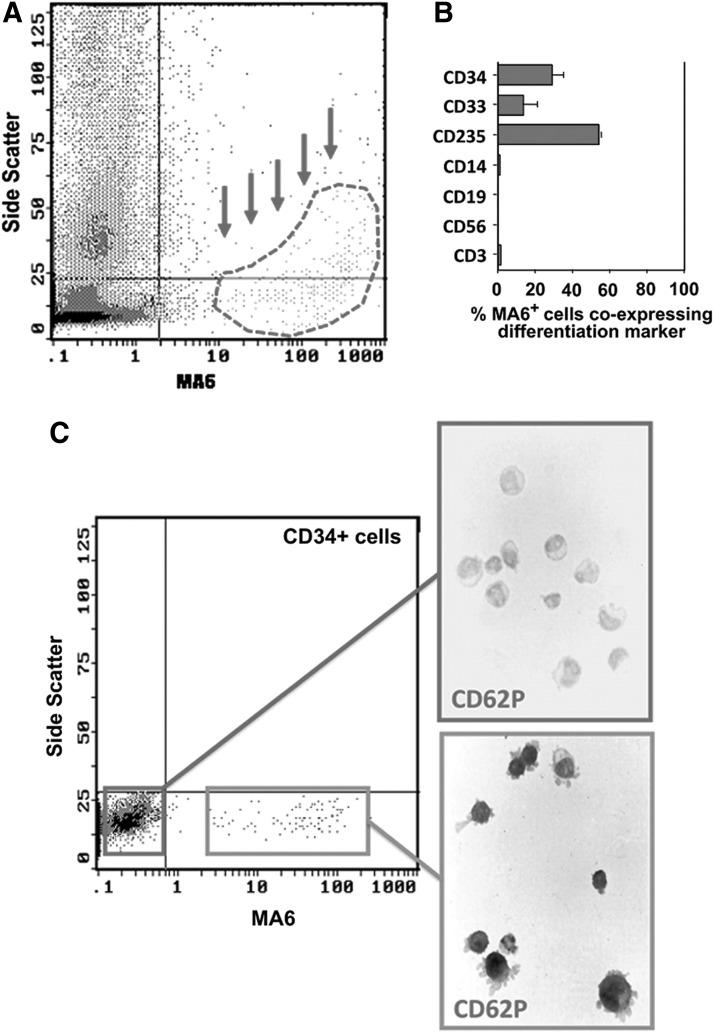
As shown in Fig. 1A, MA6 identifies a small subpopulation of BM MNC (4.6%±2.3%; n=11) characterized by low to intermediate side scatter of which ∼50% coexpressed the erythrocyte marker glycophorin A (CD235) and a further 15% expressed the myeloid marker CD33. MA6-positive cells were devoid of cell surface markers of monocytes, B cells, T cells, and NK cells (Fig. 1B). Furthermore, MA6 did not bind to platelets in BM or PB (data not shown). Notably, ∼25% of MA6-reactive cells coexpressed CD34. Approximately 5% of BM CD34+ cells exhibited a well-resolved subpopulation of MA6-reactive cells (range 2.3%–7.9%; n=6). The MK lineage identity of this population was confirmed by staining sorted CD34+ MA6-reactive cells with antibody to P-selectin (CD62P), a well-established marker of the MK lineage, an observation consistent with the hypothesis that MA6 identifies an MK progenitor (Fig. 1C).
FIG. 1.
Phenotypic characteristics of MA6-reactive (MA6+) cells. MA6+ cells comprised ∼5% of bone marrow mononuclear cells. (A) Representative flow cytometry from 17 different cord blood (CB) units revealed that MA6+ cells are low-intermediate side scatter (indicated by dashed area and arrows). (B) Multilineage flow cytometric analysis reveals that ∼50% of MA6+ cells coexpressed CD235 (Glycophorin A), 25% of MA6+ cells coexpressed CD34, and 15% of MA6+ cells coexpressed CD33. MA6+ cells do not express CD14, CD19, CD56, or CD3. Platelets do not express MA6 (data not shown). (C) Evidence of commitment to the megakaryocyte lineage by CD34+MA6+ cells was revealed by immunohistochemistry for the expression of P-selectin (CD62P). CD34+MA6+ cells were CD62P positive, while CD34+MA6− cells were CD62P negative.
