Abstract
Hematopoietic progenitor kinase 1 (HPK1) regulates stress responses, proliferation, and apoptosis in hematopoietic cells. In this study, we examined the expression, regulation, and functions of HPK1 in pancreatic ductal adenocarcinomas (PDA). We found that loss of HPK1 protein expression correlated significantly with the progression of pancreatic intraepithelial neoplasias (P = 0.001) and development of invasive PDA. Similarly, HPK1 protein was not expressed in any of eight PDA cell lines examined but was expressed in immortalized human pancreatic duct epithelial (HPDE) cells. There was no difference in HPK1 mRNA levels in PDA cell lines or primary PDA compared with those in HPDE cells or ductal epithelium in chronic pancreatitis and normal pancreas, respectively. Treatment of Panc-1 cells with a proteasome inhibitor, MG132, increased the HPK1 protein levels in a dose-dependent manner, suggesting that alteration in proteasome activity contributes to the loss of HPK1 protein expression in pancreatic cancer. Like the endogenous HPK1, both wild-type HPK1 and its kinase-dead mutant, HPK1-M46, overexpressed in Panc-1 cells, were also targeted by proteasome-mediated degradation. After MG132 withdrawal, wild-type HPK1 protein expression was markedly decreased within 24 hours, but kinase-dead HPK1 mutant protein expression was sustained for up to 96 hours. Therefore, HPK1 kinase activities were required for the loss of HPK1 protein in PDAs. Furthermore, restoring wild-type HPK1 protein in PDA cells led to the increase in p21 and p27 protein expression and cell cycle arrest. Thus, HPK1 may function as a novel tumor suppressor and its loss plays a critical role in pancreatic cancer.
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States (1). Despite the treatment modalities available for pancreatic cancer (e.g., chemoradiation, surgery, or a combination of these), it has the worst prognosis of all the major malignancies, with <5% of patients alive at 5 years after diagnosis (2). Therefore, new treatment modalities for pancreatic cancer are urgently needed. A promising approach is the targeted therapy that is directed toward aberrant molecular pathways in pancreatic cancer.
One possible avenue for such research is suggested by the fact that pancreatic ductal adenocarcinoma (PDA) develops from histologically well-defined precursor lesions, called pancreatic intraepithelial neoplasia (PanIN). With the progression of PanIN lesions to invasive PDA, there is a gradual accumulation of molecular abnormalities that are commonly seen in invasive PDA, including K-ras activation by point mutation (3) and inactivation of the tumor suppressor genes p16 (4), SMAD4/DPC4 (5), and p53 (6). Increased expression of the epidermal growth factor (EGF) family of mitogenic peptides, including EGF and transforming growth factor-α, cripto, amphiregulin, and β-cellulin, is a characteristic feature of PDA, along with elevated expression of their corresponding EGF receptor (EGFR)–related tyrosine kinase receptor subtypes, including EGFR and c-erb B/HER-2 (7). More recently, the mammalian Ste20-like serine/threonine kinase family has been shown to be involved in human malignancies (8–11). One member of this family, hematopoietic progenitor kinase 1 (HPK1; also named MAP4K1), is a downstream target of tumorigenesis suppressor Pdcd4 and plays a role in colon carcinoma cell invasion (12). Many studies have identified the role of HPK1 in T-cell receptor (TCR) signaling and a variety of other signaling pathways, including EGF (13, 14), transforming growth factor-β (15, 16), protein kinase D (17), prostaglandin E2 (18), and erythropoietin (19), in hematopoietic cells. Knockout studies have shown that HPK1 is a negative regulator of TCR signaling and T-cell–mediated immune responses (20). HPK1 functions as a MAP4K in that it activates MAP3Ks, such as MLK3 and MEKK1, which signal through the well-established MKK4/MKK7, c-Jun NH2-terminal kinase (JNK), or stress-activated protein kinase cascades (21, 22). A dominant-negative HPK1 construct that has a methionine substituted for Lys46 (HPK1-M46), which abrogates ATP binding, fails to activate JNK (21, 23). During apoptosis, HPK1 is cleaved at the DDVD motif by caspase-3 into the NH2-terminal kinase domain and the COOH-terminal region (24, 25). The NH2-terminal kinase domain of HPK1 retains the ability to activate JNK, whereas the COOH-terminal region can inhibit nuclear factor-κB (NF-κB) activation (24, 25). Thus, HPK1 promotes apoptosis by activating JNK, increasing the surface expression of FasL, and inhibiting NFκB activation. HPK1 activation also involves tyrosine phosphorylation and the formation of inducible complexes with several adaptor proteins, including Gads, Nck, and Crk (23, 26). It has been postulated that, through these adaptor proteins, HPK1 is connected to membrane tyrosine kinase receptors, which leads to JNK activation and plays a critical role in many important cellular processes. Although phosphorylation and caspase-3– mediated cleavage have been implicated in HPK1 regulation, the details of the in vivo regulation of HPK1 remain largely unknown.
Many studies have identified the role of HPK1 in stress response, proliferation, and apoptosis in hematopoietic cells; the function of HPK1 in human malignancies has, however, not been examined. In this study, we examined HPK1 protein expression in PanIN lesions of different histologic grades, human PDA samples, and their paired benign pancreatic tissue. We also examined the molecular mechanisms that regulate HPK1 expression in PDAs. Furthermore, we examined HPK1 functions in cell cycle arrest and its downstream targets in PDAs. Our data showed for the first time that HPK1 may function as a tumor suppressor, and its loss may play a critical role in the tumorigenesis and/or progression of pancreatic cancer.
Materials and Methods
Immunohistochemistry for HPK1 protein expression in human PDAs and PanIN lesions
Immunohistochemical staining for HPK1 was performed on 8 human PDA cell lines and on 79 human PDA samples and their matched benign pancreatic tissues using a PDA tissue microarray, which was constructed using formalin-fixed, paraffin-embedded archival tissue blocks as described previously (27). For each patient, two tumor cores and two cores of benign pancreatic tissue were sampled from the representative areas using a 1.0-mm punch. Immunohistochemistry was also performed on 18 PanIN lesions of different histologic grades using whole sections from the pancreatic tissue blocks. The Institutional Review Board of The University of Texas M. D. Anderson Cancer Center approved the use of human tissue for this study. The expression levels of HPK1 protein were evaluated using an indirect immunoperoxidase method (Vectastain ABC Elite standard kit, Vector Laboratories) according to the manufacturer’s protocol. Briefly, antigen retrieval was performed using a steamer for 35 min. Anti-HPK1 (N-19; Santa Cruz Biotechnology, Inc.) was used at a 1:500 dilution at 4°C overnight, secondary antibody at room temperature for 60 min, and counterstained with Mayer’s hematoxylin. Tumors or benign pancreatic ductal tissue with no or only weak focal cytoplasmic staining for HPK1 (<5% of the neoplastic cells) was regarded as negative. Tumors or benign pancreatic ductal tissue with strong cytoplasmic staining for HPK1 (z5% of the neoplastic cells) was regarded as positive.
