Abstract
Purpose
A randomized phase II trial (E1200) was designed to assess toxicities and surgical resection rates in two neoadjuvant gemcitabine-based chemoradiation regimens in patients with borderline resectable pancreatic cancer. The trial was terminated early due to poor accrual.
Patients and Methods
Patients with borderline resectable adenocarcinomas of the pancreas were enrolled. Arm A patients (n=10) received gemcitabine 500mg/m2 q6 weeks, with radiation to 50.4Gy followed by surgical resection. Arm B patients (n=11) received preoperative gemcitabine 175mg/m2 on days 1, 5, 29, and 33, cisplatin 20mg/m2 on days 1-5 and 29-32, 5-FU 600mg/m2 on days 1-5 and 29-32, followed by radiation with continuous infusion 5-FU 225mg/m2 for 6 weeks. All patients received adjuvant gemcitabine 1000 mg/m2 weekly × 3 for five cycles.
Results
3 patients in arm A, and 2 patients in arm B were resected. Hematologic toxicity was comparable between the two arms except more patients in arm B developed grade 3 or 4 thrombocytopenia than those in arm A. Arm B had fewer grade 1-2 GI toxicities although more patients (45%) experienced grade 3-4 GI toxicity.
Conclusions
This phase II trial showed that both regimens were tolerable, and resectability and survival were comparable to previous studies.
Keywords: radiotherapy, gemcitabine, pancreatic cancer
Introduction
Gemcitabine, an independent cytotoxic agent, is a nucleoside analog with potent radiosensitizing effects.1. In randomized trials, gemcitabine was superior to 5-Fluorouracil (5-FU) alone in patients with advanced pancreatic cancer.2 Clinical benefit from gemcitabine has also been observed in patients who lack an objective response to 5-FU3 and has been tested against 5-FU in the postoperative adjuvant setting (RTOG 9704) with surperior survival for pancreatic head tumors4. Preoperative chemotherapy and radiation can improve resectability rates and address systemic disease recurrence risk without delay in patients with rapidly progressive disease. Since distant disease is a component of failure in most cases of unresectable pancreatic cancer, starting systemic therapy earlier can potentially improve control of micrometastases. Various phase I studies have been carried out to define an effective and tolerable regimen combining radiation therapy with gemcitabine.7-9 Phase I studies have shown promising results by using standard doses of gemcitabine (1000mg/m2 weekly) plus small radiotherapy (RT) field.7,10-11 The maximum tolerated dose of gemcitabine during radiotherapy is largely a function of RT field size. In earlier reports, the combination of gemcitabine plus RT was unexpectedly toxic at relatively low dose levels, which was thought in part to be due to the relatively large RT fields.9,12-13 Other phase II trials using higher doses of gemcitabine with more tailored RT fields have shown more favorable toxicity profiles.8,14
The purpose of this study was to define the added efficacy of preoperative combination chemotherapy prior to chemoradiotherapy for advanced potentially resectable, localized pancreatic adenocarcinomas, as compared to a more conventional program of preoperative chemoradiotherapy.
Patients and Methods
Patient Population
Patients with potentially resectable biopsy proven pancreatic adenocarcinoma were eligible for the study. In this multicenter phase II study, patients were randomized to preoperative combination chemotherapy prior to chemoradiotherapy or a more conventional program of preoperative chemoradiotherapy with gemcitabine. Other eligibility criteria included locally advanced, potentially resectable tumor, or patient previously explored and judged unresectable by a surgeon, or patient thought by surgeon to require preoperative treatment for other reasons. Potentially resectable was defined as abutting the portal or superior mesenteric vein, abutting the hepatic or superior mesenteric artery, extending to the origin of gastroduodenal artery, occluding SMV <2 cm. Biliary stent ≥9 F or biliary bypass was required before treatment if there was biliary obstruction by tumor. Other eligibility criteria included ECOG performance status 0-2, adequate renal function (serum creatinine <1.7 mg/dL or calculated creatinine clearance >60 ml/min), bilirubin <2 mg/dL, and adequate bone marrow reserve (absolute granulocyte count >2000/mm3, platelets >100,000/mm3).