Flow cytometric characteristics of CD34+MA6+ PBPC
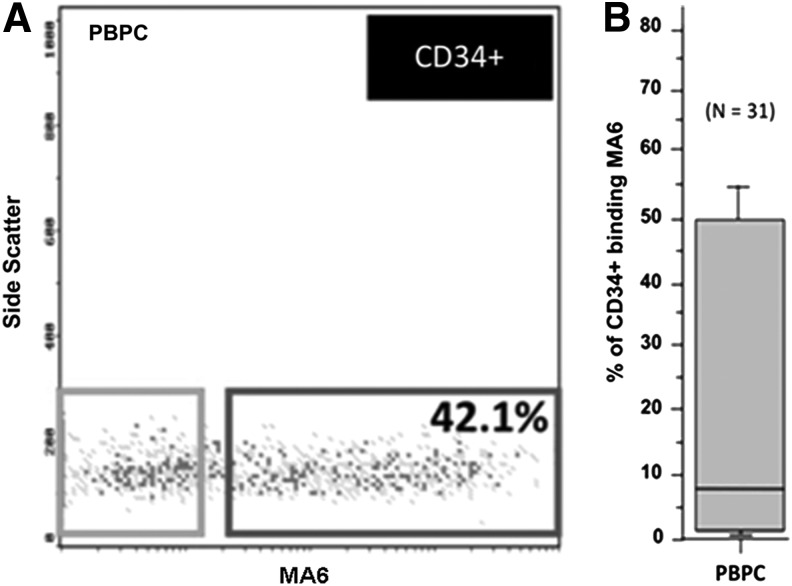
A representative flow cytometric analysis of CD34+ cells from a single PBPC product is shown (Fig. 2A). Although ∼42% of CD34+ cells coexpress MA6, significant variability was evident in the MFI of the MA6 signal (Fig. 2B). Preliminary analysis of 31 PBPC samples revealed that the variation in the proportion of CD34+ cells coexpressing MA6 was considerable. Data ranged from <5% to ∼50% (median ∼8%).
FIG. 2.
Flow cytometric characteristics of CD34+MA6+ cells. (A) Representative flow cytometric analysis of CD34+ cells from a single peripheral blood progenitor cell (PBPC) product is shown. Although ∼42% of the CD34+ coexpressed MA6, the variability in MFI of the MA6 signal was considerable. (B) Preliminary analysis of 31 PBPC samples revealed that the intersample variation in the proportion of CD34+ cells coexpressing MA6 was marked. From a median of ∼8% CD34+ cells coexpressing MA6, data ranged from <5% to ∼50%.
Patient PBPC, CD34+ and CD34+MA6+ dose
Mean patient weight was 79.2±18.2 kg (median: 78.0 kg, range: 15–125 kg) and patients received 80.5±61.8×109 TNC (median: 66.2×109 TNC, range: 5.8–106.6×109 TNC). This corresponded to a mean of 5.46±2.76×106 CD34+ cells/kg (median: 4.72 CD34+ cells/kg, range: 1.97–18.29×106 CD34+ cells/kg) and 0.24±0.32×106 CD34+MA6+ cells/kg (median: 0.16×106 CD34+MA6+ cells/kg, range: 0.01–2.10×106 CD34+MA6+ cells/kg). CD34+MA6+ cells comprised 4.07%±3.19% of the CD34+ cells (median: 3.54%, range: 0.20%–22.85%).