RNA isolation and reverse transcription-PCR
Frozen OCT-embedded human samples of primary PDA, chronic pancreatitis, and normal pancreas were microdissected and total RNA isolation was performed as described previously (28). Total RNA was isolated from pancreatic cancer cell lines or stable HPK1/Panc-1 cells using TRI Reagent (Molecular Research Center, Inc.). One microgram of total RNA was reverse transcribed using the avian myeloblastosis virus reverse transcriptase kit (Promega) according to the manufacturer’s protocol. Briefly, total RNA was denatured for 5 min at 70°C and cooled for 5 min on ice, reverse transcriptase was added to a total volume of 20 µL, and reverse transcription was conducted for 60 min at 42°C. For standard PCR, 1.0 µL of the reverse transcription products was amplified using a HotStart AmpliTaq Gold polymerase (Applied Biosystems). The primer sequences for HPK1 and β-actin and the PCR conditions were the same as described previously (21). The PCR products were separated on 1.5% agarose gels.
Quantitative reverse transcription-PCR (RT-PCR) was performed using a second set of primers specific for HPK1 (QuantiTect Primer Assays, Qiagen, Inc.). To correct for quantitative differences between samples and possible PCR artifacts, we used primers specific for RPS6 (forward, 5′-AAGGA-GAGAAGGATATTCCTGGAC-3′; reverse, 5′-AGAGAGATTGAAAAGTTTGCG-GAT-3′) as internal controls in each sample. Amplification was performed using a thermal cycler (Bio-Rad Laboratories, Inc.) for 40 cycles consisting of 20 s at 95°C (denaturation), 1 min at 60°C (annealing), and 1 min at 60°C (extension). Each quantification PCR was performed in triplicate, and the mean values were used to calculate the ratios of HPK1 to RPS6, with a value of 1 used as the control. All assays were repeated at least thrice.
Cell treatment, immunoblotting, and antibodies
Cells were treated with MG132 (Calbiochem) for the indicated times and at the indicated doses. Protein expression was analyzed by 10% SDS-PAGE, which was electroblotted onto polyvinylidene difluoride membranes (Novex), blocked in 5% skim milk in 1× TBS, and probed with the following primary antibodies: HPK1, p21, p27, and actin antibody (all from Santa Cruz Biotechnology). Proteins were detected using an enhanced chemiluminescence kit (Amersham-Pharmacia Biotech).
Cell culture and stable transfection
The human pancreatic cancer cell lines Panc-1, BxPC-3, AsPC-1, and Capan-1 were purchased from the American Type Culture Collection. The Panc-48, Panc-02, CFPAC-1, Panc-3, Panc-28, and Capan-2 pancreatic cancer cells were generously provided by Dr. Paul Chiao (The University of Texas M. D. Anderson Cancer Center). All cell lines were maintained either in DMEM or in RPMI 1640 supplemented with 10% fetal bovine serum in a humidified incubator containing 5% CO2 at 37°C. The human pancreatic duct epithelial (HPDE) cell line was provided as a generous gift from Dr. Ming-Sound Tsao (Ontario Cancer Institute, Toronto, Ontario, Canada).
To establish HPK1 stable cell lines, we transfected the Panc-1, Panc-28, or AsPC-1 cells with pCIneo-Flag–tagged vectors of wild-type HPK1 and its kinase-dead mutant HPK1-M46 using FuGENE6 Transfection Reagent (Roche Diagnostics Corp.). The transfected cells were subsequently selected in the presence of G418 (500 µg/mL) to establish the stable clones. The stable clones expressing either wild-type HPK1 or mutant HPK1-M46 constructs were screened by RT-PCR.
Immunocomplex kinase assays
Immunocomplex kinase assays were performed as described previously (21). Overexpressed HPK1 was precipitated by incubation with the anti-Flag M2 antibody (Sigma-Aldrich) and protein A/G-agarose beads (Santa Cruz Biotechnology) in incubation buffer [20 mmol/L HEPES (pH 7.4), 2 mmol/L EGTA, 50 mmol/L glycerophosphate, 1% Triton X-100, 10% glycerol, 1 mmol/L DTT, 2 µg/mL leupeptin, 5 µg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3VO4]. The precipitates were washed twice with incubation buffer, twice with LiCl buffer [500 mmol/L LiCl, 100 mmol/L Tris-Cl (pH 7.6), 0.1% Triton X-100], and twice with kinase buffer [20 mmol/L MOPS (pH 7.6), 2 mmol/L EGTA, 10 mmol/L MgCl2, 1 mmol/L DTT, 0.1% Triton X-100, 1 mmol/L Na3VO4]. They were then mixed with 5 µg of myelin basic protein (MBP) substrates, 15 µmol/L unlabeled ATP, and 10 µCi [γ-32P]ATP in 30 µL of kinase buffer. The kinase reaction was performed at 30°C for 30 min and terminated with an equal volume of SDS sampling buffer. The reaction mixtures were resolved by SDS-PAGE analysis.
Cell growth studies and fluorescence-activated cell sorting analysis
Cell growth was analyzed using the tetrazolium-based 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay referred to as CellTiter 96 (Promega) according to the manufacturer’s instructions. Briefly, cells were either untreated or treated with 0.5 µmol/L MG132 for 24, 48, and 72 h, and cell numbers were estimated using the MTS assay, with MTS added to the wells 1 h before the photometric reading. Standard propidium iodide staining by the hypotonic lysis method was used for the cell cycle studies. Panc-1, vector control, and HPK1 stable cells were seeded into 100-mm dishes and either treated with 0.5 µmol/L MG132 or left untreated. After 24 and 48 h, cells were collected by trypsinization, washed once with cold PBS, mixed with 500 µL of hypotonic solution (0.1% sodium citrate, 0.1% Triton X-100, 100 µg/mL RNase, and 50 µg/mL propidium iodide), and analyzed by flow cytometry 30 min later. Fluorescence-activated cell sorting (FACS) analysis and cell counting were done in parallel and yielded results similar to those of the MTS assay. FACS analysis and MTS assays were repeated thrice.
Statistical analysis
Statistical analysis was performed using Fisher’s exact test and unpaired t test with a P value of <0.05 was considered statistically significant.
