Abstract
Purpose
Nuclear factor κB (NFκB) activity may increase survival and protect cancer cells from chemotherapy. Therefore, NFκB activity may be prognostic, and inhibition of NFκB may be useful for pancreatic cancer therapy. To test these hypotheses, we examined NFκB activity and the effects of inhibiting NFκB in several pancreatic cancer cell lines with differing sensitivities to gemcitabine.
Experimental Design
The gemcitabine sensitivity of pancreatic cancer cell lines BxPC-3, L3.6pl, CFPAC-1, MPanc-96, PANC-1, and MIA PaCa-2 were determined by 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and fluorescence-activated cell sorting assays. NFκB levels were determined by electrophoretic mobility shift assay and reporter assays. The effects of gemcitabine on NFκB activity were determined in vitro and in vivo. NFκB was inhibited by silencing of the p65/relA subunit using small interfering RNA in vitro and by neutral liposomal delivery of small interfering RNA in vivo, and the effects were evaluated on gemcitabine sensitivity.
Results
The cell lines L3.6pl, BxPC-3, and CFPAC-1 were sensitive, whereas MPanc-96, PANC-1, and MIA PaCa-2 were resistant to gemcitabine. No significant correlation was observed between basal NFκB activity and gemcitabine sensitivity. Gemcitabine treatment did not activate NFκB either in vitro or in vivo. Silencing of p65/relA induced apoptosis and increased gemcitabine killing of all gemcitabine-sensitive pancreatic cancer cells. No significant effects, however, were observed on gemcitabine-resistant pancreatic cancer cell lines either in vitro or in vivo.
Conclusions
NFκB activity did not correlate with sensitivity to gemcitabine. Silencing of p65/relA was effective alone and in combination with gemcitabine in gemcitabine-sensitive but not gemcitabine-resistant pancreatic cancer cells. Thus, NFκB may be a useful therapeutic target for a subset of pancreatic cancers.
Pancreatic cancer has the worst prognosis of all major cancers, with an overall 5-year survival rate of around 5% (1). The current clinical standard of care for advanced pancreatic cancer is gemcitabine, a cytotoxic nucleoside analogue. Gemcitabine results in a tumor response rate of 12% and offers a median survival time of 5 months (2). Unfortunately, this means that the best current treatment offers very modest benefits. Recent studies have indicated that targeted therapies in combination with gemcitabine can have statistically significant benefits (3). However, the results to date remain meager, and new approaches to improving the effectiveness of gemcitabine are needed. One of the targets considered for combination therapy that has generated wide attention is the transcription factor NFκB (4).
NFκB is a ubiquitous transcription factor that is regulated by a vast array of stimuli, including growth factors, inflammatory mediators, cytotoxic agents like chemotherapeutic drugs, oxidative stress, UV light, and many others. NFκB is a dimer composed of various combinations of the five mammalian Rel proteins, namely, p65/RelA, c-Rel, RelB, NFκB1/p50, and NFκB2/p52 (5). The most common form of NFκB is a dimer of p65/relA and p50, and this dimer is often referred to simply as NFκB. All Rel proteins share the NH2-terminal Rel homology domain that mediates dimerization, DNA binding, and nuclear localization and interaction with the inhibitors of NFκB, the inhibitory κBs (IκB). The p65/RelA, c-Rel, and RelB proteins possess COOH-terminal transactivation domains that allow for transcriptional activation of target genes. The binding of Rel proteins with IκBs masks a nuclear localization signal and effectively sequesters them in the cytoplasm where that are inactive. The most common pathway for NFκB activation (the canonical pathway) involves upstream activation of a complex of IκB kinases leading to the phosphorylation and subsequent ubiquitination and degradation of IκBα, which releases the Rel dimer that then enters the nucleus and activates gene transcription. Activity of NFκB, however, is also regulated by a number of posttranslational modifications of Rel proteins and by interactions with other transcription factors and regulatory factors (5). Furthermore, there are several less common pathways to NFκB activation, some of which are independent of IκBs. Which of these pathways is of primary importance in cancer therapy is currently unclear.