Platelet engraftment as a function of PBPC, CD34+ and CD34+MA6+ dose
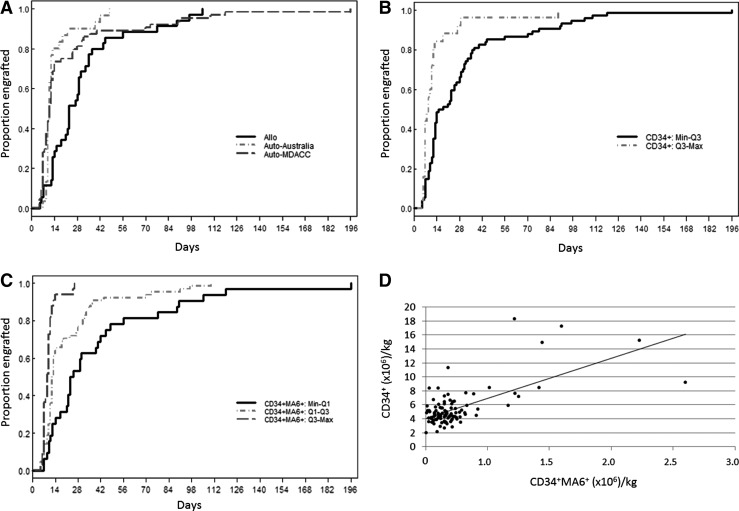
Although all 129 patients engrafted platelets, CD34+ data were only available for the 99 MDACC patients. Quartiles of the distribution of CD34+ cells/kg and the distribution of CD34+MA6+ cells/kg are shown (Table 1). Platelet engraftment was modeled as a function of patient cohort to determine whether the three patient cohorts (auto-Australia, auto-MDACC, and allo-MDACC) were similar. The two auto-cohorts (auto-Australia and auto-MDACC) each had platelet engraftment in a median of 11 days with no significant difference between the two cohorts (P=0.363) (Fig. 3A). These cohorts were therefore combined for analysis of CD34+MA6+ cells/kg data. In contrast, the allo-cohort (allo-MDACC) had platelet engraftment in a median of 23 days (Table 2). Two risk groups (Low: Min–Q3 and High: Q3–Max) for platelet engraftment were identified as a function of CD34+ cells/kg (Table 2 and Fig. 3B). When platelet engraftment was modeled as a function of the quartiles of CD34+MA6+ cells/kg, three risk groups were identified (Low: Min–Q1; Medium: Q1–Q3; High: Q3–Max) (Table 2 and Fig. 3C). When the CD34+MA6+ cell dose/kg was considered, the type of transplant (allo vs. auto) did not predict time to platelet engraftment (P=0.266) (Table 3). The relationship between MA6+CD34+ cells/kg and platelet engraftment holds when modeling the autologous transplant group (Medium: Q1–Q3, hazards ratio [HR]=1.99, P<0.05; High: Q3–Max, HR=4.46, P<0.001). For the allogeneic transplant group, there are only two patients in the high-risk group. For the medium-risk group, the relationship between MA6+CD34+ cells/kg and platelet recovery holds (Medium: Q1–Q3, HR=1.58, P=0.208), although it is not statistically significant. Data analysis revealed that 94% of transplant patients (31/33) received at least 294,408 CD34+MA6+/kg engrafted platelets within 21 days. However, if the CD34+MA6+ dose was 87,552–294,408/kg, only 72% (46/64) of patients achieved platelet engraftment within 21 days. If the dose was <87,552 CD34+MA6+/kg, the chance of platelet engraftment within 21 days was reduced to only 34% (11/32). The relationship between CD34+ cell dose and CD34+MA6+ cell dose is shown in Fig. 3D. Of note, there was a tendency to have fewer MA6+CD34+ cells/kg among the allo-transplant group (P<0.001). In addition, about half the patients in each transplant group were in the medium-risk group. We performed additional analyses of CD34+MA6+ cells/kg, comparing autologous and allogeneic transplant results. The autologous transplant group had higher CD34+MA6+ cell doses than the allo-transplant group, and the difference in median and means is statistically significant. However, the auto-transplant group also has a much higher standard deviation and range representing the greater variability seen in the autologous versus the allogeneic PBPC products (data not shown).
Table 1.
Summary Statistics (Cells/kg)
| n | Mean | Std Dev | Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|---|---|---|
| CD34+ (×106) | 99 | 5.46 | 2.76 | 1.97 | 4.07 | 4.72 | 5.85 | 18.29 |
| CD34+MA6+ | 129 | 356,057 | 594,582 | 2,326 | 87,552 | 164,776 | 294,408 | 4,577,468 |
FIG. 3.