Results
Loss of HPK1 expression is associated with the progression of PanIN lesions and invasive PDAs
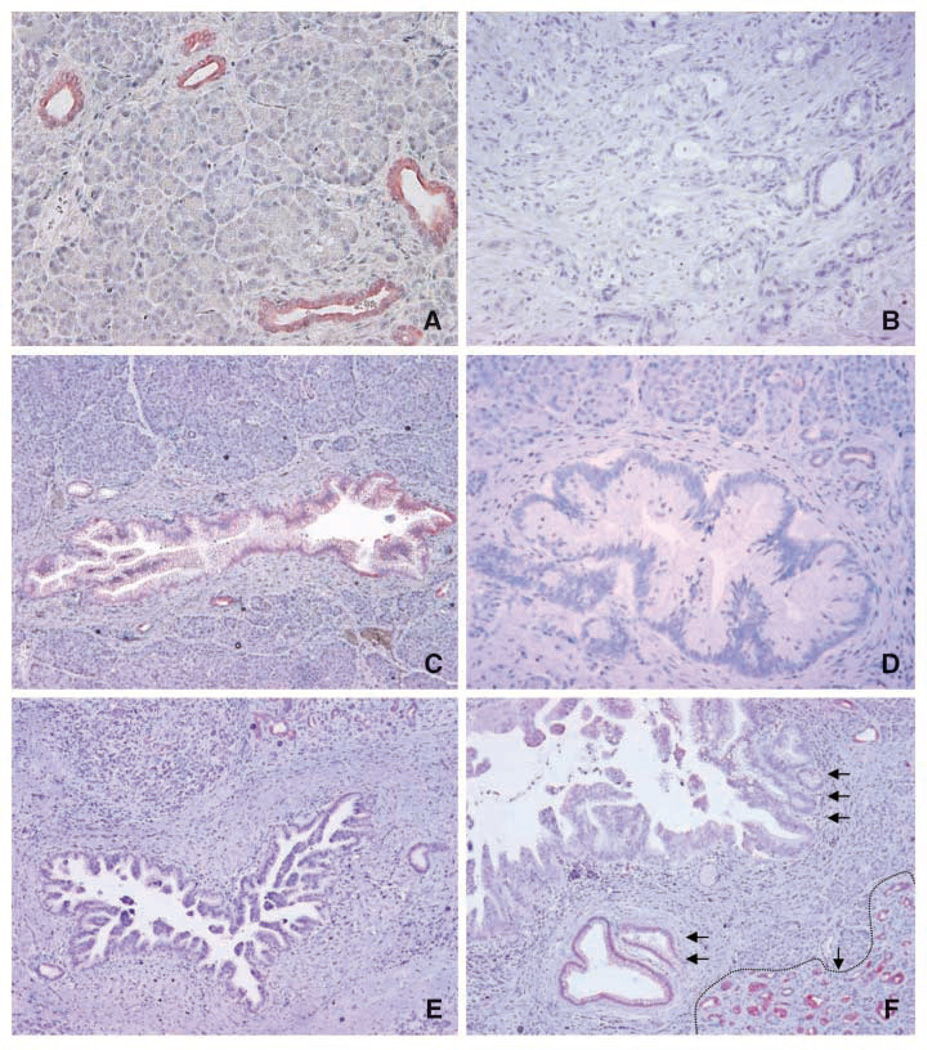
To evaluate HPK1 protein expression in PDAs and benign pancreatic tissue, we performed immunohistochemistry on 79 human PDA samples and paired samples of benign pancreatic tissues. Representative micrographs are shown in Fig. 1. In benign pancreatic tissue, HPK1 was expressed only in the ductal epithelium. Pancreatic acinar cells or islet (endocrine) cells showed either negative or only focal weak staining for HPK1 (Fig. 1A). High levels of HPK1 protein were detected in the pancreatic ducts of all 79 benign pancreatic tissue samples. In contrast, only 3 of 79 (4%) PDA samples showed positive staining for HPK1 and 76 PDA samples (96%) were negative for HPK1 protein (P < 0.0001; Fig. 1B). Furthermore, the eight PDA cell lines that were included in our tissue microarray also showed negative staining for HPK1 protein by immunohistochemistry (data not shown).
Figure 1.
Representative micrographs showing HPK1 expression in benign pancreatic tissue, invasive PDA, and PanIN lesions of different grades. A, benign pancreatic ducts that were strongly positive for HPK1, but no staining present in pancreatic acinar cells. B, invasive PDA with negative staining for HPK1. C, PanIN1 lesion with weak positive staining for HPK1. D and E, PanIN2 and PanIN3 lesions with negative staining for HPK1, respectively. F, progressive loss of HPK1 with the progression of PanIN lesions: strong positive staining for HPK1 in benign pancreatic ducts (single arrow with dotted line at the right lower corner), weak positive staining for HPK1 in PanIN1 lesions (double arrows), and negative staining in high-grade PanIN3 lesions (triple arrows).The strong positive staining for HPK1 in the adjacent benign pancreatic ducts served as internal positive control in C to E.Original magnification, ×100.
To further examine the role of HPK1 in the development of PDAs, we examined HPK1 expression in 18 PanIN lesions of different histologic grades. We found that HPK1 was expressed in six of six (100%) of the PanIN1 lesions, in two of five (40%) of the PanIN2 lesions, but in none of the seven PanIN3 lesions (P < 0.001; Fig. 1C–F). Therefore, the loss of HPK1 expression correlates with the progression of PanIN lesions and the development of invasive PDAs.
Analysis of HPK1 protein and HPK1 mRNA expression in pancreatic cancer cell lines and primary tumors
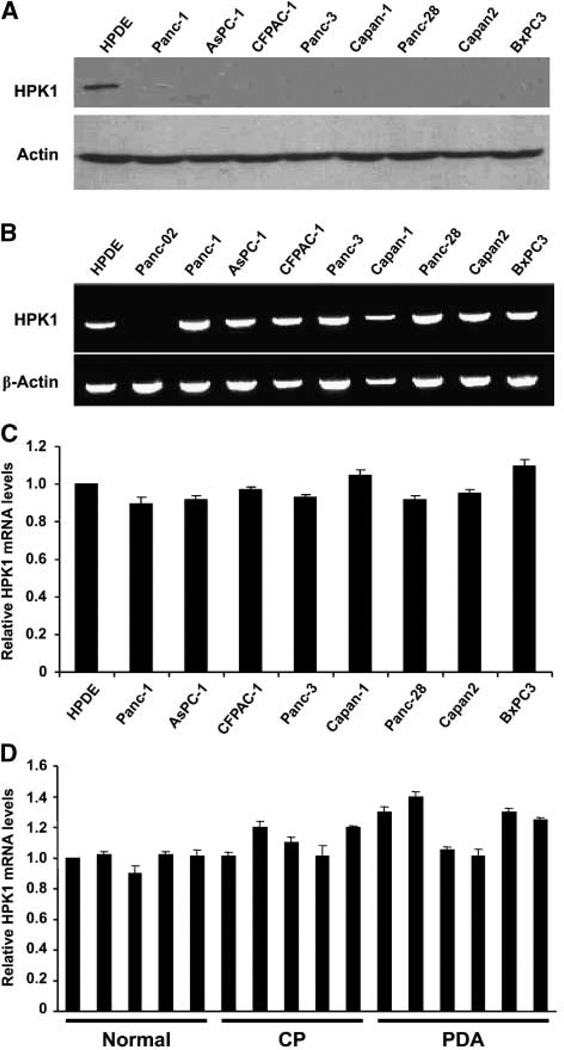
To confirm our immunohistochemical findings, we used immunoblotting to evaluate HPK1 protein expression in a panel of eight human PDA cell lines and the HPDE cells, an immortalized pancreatic ductal cell line. The Western blot results were shown in Fig. 2A. Consistent with our immunohistochemical findings, we found HPK1 protein to be expressed only in the HPDE cells, but not in any of the eight human PDA cell lines examined.
Figure 2.
HPK1 expression in HPDE cells and human PDA cell lines. A, 150 µg of cell lysate from each cell line were resolved by 10% SDS-PAGE and immunoblotted with an anti-HPK1 antibody or anti-actin as loading control. B, RT-PCR results showing HPK1 mRNA expression in the HPDE cells and eight different human PDA cell lines.Panc-02, a mouse PDA cell line, was used as the specificity control. C, QRT-PCR results showing HPK1 mRNA expression levels in eight PDA cell lines relative to those in the HPDE cells. D, QRT-PCR results showing HPK1 mRNA expression levels in the ductal epithelial cells in normal pancreas (Normal), chronic pancreatitis (CP), and PDA.