Study Design and Treatment
This was an multicenter, two-arm randomized phase II trial. Ten Eastern Cooperative Oncology Group (ECOG) institutions participated in the study.
In arm A, patients received preoperative concurrent gemicitabine plus radiation (Figure 1). Gemcitabine dose was 500mg/m2 IV over 50 minutes weekly × 6. One dose was given weekly during radiation, followed by evaluation for surgery. Surgery was done 4 to 6 weeks after completion of chemotherapy and radiation.
Figure 1. ECOG 1200 Schema 226×163mm (96 × 96 DPI).
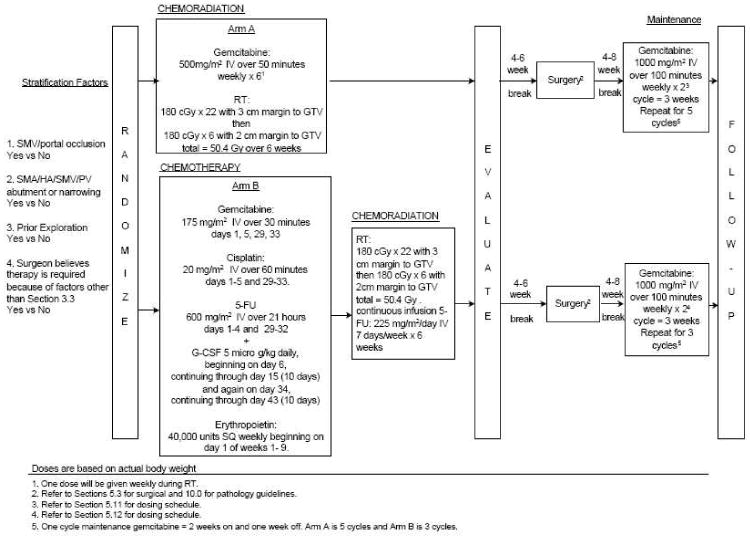
In arm B, gemcitabine 175mg/m2 IV was given over 30 minutes on days 1, 5, 29, and 33; cisplatin 20mg/m2/day IV over 60 minutes on days 1 through 5 and 29 through 33; 5-FU 600mg/m2 on days 1 through 4 and 29 through 32. Then, radiation was given with continuous infusion 5-FU (225 mg/m2/day, 7 days/week) for 6 weeks. Patients were then evaluated for surgery, which was done 4 to 6 weeks after chemotherapy and radiation. In both arms, following surgery, maintenance therapy consisted of gemcitabine 1000 mg/m2 IV over 100 minutes once a week for 2 weeks, followed by 1 week rest; five 3-week cycles were then administered.
Radiation in both arms used megavoltage photon irradiation of at least 4MV. Simulator films, first day portal films, and computer planning were submitted for quality assurance. Doses to the liver, kidneys, stomach, and spine were not to exceed the tolerance of these normal tissues. The minimal and maximal doses in the target volume were specified. All fields were treated daily. The absorbed daily dose was 1.8 Gy, with 3-cm margin to the gross tumor volume (GTV), 5 fractions a week. After 22 fractions, the total absorbed dose was 39.6 Gy. Then, GTV plus 2-cm margin was treated to 1.8 Gy per fraction for 6 fractions, for a total of 50.4 Gy.
Assessment of Efficacy and Safety
Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumor (RECIST) criteria.15 All responses had to be maintained for at least 4 weeks and be confirmed by an independent panel of radiologists. The response rate was defined as the sum of the complete plus partial responses. Safety and tolerability were assessed by monitoring adverse events, premature withdrawals, clinical laboratory tests, vital signs, physical measurements, and deaths throughout study treatment and follow-up.
Analysis of efficacy was performed on the basis of intent-to-treat. Analysis of safety was performed on safety of the population.