Platelet engraftment as a function of PBPC CD34+ and CD34+MA6+ dose. (A) Time to platelet engraftment stratified by patient cohort is shown. Auto-MDACC (n=30) and auto-Australia (n=64) cohorts have a similar pattern of platelet engraftment (median time to engraftment of 11 days) [P=0.363; hazards ratio (HR) 1.23; 95% confidence interval (CI) 0.78–1.93]. In contrast, the allo-MDACC (n=35) cohort achieved platelet engraftment only after a median of 23 days. (B) Time to platelet engraftment stratified by CD34+ cell dose is shown. The higher the CD34+ cell dose, the greater the probability of engraftment. (C) Time to platelet engraftment stratified by CD34+MA6+ cell dose is shown. The higher the CD34+MA6+ cell dose, the greater the probability of engraftment. (D) Scatter plot of CD34+MA6+ (million cells/kg) versus CD34+ (cells/kg) with regression line (CD34+=4.06+5.75×CD34+MA6+). Pearson correlation=0.657, P<0.001. Spearman correlation=0.366, P<0.001.
Table 2.
Cox Regression
| Univariate analysis | |||||
|---|---|---|---|---|---|
| n | Median (days) | P-value | HR | 95% CI | |
| Cohort | |||||
| Allo | 35 | 23 | Ref | ||
| Auto | 94 | 11 | 0.005 | 1.75 | 1.18–2.60 |
| CD34+ | |||||
| Min–Q3 | 74 | 16.5 | Ref | ||
| Q3–Max | 25 | 9 | <0.001 | 2.65 | 1.65–4.25 |
| CD34+MA6+ | |||||
| Min–Q1 | 32 | 24 | Ref | ||
| Q1–Q3 | 64 | 12.5 | 0.005 | 1.88 | 1.21–2.92 |
| Q3–Max | 33 | 9 | <0.001 | 6.03 | 3.48–10.46 |
HR, hazards ratio.
Table 3.
Cox Regression
| Multivariate analysis | |||||
|---|---|---|---|---|---|
| n | Median (days) | P-value | HR | 95% CI | |
| Cohort | |||||
| Allo | 35 | 23 | Ref | ||
| Auto | 94 | 11 | 0.266 | 1.27 | 0.83–1.94 |
| CD34+MA6+ | |||||
| Min–Q1 | 32 | 24 | Ref | ||
| Q1–Q3 | 64 | 12.5 | 0.008 | 1.83 | 1.17–2.85 |
| Q3–Max | 33 | 9 | <0.001 | 5.40 | 3.03–9.64 |
CI, confidence interval.
CD34+MA6+ cells in PBPC and CB samples
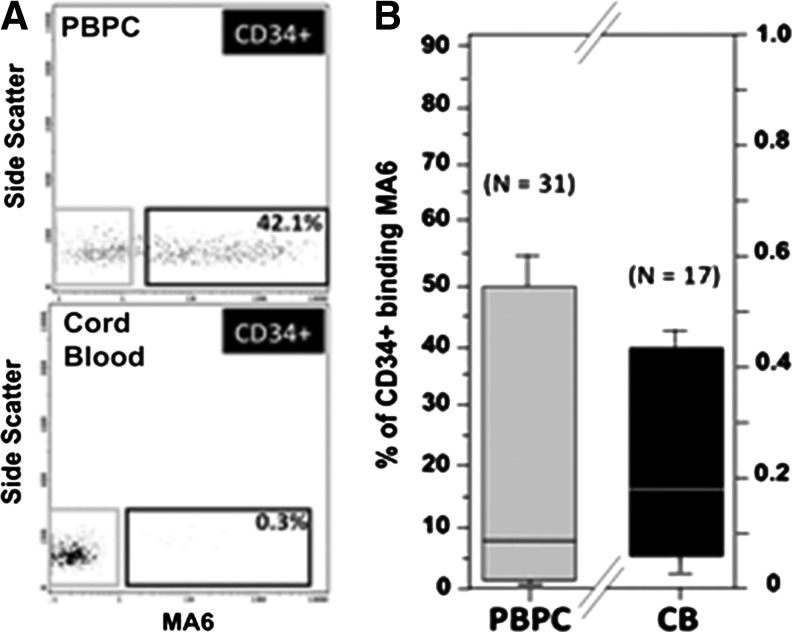
Compared with the median of ∼8% of PBPC CD34+ cells that coexpressed MA6, only 0.2% of the CB CD34+ cells coexpressed MA6 (Fig. 4A, B).