To understand the loss of HPK1 protein expression in PDAs, we measured HPK1 mRNA levels in eight human PDA cell lines and the HPDE cells by RT-PCR and quantitative RT-PCR (QRT-PCR) using two sets of primers targeting the different regions of the HPK1 sequences. RT-PCR results for HPK1 mRNA levels in HPDE cells and eight human PDA cell lines are shown in Fig. 2B. Panc-02, a mouse PDA cell line, served as specificity control for human HPK1 primers. QRT-PCR results for HPK1 mRNA levels in HPDE cells and these eight human PDA cell lines are shown in Fig. 2C. We observed no significant difference in HPK1 mRNA levels between the eight human PDA cell lines and the HPDE cells using either RTPCR or QRT-PCR. In addition, we also examined HPK1 mRNA expression levels in the microdissected ductal epithelial cells from six cases of PDA, five cases of chronic pancreatitis, and five samples of normal pancreas. No significant difference in HPK1 mRNA levels was observed among primary PDA, chronic pancreatitis, and normal pancreas (Fig. 2D). These findings indicate that HPK1 is down-regulated at the protein level, not at the mRNA level.
HPK1 is down-regulated through a proteasome-dependent pathway in pancreatic cancer
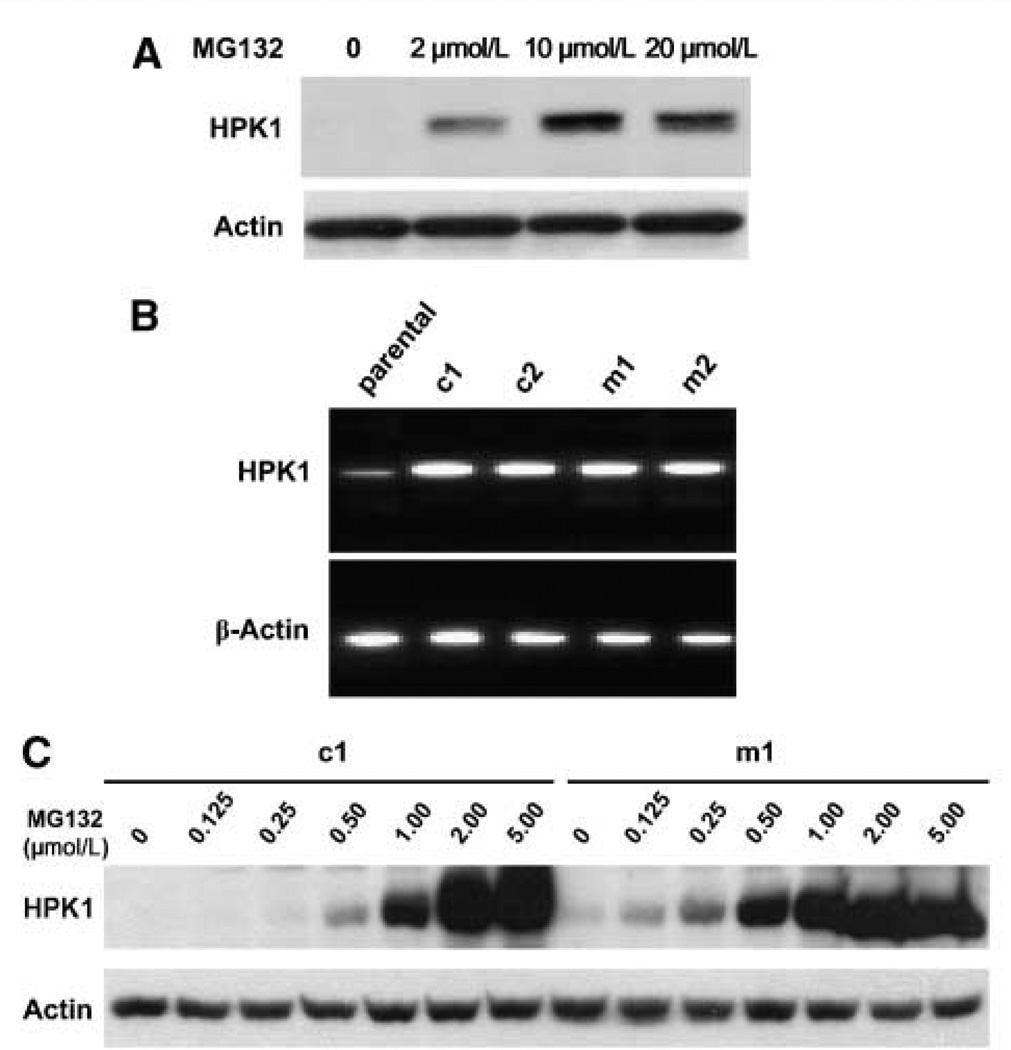
Using HEK293Tcells and cotransfection assays, we previously showed that HPK1 protein is ubiquitinated and targeted by proteasome-mediated degradation (29). Therefore, we examined whether treatment with a proteasome inhibitor, MG132, would restore HPK1 protein expression in PDA cell lines. We found that MG132 enhanced HPK1 protein expression in a dose-dependent manner in Panc-1 cells (Fig. 3A), indicating that HPK1 is subject to ubiquitin-targeted degradation in PDA cells. Similar results were obtained in Panc-1 cells treated with another proteasome inhibitor, PS-341, and in BxPC3 cells treated with either MG132 or PS-341 (data not shown). Therefore, alterations in proteasome activities may lead to the loss of HPK1 protein expression in pancreatic cancer.
Figure 3.
HPK1 proteins were targeted by proteasome-mediated degradation in PDA cell lines. A, Western blots showing that MG132 treatment enhanced HPK1 protein expression in a dose-dependent manner in Panc-1 cells.Panc-1 cells were treated with 2.0 to 20.0 µmol/L of MG132 for 16 h. B, RT-PCR results showing HPK1 mRNA expression in Panc-1 parental cells, wild-type HPK1/ Panc-1 stable clones (c1 and c2), and kinase-dead mutant HPK1/Panc-1 stable clones (m1 and m2). C, Western blots showing that MG132 treatment (0.125–5.0 µmol/L for 16 h) enhanced HPK1 protein levels in a dose-dependent manner in c1 and m1 stable cells. Actin was used as the loading control.
HPK1 kinase activity is required for its degradation
To examine the role of HPK1 in pancreatic cancer, we established the stable cell lines overexpressing wild-type HPK1 or kinase-dead mutant HPK1-M46 using Panc-1 cells. The clones that had significantly higher levels of HPK1 mRNA than the parental control cells were selected as stable clones (Fig. 3B). The clones that had HPK1 mRNA levels similar to the parental control cells were used as vector control. We then verified HPK1 protein expression by Western blotting using an anti-HPK1 antibody. HPK1 protein expression was barely detectable in the HPK1/Panc-1 stable clone. Similarly, mutant HPK1-M46 protein expression was also very low in the HPK1-M46/Panc-1 stable clone (Fig. 3C). Both wild-type and mutant HPK1-M46 protein expression were markedly increased in a dose-dependent manner by MG132 treatment (Fig. 3C). These data indicate that, like the endogenous HPK1 protein, the overexpressed wild-type and mutant HPK1-M46 proteins are also targeted by proteasome degradation in PDAs.
