Abstract
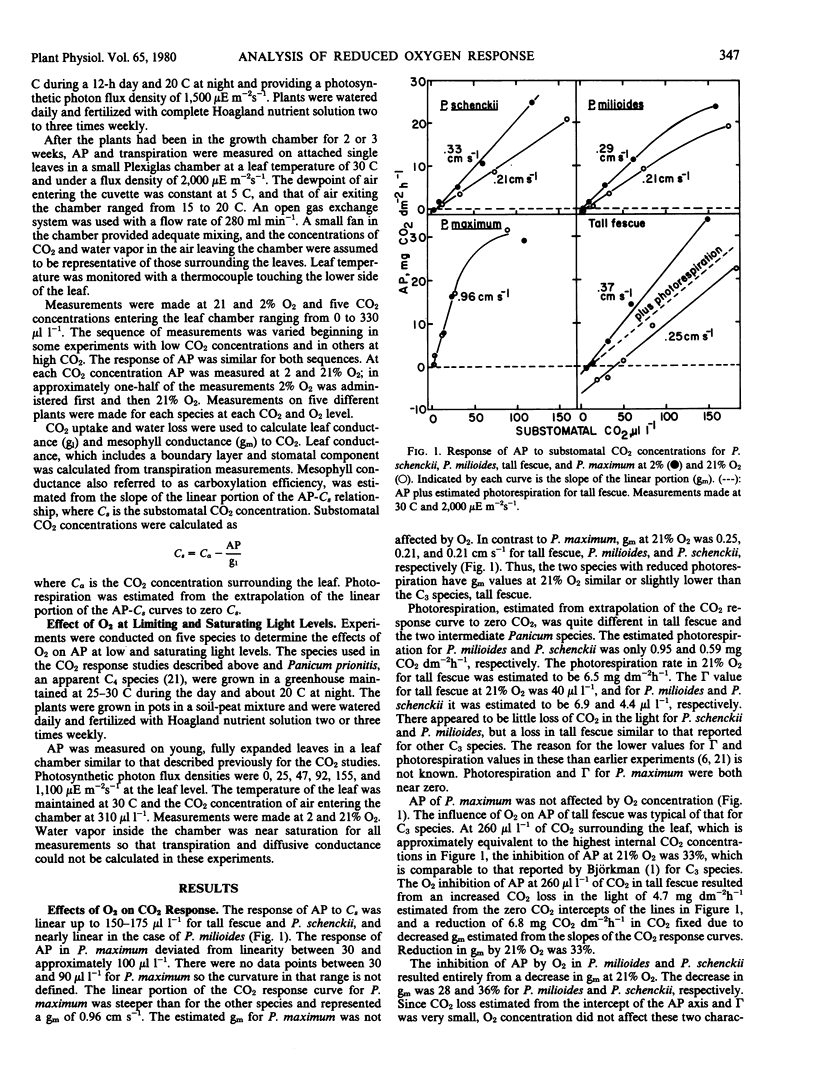
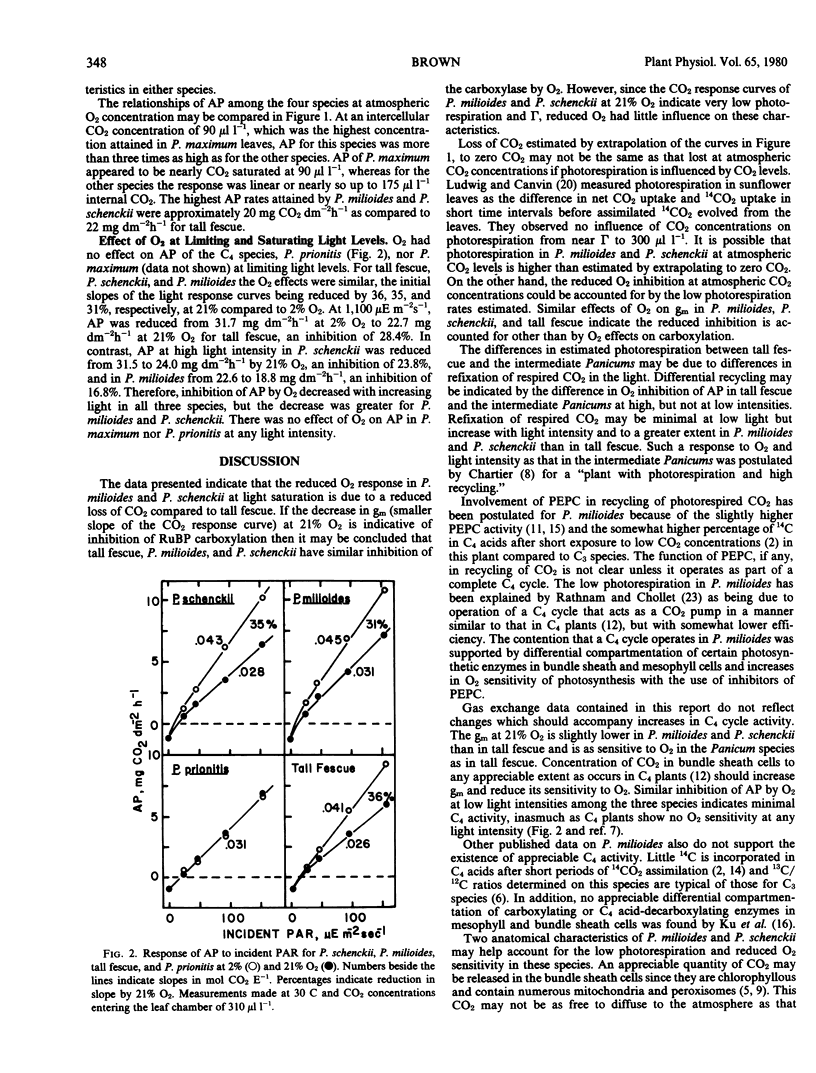
Reduced photorespiration has been reported in Panicum milioides on the basis of lower CO2 compensation concentrations than in C3 species, lower CO2 evolution in the light, and less response of apparent photosynthesis to O2 concentration. The lower response to O2 in P. milioides could be due to reduced O2 competition with CO2 for reaction with ribulose 1,5-bisphosphate, to a reduced loss of CO2, or to an initial fixation of CO2 by phosphoenolpyruvate carboxylase. Experiments were carried out with Panicum maximum Jacq., a C4 species having no apparent photorespiration; tall fescue (Festuca arundinacea Schreb.), a C3 species; P. milioides Nees ex Trin.; and Panicum schenckii Hack. The latter two species are closely related and have low photorespiration rates. CO2 exchange was measured at five CO2 concentrations ranging from 0 to 260 microliters per liter at both 2 and 21% O2. Mesophyll conductance or carboxylation efficiency was estimated by plotting substomatal CO2 concentrations against apparent photosynthesis. In the C4 species P. maximum, mesophyll conductance was 0.96 centimeters per second and was unaffected by O2 concentration. At 21% O2 mesophyll conductance of tall fescue was decreased 32% below the value at 2% O2. Decreases in mesophyll conductance at 21% O2 for P. milioides and P. schenckii were similar to that for tall fescue. On the other hand, loss of CO2 in CO2-free air, estimated by extrapolating the CO2 response curve to zero CO2, was increased from 1.8 to 6.5 milligrams per square decimeter per hour in tall fescue as O2 was raised from 2-21%. Loss of CO2 was less than 1 milligram per square decimeter per hour for P. milioides and P. schenckii and was unaffected by O2. The results suggest that the reduced O2 response in P. milioides and P. schenckii is due to a lower loss of CO2 in the light rather than less inhibition of carboxylation by O2, since the decrease in carboxylation efficiency at 21% O2 was similar for P. milioides, P. schenckii, and tall fescue. The inhibition of apparent photosynthesis by 21% O2 in these three species at low light intensities was similar at 31 to 36% which also indicates