FIG. 4.
Comparison of the MA6 reactivity of PBPC and CB CD34+ cells. (A) Shows representative flow cytometric analyses of PBPC (n=31) and CB (n=17) CD34+ fraction cells. While ∼42% of PBPC CD34+ cells coexpressed MA6, only 0.3% of CB CD34+ cells coexpressed MA6. (B) Flow cytometric analyses of multiple samples revealed that 8% (range: ∼ <1%–50%, n=31) PBPC CD34+ cells coexpressed MA6. By comparison, only 0.2% (range: ∼0.1%–0.4%, n=17) CB CD34+ cells coexpressed MA6.
Expression of MA6 by CB CD34+ cells before and after ex vivo expansion
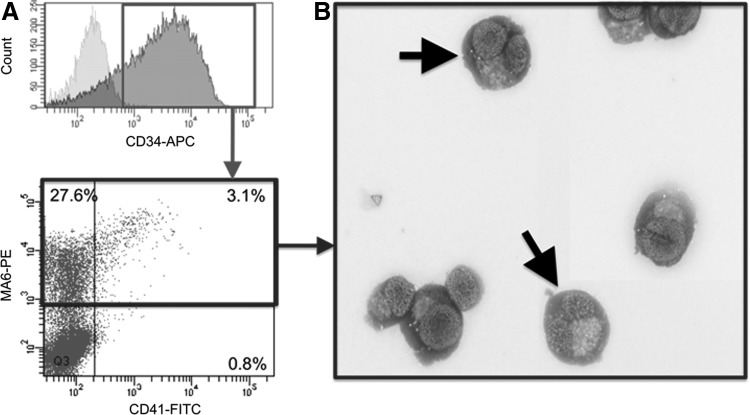
Flow cytometry revealed that following ex vivo culture, approximately one-third of CB CD34+ cells was MA6+ compared to only 0.2% in the unexpanded CB (Fig. 5A). Giemsa staining revealed that the CD34+MA6+ CB cells showed MK morphology (Fig. 5B). These data demonstrate that CD34+MA6+ progenitors can be generated from CB following ex vivo culture.
FIG. 5.
Expression of MA6+ by CB CD34+ cells is increased by ex vivo expansion. (A) A representative flow cytometric analysis (n=3) revealed that following ex vivo culture, approximately one-third of the CB CD34+ cells coexpressed MA6+. (B) Histologic staining (Giemsa) of cytospin preparations of ex vivo-generated CD34+MA6+ CB cells revealed a megakaryocyte-like morphology (arrows).