Statistical Analysis
Actuarial survival curves were calculated using the Kaplan-Meier method. Due to the small number of patients, the Log-rank to determine statistical significance in survival between the groups and a p-value of ≤ 0.05 was not performed. Safety analyses were based on all eligible patients who started treatment. The primary endpoint of this study was the percentage of margin-free resections produced by each treatment arm. Secondary endpoints were disease-free and overall survival, toxicity, efficacy measured by CT scan response and duration of objective responses. Duration of response was calculated from the day a response was first demonstrated until documented disease progression. Overall survival was calculated from the day of enrollment until death. Progression-free survival was calculated from the day of study entry until documented disease progression. Due to the small number of patients enrolled on the study, complete resections rates, response rate and duration of response were not statistically analyzed.
Results
Patient Characteristics
Between October 13, 2003 and June 21, 2005, 23 patients were enrolled in this multicenter randomized trial. Patient and primary tumor characteristics are outlined in table 1. Mean patient age was 60.2 years.
Table 1. Patient characteristics for randomized treatment of pancreatic adenocarcinoma.
| Characteristics of Eligible Patients | |||
|---|---|---|---|
| Characteristic | Arm A (n=10) | Arm B (n=11) | |
| Age | Mean (years) | 60.2 | 60 |
| Median (years) | 53.6 | 53 | |
| Number (%) | |||
| Sex | Male | 6 (60) | 6 (54.5) |
| Female | 4 (40) | 5 (45.5) | |
| Performance status | 0 | 5 (50) | 5 (45.5) |
| 1 | 5 (50) | 5 (45.5) | |
| 2 | 0 (0) | 1 (9.1) | |
| Race | White | 10 (100) | 10 (90.9) |
| Black | 0 (0) | 1 (9.1) | |
| SMV/portal occlusion | No | 9 (90) | 11 (100) |
| Yes | 1 (10) | 0 (0) | |
| SMA/SMV/PV abutment or narrowing | No | 2 (20) | 1 (9.1) |
| Yes | 8 (80) | 10 (90.9) | |
| Prior Exploration | No | 3 (30) | 4 (36.4) |
| Yes | 7 (70) | 7 (63.6) | |
| Tumor Characteristics | Unresectable | 5 (50) | 3 (27.3) |
| Locally Advanced Resectable | 4 (40) | 6 54.5) | |
| Requires Preoperative Treatment for Other Reasons | 1 (10) | 2 (18.2) | |
| Primary Site | Head of Pancreas | 8 (80) | 7 (63.6) |
| Body of Pancreas | 1 (10) | 1 (9.1) | |
| Tail of Pancreas | 1 (10) | 0 (0) | |
| Uncinate Process | 0 (0) | 2 (18.2) | |
| Head and Body | 0 (0) | 1 (9.1) | |
| Histology | Ductal Adenocarcinoma | 9 (90) | 8 (72.7) |
| Other | 1 (10) | 3 (27.3) | |
| Histologic Grade | Well Differentiated (Grade I) | 2 (28.6) | 1 (16.7) |
| Moderately Differentiated (Grade II) | 3 (42.6) | 3 (50.0) | |
| Poorly Differentiated (Grade III) | 1 (14.3) | 1 (16.7) | |
| Undifferentiated (Grade IV) | 1 (14.3) | 1 (16.7) | |
| Histologic proof of residual or metastatic disease | No | 9 (90) | 9 (90) |
| Yes | 1 (10) | 1 (10) | |
| Regional Node | No | 6 (75) | 8 (80) |
| Involvement | Yes | 2 (25) | 2 (20) |
| Adjacent | No | 5 (50) | 6 (60) |
| viscera/vessels Involvement | Yes | 5 (50) | 4 (40) |
Two patients randomized to arm A were ineligible, the first due to lack of baseline scans and the second due to metastatic sites at entry. The second patient never received assigned therapy and was excluded from all further analyses. The first patient received preoperative chemotherapy, and was included in the toxicity report but excluded from other analyses. Twenty-one analyzable patients remained, 10 on arm A and 11 on arm B. The arms were generally well balanced with respect to characteristics at entry. One exception was the initial resectability assessment of the tumor; five patients on arm A were initially thought to be unresectable while only three patients with unresectable disease were randomized to arm B.