similar O2 effects on carboxylation. Apparent photosynthesis at high light intensity was inhibited less by 21% O2 in P. milioides (16.8%) and P. schenckii (23.8%) than in tall fescue (28.4%). This lower inhibition in the Panicum species may have been due to a higher degree of recycling of photorespired CO2 in these species than in tall fescue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowes G., Ogren W. L., Hageman R. H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1971 Nov 5;45(3):716–722. doi: 10.1016/0006-291x(71)90475-x. [DOI] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L. Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1972 Apr 10;247(7):2171–2176. [PubMed] [Google Scholar]
- Keck R. W. Differential Oxygen Response of Photosynthesis in Soybean and Panicum milioides. Plant Physiol. 1976 Oct;58(4):552–555. doi: 10.1104/pp.58.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S. B., Edwards G. E., Tanner C. B. Effects of Light, Carbon Dioxide, and Temperature on Photosynthesis, Oxygen Inhibition of Photosynthesis, and Transpiration in Solanum tuberosum. Plant Physiol. 1977 May;59(5):868–872. doi: 10.1104/pp.59.5.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig L. J., Canvin D. T. The Rate of Photorespiration during Photosynthesis and the Relationship of the Substrate of Light Respiration to the Products of Photosynthesis in Sunflower Leaves. Plant Physiol. 1971 Dec;48(6):712–719. doi: 10.1104/pp.48.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Brown R. H. Photosynthesis in Grass Species Differing in Carbon Dioxide Fixation Pathways: II. A Search for Species with Intermediate Gas Exchange and Anatomical Characteristics. Plant Physiol. 1979 Aug;64(2):257–262. doi: 10.1104/pp.64.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Comparative growth analyses of panicum species with differing rates of photorespiration. Plant Physiol. 1977 Jan;59(1):42–44. doi: 10.1104/pp.59.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. CO2 donation by malate and aspartate reduces photorespiration in Panicum milioides, a C3-C4 intermediate species. Biochem Biophys Res Commun. 1978 Nov 29;85(2):801–808. doi: 10.1016/0006-291x(78)91233-0. [DOI] [PubMed] [Google Scholar]