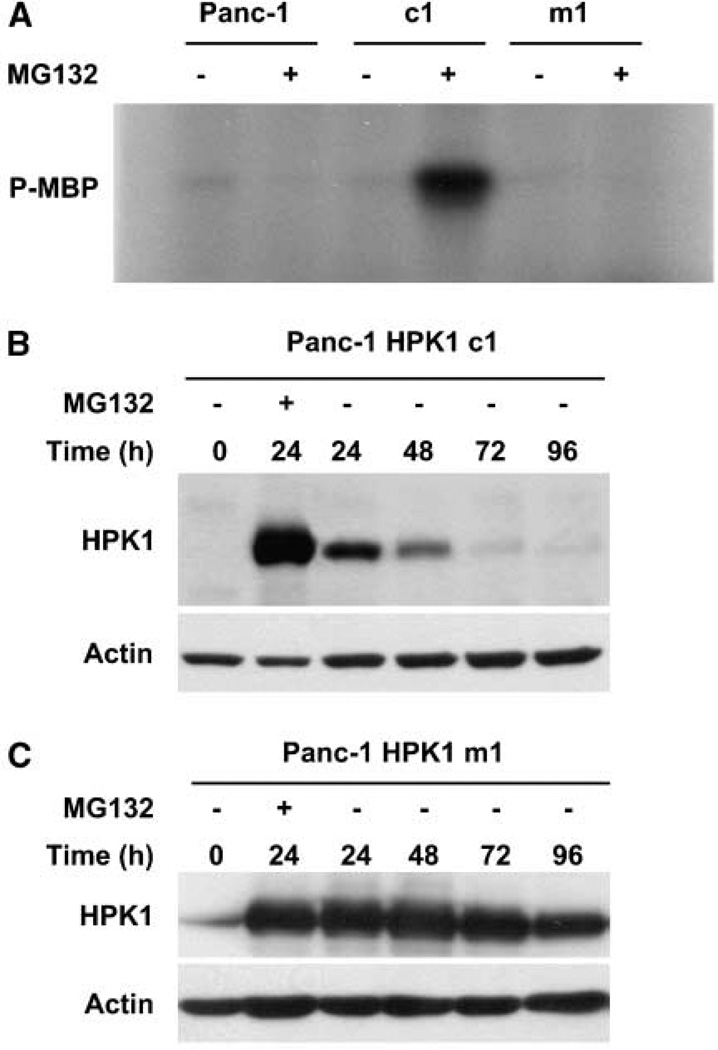
A notable feature of mammalian Ste20-like kinases, including HPK1, is that the overexpressed kinases are somehow activated, at least partially, in the absence of external stimuli. To verify whether overexpressed HPK1 is activated in the absence of external stimuli, we performed immunocomplex kinase assays using stable clones treated with MG132. We found that clones overexpressing wild-type Flag-HPK1 (c1) showed strong kinase activity toward MBP (Fig. 4A). However, the kinase activities were not detectable in parental Panc-1 cells or in the stable clones overexpressing kinase-dead mutant HPK1-M46 (m1, Fig. 4A). To examine whether HPK1 kinase activities affected the stability of HPK1 proteins in PDA cells, we treated Panc-1 stable clones c1 or m1 with 2.0 µmol/L MG132 for 24 hours and then measured HPK1 protein expression by Western blotting at 0 (24 hours after treatment), 24, 48, 72, and 96 hours after withdrawal of MG132. Wild-type HPK1 protein expression in c1 stable cells was markedly reduced during the first 24 hours and was barely detectable at 72 or 96 hours after MG132 withdrawal (Fig. 4B). In contrast, the kinase-dead mutant HPK1- M46 protein expression in m1 stable cells was sustained even at 96 hours after MG132 withdrawal (Fig. 4C), indicating that HPK1 kinase activity is required for its degradation.
Figure 4.
HPK1 kinase activity is required for its proteasome-mediated degradation. A, after MG132 treatment, HPK1 kinase activity was present only in wild-type HPK1/Panc-1 stable clones (c1), not in kinase-dead mutant HPK1/ Panc-1 stable clones (m1) or in parental Panc-1 cells.Panc-1 cells and stable clones, c1 and m1, were either treated or untreated with 0.5 µmol/L MG132 for 24 h, and cell lysates were immunoprecipitated with an anti-Flag M2 antibody. Immunocomplex kinase assays were performed using the MBP as a substrate. B and C, time courses of the wild-type HPK1 protein and the kinase-dead mutant HPK1 protein after MG132 withdrawal. Wild-type HPK1 and kinase-dead mutant HPK1 proteins were monitored by Western blotting. Stable clones, c1 and m1, were either untreated (control) or treated with 2.0 µmol/L MG132 for 24 h. Cells were then washed and maintained in fresh medium without MG132 and harvested at 0, 24, 48, 72, and 96 h after MG132 withdrawal.
HPK1 inhibits cell proliferation and increases the expression of cyclin-dependent kinase inhibitors p21 and p27
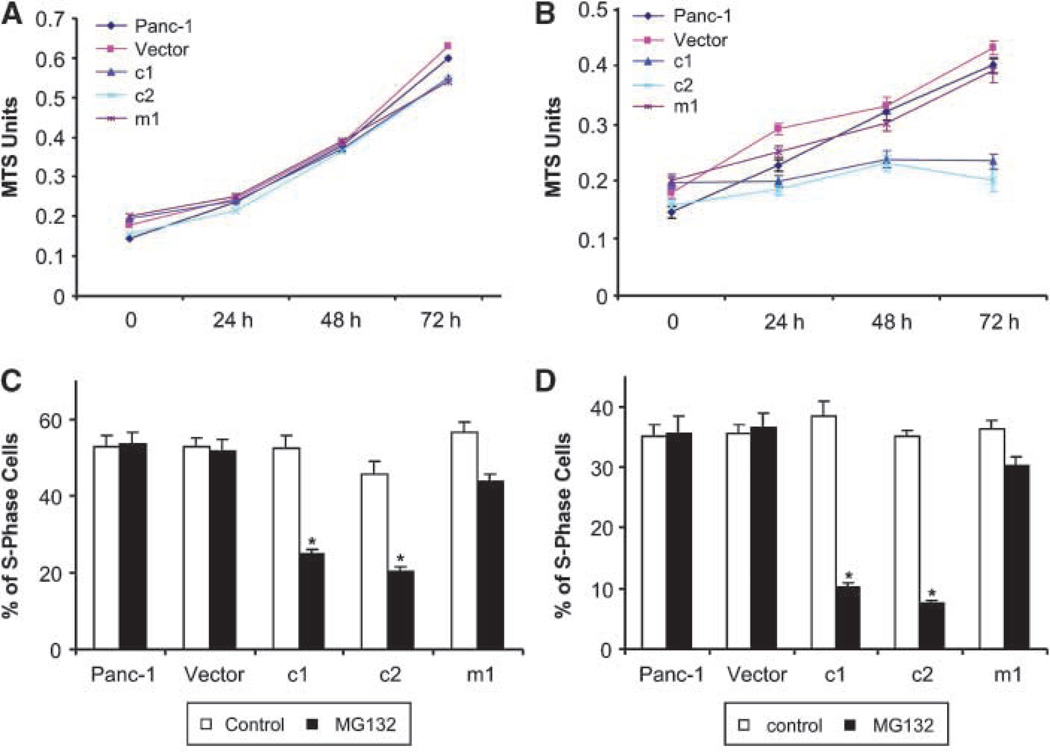
To investigate the potential role of HPK1 proteins in pancreatic cancer cell proliferation, we compared cell growth rates by performing MTS assays. Under normal culture conditions without MG132 treatment, HPK1 was not expressed or activated in any cell lines. All PDA cell lines had similar growth rates (Fig. 5A). In contrast, when the cell lines were treated with 0.5 µmol/L MG132, which stabilized and activated wild-type HPK1 proteins in c1 and c2, c1 and c2 had significantly lower proliferation rates than the parental Panc-1 cells, vector control cells, and m1 cells (Fig. 5B), suggesting that HPK1 kinase activity was required for HPK1-mediated cell growth inhibition. We further confirmed these findings by FACS analysis (Fig. 5C and D). After 24-hour treatment of 0.5 µmol/L MG132, the percentages of S-phase cells were 25.0% and 20.5% for the c1 and c2 stable cells, respectively, which were significantly lower than those for the Panc-1 parental cells (53.4%), vector control cells (51.5%), and m1 cells (43.5%; Fig. 5C). Similar results were observed after 48 hours of MG132 treatment (Fig. 5D). Consistent with the MTS assays, we found no significant difference in the percentage of S-phase cells among the untreated c1, c2, Panc-1 parental, vector control, and m1 cells (Fig. 5C and D). These data suggest that HPK1 played a role in inhibiting the growth of pancreatic cancer cells.