Treatment
Of the five patients who received resections per protocol, three were from arm A, and two were from arm B. One patient from arm A and two patients from arm B had palliative surgeries only. Surgery status is unknown for one patient due to lack of follow-up data. The 90% confidence intervals on resection rate are 8.7% and 60.7% for arm A and 3.3% and 47.0% for arm B. Of the three patients in arm A who received resection, two were classic Whipple and one was distal pancreatectomy. Both resections in arm B were classic Whipples. Among the three resected patients in arm A, one was resected with clear margins, one had positive margins, and one had close margins <1 mm. In arm B, one patient was resected with clear margins, and one had positive margins. Of the five patients who received protocol surgery, one received two post-operative cycles on arm B, two patients received all five cycles on arm A, one patient was not ready to begin adjuvant treatment 12 weeks after surgery and went off study, and one patient received three doses of gemcitabine as non-protocol therapy before going to surgery.
Response
Response was assessed by imaging. Complete response was defined as the disappearance of all target lesions. Partial response was defined as at least a 30% decrease in the sum of the longest diameter of target lesions. No complete responses were observed. Partial responses were seen in 10% of patients in arm A and 18.2% of patients in arm B. Ninety-percent confidence intervals for partial response are 0.5% and 39.4% for arm A, and 3.3% and 47.0% for arm B. Detailed results for objective response to neoadjuvant treatment are summarized in Table 2.
Table 2. Results for objective response to neoadjuvant treatment in 21 patients with pancreatic adenocarcinoma.
| Treatment | ||
|---|---|---|
| Arm A | Arm B | |
| Objective Response to Neoadjuvant Treatment | N patients (%) | |
| Partial Response | 1 (10) | 2 (18.2) |
| No Change/Stable | 6 (60) | 4 (36.4) |
| Progression | 1 (10) | 4 (36.4) |
| Unevaluable | 2 (20) | 1 (9) |
| Total | 10 | 11 |
Survival
At final analysis, 6 of 10 patients (60%) in arm A evaluated for OS had died, and median survival was 19.4 months. For arm B, 8 of 11 patients (73%) in had died and median survival was 13.4 months. Among the five resected patients, four have died and one is still alive with a follow-up time of 48.8 months. The median overall survival of the resected patients is 26.3 months.
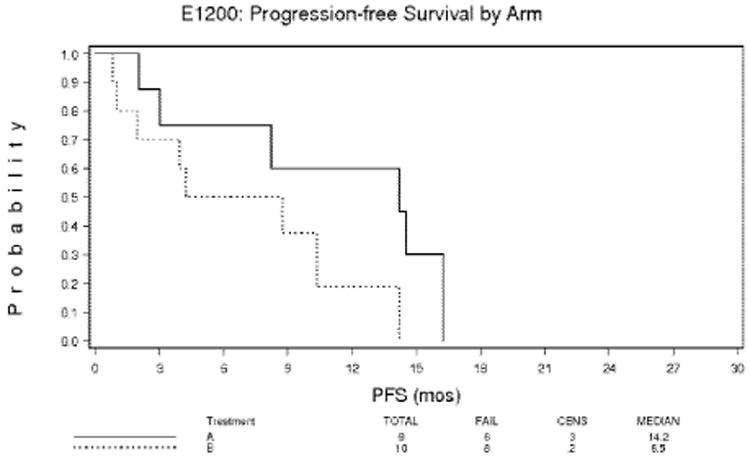
Median progression-free survival in arm A was 14.2 months. The 1-year actuarial overall survival rate was 69% for arm A and 61% for arm B. The 2-year actuarial survival rate was 32% for arm A and 13% for arm B (Figure 2). The 1-year actuarial progression-free survival rate for arm A was 59% and for arm B, 15% (Figure 3).
Figure 2. Overall survival by arm for patients with pancreatic adenocarcinoma 215×279mm (300 × 300 DPI).