Discussion
Measures of a hematopoietic graft that are strongly predictive of platelet engraftment following hematopoietic transplantation currently remain to be identified. In this study, we demonstrate that an antibody produced by the MA6 hybridoma identified a primitive MK progenitor (CD34+MA6+). The MA6 antigen is expressed by CFU-MK, is rapidly lost upon further maturation, and not expressed by platelets. The identity of the cell surface moiety identified by MA6 is currently being sought in studies outside the scope of this article, but the utility of the CD34+MA6+ phenotype as a flow cytometric measure of MK-committed progenitors prompted us to examine whether a correlation between the number of CD34+MA6+ cells transplanted and platelet recovery could be identified in recipients of G-CSF-mobilized PBPC. A retrospective study demonstrated that although a correlation was identified between CD34+ dose transplanted and platelet engraftment, an even stronger correlation was identified between CD34+MA6+ dose transplanted and platelet engraftment suggesting that MA6 expression by CD34+ cells may present as a surrogate measure that is predictive of platelet engraftment. The authors acknowledge the large variability in the expression of MA6 by CD34+ cells in the different PBPC samples. Inherent variations in the adult donor PBPC and/or perhaps variable responsiveness of the MA6+ population to the G-CSF used for mobilization are hypothetical explanations. This finding was in contrast to the consistently low levels of MA6 expression by CD34+ cells present in CB samples. These data support the hypothesis that the delayed platelet engraftment observed in CB recipients (when compared to PBPC) may be, at least in part, a consequence of the low dose of CD34+MA6+ cells transplanted and potentially providing a target cell population for ex vivo expansion protocols. It is possible that since all of the PBPC products were mobilized with G-CSF, there was a differential effect on the frequency of CD34+MA6+ cells that were mobilized, which was patient or donor dependent. While our data suggest that the dose of CD34+MA6+ cells is positively correlated with platelet engraftment in a transplant setting, the role and contribution of more mature megakaryopoietic progenitors (present as CD34+MA6− cells) in platelet engraftment is unclear. The potentially more rapid, although short-lived, contribution to platelet production by these more mature, lineage-committed progenitors might be countered by their exposure (upon transplantation) to the rigors of the bloodstream and microvasculature environmental conditions to which they are not physiologically adapted.
Despite our findings that the CD34+MA6+ dose provides a better predictor of platelet engraftment than the CD34+ dose alone, we acknowledge that it is unlikely that it will replace an assessment of CD34+ cell dose in the clinical PBPC transplant setting. However, it is noteworthy that we detected that the CD34+MA6+ population is markedly rarer in CB products than in PBPC products. This observation may be consistent with the markedly delayed platelet engraftment observed after CB transplantation when compared to PBPC transplantation. However, as shown here, in preliminary experiments, ex vivo expansion of CB significantly increased the proportion of CB CD34+ cells expressing MA6. This may be responsible, at least in part, for the increased rate of platelet engraftment following transplantation of the ex vivo-expanded CB product. It is therefore conceivable that CD34+MA6+ cells could be targeted as a population for expansion during ex vivo CB expansion studies in the future. Such an approach may further speed platelet engraftment in CB recipients to levels that match those currently achieved by PBPC or BM recipients and thereby improve the overall efficacy of CB transplantation.
Acknowledgments
This research was supported, in part, by the National Cancer Institute (NCI) Grant RO1 R01CA61508-20, the Cancer Prevention & Research Institute of Texas (CPRIT) Grant RP100469, and the National Institutes of Health (NIH) MD Anderson's Cancer Center Support Grant CA016672. We also acknowledge Jason A. Williams and Kevin H. Cao for their invaluable support of the laboratory experiments described in this report and M. Norma Dominguez for her secretarial expertise.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, et al. (2002). Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment related mortality and survival. Blood 100:1611–1618 [DOI] [PubMed] [Google Scholar]