Figure 5.
Wild-type HPK1 protein expression and activation inhibits pancreatic cancer cell proliferation. A and B, Panc-1 parental, vector control, c1, c2, and m1 cells were plated at an equal density and cultured under normal conditions without MG132 treatment (A) or treated with 0.5 µmol/L MG132 (B) for the indicated time periods. Cell numbers were estimated using the MTS assays. C and D, percentages of S-phase cells in Panc-1 parental, vector control, c1, c2, and m1 cells either untreated or treated with 0.5 µmol/L MG132 for 24 h (C) or 48 h (D). Cells were plated at an equal density, and the percentage of S-phase cells was analyzed by FACS analysis. Columns, mean from three experiments.*, P < 0.05.
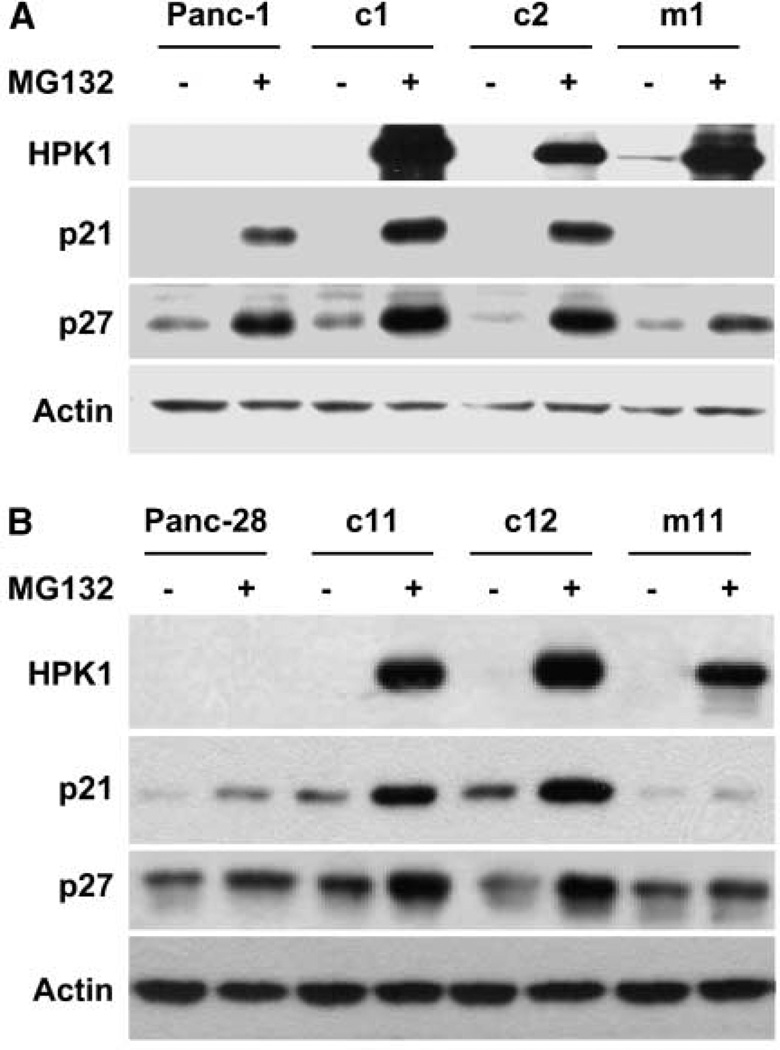
Loss of cell cycle control is the key feature in tumor cell growth. p21 and p27 are two important cyclin-dependent kinase (CDK) inhibitors and negative regulators of the G1-to-S phase transition. While investigating the negative regulatory role of HPK1 in cell cycle distribution, we found that HPK1 overexpression/activation led to increased protein expression of both p21 and p27 in c1 and c2 clones compared with the parental Panc-1 cells after treatment with 0.5 µmol/L MG132 for 48 hours (Fig. 6A). However, no increase in either p21 or p27 protein levels was detected in kinase-dead mutant m1 cells after MG132 treatment compared with the treated parental Panc-1 cells (Fig. 6A). Similar results were obtained using Panc-28/wild-type HPK1 stable clones (c11 and c12) and Panc-28/kinase-dead mutant stable cells (m11) after MG132 treatment (Fig. 6B) and AsPC-1 cells (data not shown). These findings indicate that HPK1 contributes to the increase of p21 and p27 through its kinase activity, which provides direct evidence of a functional relationship between HPK1 and cell growth inhibition through p21 and p27 stabilization or induction.
Figure 6.
HPK1-mediated cell cycle arrest was associated with p21 and p27 protein stabilization in Panc-1 (A) and Panc-28 cells (B).Cells were either untreated or treated with 0.5 µmol/L MG132 for 48 h; cell extracts were subjected to immunoblotting for HPK1, p21, and p27 expression. Actin was used as the loading control.
Discussion
Considerable evidence has suggested that HPK1 plays a critical role in the regulation of the JNK pathway in hematopoietic cells (21). It has been shown that the JNK pathway is activated in pancreatic cancer cells by tumor necrosis factor-α and EGF (30–32). However, the JNK cascade has not been systematically studied in pancreatic cancer cells. Little is known about the upstream regulator of JNK pathway in pancreatic cancer. In this study, we first examined HPK1 protein expression in 79 human PDA samples and their paired benign pancreatic tissue, 18 PanIN lesions of different histologic grades, 8 PDA cell lines, and HPDE cells. We found that loss of HPK1 protein expression was strongly associated with the progression of PanIN lesions and the development of invasive PDAs. Although there was no detectable HPK1 protein expression in any of the eight PDA cell lines examined, the HPK1 mRNA levels in these cell lines were similar to those in the HPDE cells, which had HPK1 protein expression. Proteasome inhibitor treatment increased HPK1 protein expression in a dose-dependent manner in the Panc-1 cells. Thus, our data suggest that alterations in proteasome activity contribute to the loss of HPK1 protein expression in PDAs. Using the stable cell lines, we found that the kinase-dead mutant HPK1 protein was much more stable than the wild-type HPK1 protein after MG132 withdrawal, suggesting that HPK1 kinase activity was required for its degradation. Furthermore, we found that wild-type HPK1 protein overexpression and activation in pancreatic cell lines led to increase of p21 and p27 protein expression and inhibition of G1-to-S phase progression compared with parental cells or kinase-dead stable cells, indicating that HPK1 kinase activity contributes to the stability of p21 and p27 and cell growth arrest. Thus, HPK1 may function as a tumor suppressor in PDAs, and its loss may play a critical role in the tumorigenesis of pancreatic cancer.