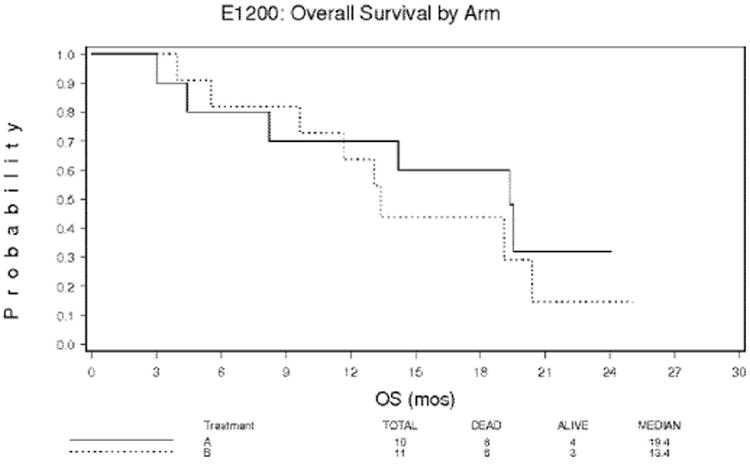
Figure 3. Progression-free survival for patients with pancreatic adenocarcinoma 215×279mm (300 × 300 DPI).
Safety Results
All patients were evaluated for safety. Hematologic toxicity was comparable between the two arms except more patients in arm B developed grade 3 or 4 thrombocytopenia than in arm A (five and two patients, respectively). Grade 3 or 4 neutropenia was not significantly different between the two arms. There was more grade 1-2 GI toxicity in arm A than arm B, however arm B had more grade 3-4 GI toxicities.
Grade 4 toxicity was experienced by 36% of patients in arm A and 18% of patients in arm B. In Arm A, grade 3-4 hematological toxicity included neutropenia in 4 of 11 patients (36.4%). In arm B, grade 4 hematologic toxicities included neutropenia in 5 of 11 patients (45%). In arm A, grade 3 or above non-hematological toxicities included nausea in 0 of 11 patients (0%). In arm B, grade 3 or above non-hematological toxicities included vomiting in 5 of 11 patients (45%). There was a marginal difference in nausea between arm A and B (p = 0.06).
There was a significant increase in grade 1-2 toxicity between arms A and B (p = 0.02). However, there was no difference in toxicity otherwise (Table 4).
Discussion
It is likely that the addition of radiation therapy and chemotherapy (5-FU) to resectional surgery is of survival benefit for those with resectable pancreatic adenocarcinoma.16,17 For unresectable pancreatic cancer, combined chemotherapy and radiation is the treatment of choice. However, the survival benefit from combined chemotherapy and radiation therapy remains modest with poor local control. In the phase III RTOG 9704 trial, gemcitabine was superior to 5-FU in patients with stage IV cancers, and has been shown to be superior to 5-FU when given prior to chemoradiation in the postoperative adjuvant setting.18 The ECOG Gastrointestinal Committee selected two phase I/II programs that contain gemcitabine—either in combination with RT or in combination with other drugs—preceding RT with conventional 5-FU. The Fox Chase Cancer Center/University of Michigan trials of gemcitabine during RT were conducted separately for patients with locally advanced unresectable pancreatic cancer (protocol JHFA) and locally advanced resectable (protocol JHEM) pancreatic cancer.7,8 For both groups, the maximum tolerated dose (MTD) was defined as 600 mg/m2 weekly given over 30 minutes during RT, with RT fields (3 cm margin around the gross tumor volume [GTV]) to 39.6 Gy, then reduced to 2 cm around GTV to 50.4 Gy, in 1.8 Gy fractions.