- 2.Glaspy JA, Shpall EJ, LeMaistre CF, Briddell RA, Menchaca DM, Turner SA, Lill M, Chap L, Jones R, et al. (1997). Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood 90:2939–2951 [PubMed] [Google Scholar]
- 3.Robinson S, Freedman A, Neuberg D, Nadler L. and Mauch P. (2000). Loss of marrow reserve from dose-intensified chemotherapy results in impaired hematopoietic reconstitution after autologous transplantation: CD34+, CD34+38- and week 6 CAFC assays predict poor engraftment. Exp Hematol 28:1325–1333 [DOI] [PubMed] [Google Scholar]
- 4.Kanamaru S, Kawano Y, Watanabe T, Nakagawa R, Suzuya H, Onishi T, Yamazaki J, Nakayama T, Kuroda Y, and Takaue Y. (2000). Low numbers of megakaryocyte progenitors in grafts of cord blood cells may result in delayed platelet recovery after cord blood cell transplant. Stem Cells 18:190–195 [DOI] [PubMed] [Google Scholar]
- 5.Page KM, Zhang L, Mendizabal A, Wease S, Carter S, Gentry T, Balber AE. and Kurtzberg J. (2011). Total colony forming units are a strong, independent predictor of neutrophil and platelet engraftment after unrelated umbilical cord blood transplantation: a single-center analysis of 435 cord blood transplants. Biol Blood Marrow Transplant 17:1362–1374 [DOI] [PubMed] [Google Scholar]
- 6.Migliaccio AR, Adamson JW, Stevens CE, Dobrila N, Carrier CM. and Rubinstein P. (2000). Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: graft progenitor cell content is a better predictor than nucleated cell quantity. Blood 96:2717–2722 [PubMed] [Google Scholar]
- 7.von Drygalski A, Xu G, Constantinescu D, Kashiwakura I, Farley T, Dobrila L, Rubinstein P. and Adamson JW. (2000). The frequency and proliferative potential of megakaryocytic colony forming cells (Meg-CFC) in cord blood, cytokine-mobilized peripheral blood and bone marrow, and their correlation with total CFC numbers: implications for the quantitation of Meg-CFC to predict platelet engraftment following cord blood transplantation. Bone Marrow Transplant 25:1029–1034 [DOI] [PubMed] [Google Scholar]
- 8.Sartor M, Garvin F, Antonenas V, Bradstock K. and Gottlieb D. (2007) Failure to achieve a threshold dose of CD34+ CD110+ progenitor cells in the graft predicts delayed platelet engraftment after autologous stem cell transplantation. Bone Marrow Transplant 40:851–857 [DOI] [PubMed] [Google Scholar]
- 9.Vinci G, Tabilio A, Deschamps JF, Van Haeke D, Henri A, Guichard J, Tetteroo P, Lansdorp PM, Hercend T, Vainchenker W, et al. (1984). Immunological study of in vitro maturation of human megakaryocytes. Br J Haematol 56:589–605 [DOI] [PubMed] [Google Scholar]
- 10.Chang Y, Bluteau D, Debili N. and Vainchenker W. (2007). From hematopoietic stem cells to platelets. J Thromb Haemost 5:318–327 [DOI] [PubMed] [Google Scholar]
- 11.Briddell RA, Brandt JE, Straneva JE, Srour EF. and Hoffman R. (1989). Characterization of the human burst-forming unit-megakaryocyte. Blood 74:145–151 [PubMed] [Google Scholar]
- 12.Vainchenker W, Bouguet J, Guichard J. and Breton-Gorius J. (1979). Megakaryocyte colony formation from human bone marrow precursors. Blood 54:940–945 [PubMed] [Google Scholar]
- 13.Mazur EM, Hoffman R, Chasis J, Marchesi S. and Bruno E. (1981). Immunofluorescent identification of human megakaryocyte colonies using an antiplatelet glycoprotein antiserum. Blood 57:277–286 [PubMed] [Google Scholar]
- 14.Schipper LF, Brand A, Reniers N, Melief CJ, Willemze R. and Fibbe WE. (2003). Differential maturation of megakaryocyte progenitor cells from cord blood and mobilized peripheral blood. Exp Hematol 31:324–330 [DOI] [PubMed] [Google Scholar]
- 15.Schipper LF, Brand A, Fibbe WE. and Van Hensbergen Y. (2012). Functional characterization of TPO-expanded CD34+ cord blood cells identifies CD34- CD61- cells as platelet producing cells early after transplantation in NOD/SCID mice and rCD34+ cells as CAFC colony-forming cells. Stem Cells 30:988–996 [DOI] [PubMed] [Google Scholar]
- 16.Horsfall MJ, Zannettino ACW, Nichol JL, Dyson P. and Simmons PJ. (1998). The monoclonal antibody MA6 identifies a cell surface antigen expressed on late bone marrow megakaryocytic progenitor cells. Exp Hematol 26:740 [Google Scholar]
- 17.Cox DR. (1972). Regression models and life tables (with discussion). J R Stat Soc Series B Stat Methodol 34:187–220 [Google Scholar]
- 18.Kaplan EL. and Meier P. (1958). Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 [Google Scholar]
- 19.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, Kaur I, Fu P, Del Angel M, et al. (2006). Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant 37:359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]