Although previous studies have shown that HPK1 is predominantly expressed in the hematopoietic cells, our data showed that HPK1 is expressed at high levels in benign pancreatic ducts but not in the acinar cells or islet cells in benign pancreatic tissue. What is more important is that there was a progressive loss of HPK1 protein with the progression of PanIN lesions from low-grade PanIN to high-grade PanIN3 and invasive PDAs. Our data showed for the first time that the loss of HPK1 in pancreatic cancer was mainly due to ubiquitin proteasome-mediated degradation, not due to the down-regulation of HPK1 mRNA. The proteasome-mediated degradation of signaling molecules of the mitogen-activated protein kinase pathways has been reported previously (33). In addition, other members of the Ste20 family, germinal center kinase (GCK) and GCK-related kinase, are regulated by proteasomal degradation, which requires kinase activity (34). The specificity of ubiquitination is typically governed by the E3 enzymes. It would be interesting to determine which E3 ligase is responsible for HPK1 degradation in pancreatic cancer. Several activated receptor and cytoplasmic tyrosine kinases, including the EGFR, have been shown to stimulate the tyrosine phosphorylation of the HPK1 serine/threonine kinase (13). Because HPK1 kinase activity may be responsible for its degradation in pancreatic cancer, it will be interesting to investigate whether the EGF pathway, which plays a critical role in pancreatic cancer tumorigenesis (35, 36), activates HPK1 and contributes to its degradation in pancreatic cancer.
HPK1 has been shown to play an important role in stress response, proliferation, and apoptosis in hematopoietic cells through NF-κB, JNK, and extracellular signal-regulated kinase (ERK) 2 (20, 21, 24). However, the role of HPK1 in human malignancies has not been reported. In this study, we showed for the first time that restoration of wild-type HPK1 protein expression and its kinase activities in pancreatic cell lines increased the expression of the two CDK inhibitors, p21 and p27, which have been shown to mediate antiproliferative response in many types of human tumors, including pancreatic cancer (37–39). By showing that HPK1 induced p21 and p27 in pancreatic cancer and led to cell cycle arrest, we provided further evidence that HPK1 functions as a tumor suppressor gene in PDAs. However, the mechanism by which HPK1 induces p21 and p27 protein expression in pancreatic cell lines is not currently known and will be the subject of future investigation. It has been reported that JNK1 is dynamically associated with p21 and increases its stability by inducing phosphorylation at Ser130, which inhibits the ubiquitination of p21 (40). It is possible that HPK1 activates JNK and stabilizes p21 through JNK1 activation. Several studies have suggested that the Ras-ERK1/2 signaling pathway is involved in the mitogen-induced down-regulation of p27 (41, 42). Because HPK1 has been reported to be a negative regulator of ERK2 (20), it may regulate p27 levels through HPK1-mediated ERK2 inhibition.
Many questions remain about the mechanisms by which HPK1 up-regulates p21 and p27 protein expression and about the importance of their induction in HPK1-mediated cell cycle arrest in pancreatic cancer. Although HPK1 may regulate p21 or p27 protein stability through its phosphorylation mediated by JNK, ERK2, or other pathways that are presently unknown, it is possible that HPK1 increases p21 and p27 mRNA levels through transcriptional mechanisms. On the other hand, it might be necessary to silence p21 and p27 individually or together by RNA interference to further evaluate their role in HPK1-mediated cell cycle arrest. A more detailed investigation of HPK1 functions in pancreatic cancer will be the focus of future studies.
In summary, we showed that the serine/threonine kinase HPK1 is lost in pancreatic cancer. The loss of HPK1 is associated with the progression of PanIN lesions and invasive PDA, and thus, it may contribute to the tumorigenesis of pancreatic cancer. We also showed that the loss of HPK1 protein expression in PDA was through proteasome pathway, which required HPK1 kinase activity for its degradation. By showing that HPK1 stabilized p21 and p27 in pancreatic cancer and led to cell cycle arrest, we provide further evidence that HPK1 may function as a tumor suppressor gene in PDA. The development of novel treatment strategies directed toward HPK1 may be an important approach for controlling pancreatic tumor growth.
Acknowledgments
Grant support: AACR PanCAN Career Development Award and The University of Texas M. D. Anderson Cancer Center Institutional Research Grant.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 3.Klimstra DS, Longnecker DS. K-ras mutations in pancreatic ductal proliferative lesions. Am J Pathol. 1994;145:1547–1550. [PMC free article] [PubMed] [Google Scholar]
- 4.Caldas C, Hahn SA, da Costa LT, et al. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994;8:27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- 5.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 6.Rozenblum E, Schutte M, Goggins M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 7.Ruggeri BA, Huang L, Berger D, et al. Molecular pathology of primary and metastatic ductal pancreatic lesions: analyses of mutations and expression of the p53, mdm-2, and p21/WAF-1 genes in sporadic and familial lesions. Cancer. 1997;79:700–716. [PubMed] [Google Scholar]
- 8.Moore TM, Garg R, Johnson C, Coptcoat MJ, Ridley AJ, Morris JD. PSK, a novel STE20-like kinase derived from prostatic carcinoma that activates the c-Jun N-terminal kinase mitogen-activated protein kinase pathway and regulates actin cytoskeletal organization. J Biol Chem. 2000;275:4311–4322. doi: 10.1074/jbc.275.6.4311. [DOI] [PubMed] [Google Scholar]
- 9.Lin JL, Chen HC, Fang HI, Robinson D, Kung HJ, Shih HM. MST4, a new Ste20-related kinase that mediates cell growth and transformation via modulating ERK pathway. Oncogene. 2001;20:6559–6569. doi: 10.1038/sj.onc.1204818. [DOI] [PubMed] [Google Scholar]
- 10.Wright JH, Wang X, Manning G, et al. The STE20 kinase HGK is broadly expressed in human tumor cells and can modulate cellular transformation, invasion, and adhesion. Mol Cell Biol. 2003;23:2068–2082. doi: 10.1128/MCB.23.6.2068-2082.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicke B, Bastien J, Khanna SJ, et al. Involvement of MINK, a Ste20 family kinase, in Ras oncogene-induced growth arrest in human ovarian surface epithelial cells. Mol Cell. 2005;20:673–685. doi: 10.1016/j.molcel.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Yang HS, Matthews CP, Clair T, et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated protein kinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol. 2006;26:1297–1306. doi: 10.1128/MCB.26.4.1297-1306.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anafi M, Kiefer F, Gish GD, Mbamalu G, Iscove NN, Pawson T. SH2/SH3 adaptor proteins can link tyrosine kinases to a Ste20-related protein kinase, HPK1. J Biol Chem. 1997;272:27804–27811. doi: 10.1074/jbc.272.44.27804. [DOI] [PubMed] [Google Scholar]