Dose limiting toxicity was grade 2 thrombocytopenia and distal gastric ulceration requiring hospitalization and transfusion (grade 4 GI toxicity) in two different patients given 700 mg/m2/week. In the JHEM protocol, 17 of 25 patients underwent subsequent pancreatic resection. Median survival for that group was 19 months. The Emory trial was a phase I/II effort to determine the MTD of combination chemotherapy before RT. Cisplatin, gemcitabine, and 5-FU were given in combination for 2 cycles over 56 days, followed by RT combined with 5-FU. G-CSF was required routinely for neutropenia. Of 11 patients with locally advanced pancreatic cancer treated by this regimen, there were four partial responses and one complete response. Although these patients were considered to have unresectable tumors, one had a successful resection with almost no cancer noted in the resected specimen. The most common toxicities were thrombocytopenia and neutropenia. Most patients required dose reductions because of excessive toxicity despite the use of G-CSF. As originally designed, the treatment plan consisted of the regimen of gemcitabine, cisplatin, leucovorin, and 5-FU (GPLF). Leucovorin and 5-FU were given by bolus infusion. Because of excessive toxicity, the GPLF regimen was subsequently modified to a GPF regimen in which leucovorin was no longer used and 5-FU was administered by continuous infusion. The median survival from the aforementioned trial was 11.7 months.19
In the present trial, median survival was 19.4 months for arm A and 14.2 months for arm B. The median survival in the resected patients was better than in the non resected patients. Due to the small numbers of patients, the observation should be viewed with caution but is consistent with the 22 month median survival reported by Staley et al.20
Hematologic toxicity was comparable between the two arms except more patients in arm B developed grade 3 or 4 thrombocytopenia than those in arm A. Arm B—more chemotherapy intensive—had fewer grade 1-2 GI toxicities although 5 of 11 patients experienced grade 3-4 GI toxicity. It cannot be overemphasized that the MTD of gemcitabine during RT is largely a function of the RT field size. A trial done in locally unresectable patients at the MD Anderson Cancer Center using conventional RT fields with larger fractions defined the MTD of gemcitabine at 350 mg/m2/week.9 Their current trial in patients with resectable disease uses 400 mg/m2/week over 40 minutes and conventional RT fields. McGinn et al at the University of Michigan have given 1000 mg/m2 over 30 minutes weekly of gemcitabine in conjunction with very tight RT fields (1 cm margin around GTV, but also including any enlarged nodes), further emphasizing the impact of RT treatment volumes on gemcitabine MTD.7
Tempero et al have shown that a fixed dose delivery (10 mg/m2/min) of gemcitabine is more effective and able to produce higher levels of intracellular gemcitabine triphosphate than a 30-minute delivery.21 They reported their experience regarding a fixed dose rate of gemcitabine in four patients at 400 mg/m2/week and seven patients at 500 mg/m2/week during RT. One patient had grade 2 and 3 thrombocytopenia requiring two dose withholdings, and two required 25% dose reductions for 1 week because of grade 1 thrombocytopenia and grade 3 neutropenia. Those having resection after this regimen had tumor reduction as complete as those with 700 mg/m2/week at 30-minute delivery. It appears that 500 mg/m2/week over 50 minutes is the optimal fixed dose rate infusion dosage with smaller RT fields. Sixty-nine percent of those with dose attenuation during post-resectional maintenance gemcitabine in the JHEM trial required attenuation on day 21 or in the third week of a 4-week cycle. The cycles of maintenance gemcitabine were therefore reduced in this trial to 3-week cycles in an attempt to reduce toxicity. Gemcitabine was delivered by fixed dose rate delivery in the present study.
Ammori et al at the University of Michigan reported a 13% resection rate (9 of 67 patients) after preoperative concurrent gemcitabine and radiotherapy in unresectable patients.22 In this ECOG study, a resection rate of 18.2% (2 of 11 patients) was achieved in arm B. One of the two resected patients in arm B had negative margins. One patient in arm A also had a resection with negative margins. There was no increased perioperative morbidity and no treatment-related mortality.
Toxicity in the present study is tolerable, and resectability and survival are comparable to previous studies. Future attempts to improve efficacy of chemotherapy includes optimizing concurrent radiation through intensity modulated radiotherapy and concomitant use of targeted therapy. Neoadjuvant chemotherapy and radiation may also be extended to patients with lower stage disease as they may benefit the most from increased systemic and local control.
Acknowledgments
grant or other support: Conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, MD, Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA27525, CA07190, CA21076, CA49957, CA17145 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Presented in part at 48th annual meeting of the American Society for Therapeutic Radiology Oncology (ASTRO) in 2006.