- 14.Ling P, Yao Z, Meyer CF, et al. Interaction of hematopoietic progenitor kinase 1 with adapter proteins Crk and CrkL leads to synergistic activation of c-Jun N-terminal kinase. Mol Cell Biol. 1999;19:1359–1368. doi: 10.1128/mcb.19.2.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Zhou G, Hu MC, Yao Z, Tan TH. Activation of the hematopoietic progenitor kinase-1 (HPK1)-dependent, stress-activated c-Jun N-terminal kinase (JNK) pathway by transforming growth factor β (TGF-β)-activated kinase (TAK1), a kinase mediator of TGFβ signal transduction. J Biol Chem. 1997;272:22771–22775. doi: 10.1074/jbc.272.36.22771. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Lee SC, Yao Z, Tan TH. Hematopoietic progenitor kinase 1 is a component of transforming growth factor β-induced c-Jun N-terminal kinase signaling cascade. J Biol Chem. 1999;274:13133–13138. doi: 10.1074/jbc.274.19.13133. [DOI] [PubMed] [Google Scholar]
- 17.Arnold R, Patzak IM, Neuhaus B, et al. Activation of hematopoietic progenitor kinase 1 involves relocation, autophosphorylation, and transphosphorylation by protein kinase D1. Mol Cell Biol. 2005;25:2364–2383. doi: 10.1128/MCB.25.6.2364-2383.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawasdikosol S, Pyarajan S, Alzabin S, Matejovic G, Burakoff SJ. Prostaglandin E2 activates HPK1 kinase activity via a PKA-dependent pathway. J Biol Chem. 2007;282:34693–34699. doi: 10.1074/jbc.M707425200. [DOI] [PubMed] [Google Scholar]
- 19.Nagata Y, Kiefer F, Watanabe T, Todokoro K. Activation of hematopoietic progenitor kinase-1 by erythropoietin. Blood. 1999;93:3347–3354. [PubMed] [Google Scholar]
- 20.Shui JW, Boomer JS, Han J, et al. Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell-mediated immune responses. Nat Immunol. 2007;8:84–91. doi: 10.1038/ni1416. [DOI] [PubMed] [Google Scholar]
- 21.Hu MC, Qiu WR, Wang X, Meyer CF, Tan TH. Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade. Genes Dev. 1996;10:2251–2264. doi: 10.1101/gad.10.18.2251. [DOI] [PubMed] [Google Scholar]
- 22.Kiefer F, Tibbles LA, Anafi M, et al. HPK1, a hematopoietic protein kinase activating the SAPK/JNK pathway. EMBO J. 1996;15:7013–7025. [PMC free article] [PubMed] [Google Scholar]
- 23.Ling P, Meyer CF, Redmond LP, et al. Involvement of hematopoietic progenitor kinase 1 in T cell receptor signaling. J Biol Chem. 2001;276:18908–18914. doi: 10.1074/jbc.M101485200. [DOI] [PubMed] [Google Scholar]
- 24.Arnold R, Liou J, Drexler HC, Weiss A, Kiefer F. Caspase-mediated cleavage of hematopoietic progenitor kinase 1 (HPK1) converts an activator of NFκB into an inhibitor of NFκB. J Biol Chem. 2001;276:14675–14684. doi: 10.1074/jbc.M008343200. [DOI] [PubMed] [Google Scholar]
- 25.Chen YR, Meyer CF, Ahmed B, Yao Z, Tan TH. Caspase-mediated cleavage and functional changes of hematopoietic progenitor kinase 1 (HPK1) Oncogene. 1999;18:7370–7377. doi: 10.1038/sj.onc.1203116. [DOI] [PubMed] [Google Scholar]
- 26.Liu SK, Smith CA, Arnold R, Kiefer F, McGlade CJ. The adaptor protein Gads (Grb2-related adaptor downstream of Shc) is implicated in coupling hemopoietic progenitor kinase-1 to the activated TCR. J Immunol. 2000;165:1417–1426. doi: 10.4049/jimmunol.165.3.1417. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Wang H, Shen W, et al. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315–4321. [PubMed] [Google Scholar]
- 28.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 29.Zhou G, Boomer JS, Tan TH. Protein phosphatase 4 is a positive regulator of hematopoietic progenitor kinase 1. J Biol Chem. 2004;279:49551–49561. doi: 10.1074/jbc.M410317200. [DOI] [PubMed] [Google Scholar]
- 30.Kolb A, Kleeff J, Arnold N, et al. Expression and differential signaling of heregulins in pancreatic cancer cells. Int J Cancer. 2007;120:514–523. doi: 10.1002/ijc.22360. [DOI] [PubMed] [Google Scholar]
- 31.Hurd C, Rozengurt E. Uncoupling of protein kinase D from suppression of EGF-dependent c-Jun phosphorylation in cancer cells. Biochem Biophys Res Commun. 2003;302:800–804. doi: 10.1016/s0006-291x(03)00268-7. [DOI] [PubMed] [Google Scholar]
- 32.Jonckheere N, Perrais M, Mariette C, et al. A role for human MUC4 mucin gene, the ErbB2 ligand, as a target of TGF-β in pancreatic carcinogenesis. Oncogene. 2004;23:5729–5738. doi: 10.1038/sj.onc.1207769. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 34.Zhong J, Kyriakis JM. Germinal center kinase is required for optimal Jun N-terminal kinase activation by Toll-like receptor agonists and is regulated by the ubiquitin proteasome system and agonist-induced, TRAF6-dependent stabilization. Mol Cell Biol. 2004;24:9165–9175. doi: 10.1128/MCB.24.20.9165-9175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda K, Idezawa T, You XJ, Kothari NH, Fan H, Korc M. Multiple mitogenic pathways in pancreatic cancer cells are blocked by a truncated epidermal growth factor receptor. Cancer Res. 2002;62:5611–5617. [PubMed] [Google Scholar]
- 36.Wang Z, Sengupta R, Banerjee S, et al. Epidermal growth factor receptor-related protein inhibits cell growth and invasion in pancreatic cancer. Cancer Res. 2006;66:7653–7660. doi: 10.1158/0008-5472.CAN-06-1019. [DOI] [PubMed] [Google Scholar]
- 37.Vogt A, Sun J, Qian Y, Hamilton AD, Sebti SM. The geranylgeranyltransferase-I inhibitor GGTI-298 arrests human tumor cells in G0/G1 and induces p21(WAF1/ CIP1/SDI1) in a p53-independent manner. J Biol Chem. 1997;272:27224–27229. doi: 10.1074/jbc.272.43.27224. [DOI] [PubMed] [Google Scholar]
- 38.Wiseman DA, Werner SR, Crowell PL. Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21(Cip1) and p27(Kip1) in human pancreatic adenocarcinoma cells. J Pharmacol Exp Ther. 2007;320:1163–1170. doi: 10.1124/jpet.106.111666. [DOI] [PubMed] [Google Scholar]
- 39.Gysin S, Lee SH, Dean NM, McMahon M. Pharmacologic inhibition of RAF→MEK→ERK signaling elicits pancreatic cancer cell cycle arrest through induced expression of p27Kip1. Cancer Res. 2005;65:4870–4880. doi: 10.1158/0008-5472.CAN-04-2848. [DOI] [PubMed] [Google Scholar]
- 40.Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38α and JNK1 stabilize p21(Cip1) by phosphorylation. J Biol Chem. 2002;277:29792–29802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- 41.Lenferink AE, Simpson JF, Shawver LK, Coffey RJ, Forbes JT, Arteaga CL. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu + MMTV/TGF-α bigenic mice. Proc Natl Acad Sci U S A. 2000;97:9609–9614. doi: 10.1073/pnas.160564197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster JS, Fernando RI, Ishida N, Nakayama KI, Wimalasena J. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J Biol Chem. 2003;278:41355–41366. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]