References
- 1.Lawrence TS, Eisbruch A, Shewach DS. Gemcitabine-mediated radiosensitization. Semin Oncol. 24(2 Suppl 7):S7-24–S7-28. 1997. [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Anderson J, et al. Phase I and pharmacologic study of oral topotecan administered twice daily for 21 days to adult patients with solid tumors. J Clin Oncol. 15:1087–93. 1997. doi: 10.1200/JCO.1997.15.3.1087. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg ML, Benedetti JK, Macdonald JS, et al. Phase II trial of 5-fluorouracil plus eniluracil in patients with advanced pancreatic cancer: a Southwest Oncology Group study. Ann Oncol. 2002;13:1576–82. doi: 10.1093/annonc/mdf274. [DOI] [PubMed] [Google Scholar]
- 4.Moossa AR, Lewis MH, Mackie CR. Surgical treatment of pancreatic cancer. Mayo Clinic Proceedings. 1979 Jul;54(7):468–74. [PubMed] [Google Scholar]
- 5.Regine WF, Winter KA, Abrams RA, et al. Flurouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 6.Connolly MM, Dawson PJ, Michelassi F, et al. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg. 1987;206:366–73. doi: 10.1097/00000658-198709000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGinn CJ, Zalupski MM, Shureiqui I, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman JP, McGinn CJ, Ross E, et al. A phase I trial of preoperative gemcitabine and radiotherapy followed by postoperative gemcitabine for patients with localized, resectable pancreatic adenocarcinoma. Cancer Investigation. 1999;17(Suppl1):30–32. [Google Scholar]
- 9.Wolff RA, Evans DB, Gravel DM, et al. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res. 2001;7:2246–53. [PubMed] [Google Scholar]
- 10.Murphy JD, Adusumili S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–8. doi: 10.1016/j.ijrobp.2006.12.053. Epub 2007 Mar 26. [DOI] [PubMed] [Google Scholar]
- 11.Talamonti MS, Small W, Jr, Mulcahy MF, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150–8. doi: 10.1245/ASO.2006.03.039. Epub 2006 Jan 19. [DOI] [PubMed] [Google Scholar]
- 12.Blackstock AW, Bernard SA, Richards F, et al. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208–12. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 13.Joensuu TK, Kiviluoto T, Karkkainen P, et al. Phase I-II trial of twice-weekly gemcitabine and concomitant irradiation in patients undergoing pancreaticoduodenectomy with extended lymphadenectomy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;60:444–52. doi: 10.1016/j.ijrobp.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Epelbaum R, Rosenblatt E, Nasrallah S, et al. Phase II study of gemcitabine combined with radiation therapy in patients with localized, unresectable pancreatic cancer. J Surg Oncol. 2002;81:138–43. doi: 10.1002/jso.10159. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. RECIST: New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–2. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 17.Klinkenbijl HH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776–784. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regine WF, Winter KW, Abrams R, et al. RTOG 9704: A phase III study of adjuvant pre- and post-chemoradiation (CRT) 5-FU vs. gemcitibine (G) for resected pancreatic adenocarcinoma. J Clin Oncol. 2006 ASCO Annual Meeting, Part I, Vol 24, No 18S. abstr 14056. [Google Scholar]
- 19.Staley CA, Harris WB, Landry J, et al. Neoadjuvant induction chemotherapy followed by chemoradiation: a phase I trial of gemcitabine, cisplatin, and 5-fluorouracil for advanced pancreatic/gastrointestinal malignancies. Surg Oncol Clin N Am. 2004;13:697–709. doi: 10.1016/j.soc.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Staley CA, Lee JE, Cleary KR, et al. Preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg. 1996 Jan;171(1):118–24. doi: 10.1016/S0002-9610(99)80085-3. discussion 124-5. [DOI] [PubMed] [Google Scholar]
- 21.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–8. doi: 10.1200/JCO.2003.09.140. Epub 2003 Jul 28. [DOI] [PubMed] [Google Scholar]
- 22.Ammori JB, Colletti LM, Zalupski MM, et al. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2003;7:766–72. doi: 10.1016/s1091-255x(03)00113-6. [DOI] [PubMed] [Google Scholar]