In cancer cells, NFκB is thought to play an antiapoptotic role through its ability to induce the expression of several molecules, including inhibitors of apoptosis family members as well as Bcl-2 homologues (6). Thus, NFκB is often regarded as being important for chemoresistance. This being the case, then the level of NFκB may be an indicator of chemoresistance and NFκB may be an excellent target for combined therapy with a cytotoxic such as gemcitabine. That NFκB activity might be an indicator of chemoresistance is supported by the observation that in pancreatic cancer, which is highly chemoresistant, NFκB is often constitutively activated (7–9). Furthermore, data suggesting that basal NFκB activity is higher in highly resistant cells compared with more sensitive cells have previously been reported (10). The concept of NFκB as a therapeutic target in cancer is supported by the observation that inhibition of NFκB seems to sensitize a wide variety of cancer cells to cytotoxics (11, 12) and this has also been reported for pancreatic cancer cells (10, 13–15).
Despite the abundance of data supporting the role of NFκB in chemoresistance, there are also data that suggest this may be an oversimplification. Many studies have now shown that NFκB can have either proapoptotic or antiapoptotic effects depending on the status of tumor suppressors and other mechanisms within a specific cell (16–18). The role of NFκB is also determined by the specific cytotoxic being tested; for example, NFκB activity is required for doxorubicin-induced cell death in N-type neuroblastoma cells (19). In addition, the effects of pharmacologic and molecular inhibition of NFκB are often divergent (20). Unfortunately, most of the studies supporting NFκB as a target for sensitization to cytotoxics have relied on pharmacologic inhibitors that are not specific for NFκB. In summary, based on the complex pathways of NFκB activity and function, the effects of NFκB inhibition can be cell-type specific and may depend on the method of inhibition and on the specific cytotoxic stress being applied. Therefore, whether or not inhibiting NFκB might improve gemcitabine therapy in patients with gemcitabine-resistant cancer cells remains a relevant issue.
The aim of the current study was to test the hypotheses that (a) levels of NFκB activity could distinguish sensitive from resistant pancreatic cancer cells; and (b) that inhibition of NFκB by small interfering RNA (siRNA)–directed gene silencing of the major DNA binding component p65/relA would increase the sensitivity of pancreatic cancer cell lines to gemcitabine-mediated apoptosis. The first hypothesis was not supported. The second hypothesis was partially supported, as we found that silencing p65/relA was effective alone and in combination with gemcitabine, but only for the subset of pancreatic cancer cells with native sensitivity to gemcitabine. Thus, our results suggest that the regulation and role of NFκB in pancreatic cancer is more complex than previously recognized and do not support targeting NFκB as a universal treatment for this disease.
Materials and Methods
Cell lines and culture conditions
Human pancreatic cancer cell lines BxPC-3, L3.6pl, CFPAC-1, MPanc-96, PANC-1 and MIA PaCa-2 were obtained from the American Type Culture Collection, and all except BxPC3 cells were routinely cultured in DMEM (BxPC-3 were in RPMI) supplemented with 10% fetal bovine serum in a 37°C incubator in a humidified atmosphere of 5% CO2. Human pancreatic duct epithelial (HPDE) cells (21) kindly provided by Dr. Tsao (University of Toronto) were cultured in keratinocyte serum-free media.
Lentiviral constructs
To allow monitoring of NFκB activity in living cells, a lentivirus NFκB luciferase reporter gene was constructed. A NFκB luciferase reporter gene consisting of repeats of a consensus κB binding site coupled with a minimal promoter to drive firefly luciferase was excised from the pNFκB-luc vector (Clonetec) and cloned into the lentiviral vector FG9 (ref. 22; a gift from Dr. Xiao-Feng Qin, Department of Immunology, M. D. Anderson Cancer Center, Houston, TX), replacing UBiC promoters, to form p-Lenti-NFκB-luc. As a control for infection efficiency, we used a modified version of the same FG9 lentiviral plasmid in which the ubiquitin promoter drives renilla luciferase gene expression (Lenti-Ubiquitin-renilla-luc), also from Dr. Xiao-Feng Qin. To study pancreatic cancer growth in vivo, we used a control lentiviral luciferase construct in which the luciferase coding sequence was isolated from the pGL-3 vector (Promega) and cloned into the lentiviral vector FG9 behind the UBiC promoter to form the luciferase-expressing p-Lenti-luc. Lentiviral plasmid vectors were cotransfected with packaging vectors in 293T cells by the calcium transfection method, and Lenti-NFκB-luc and Lenti-luc viruses were produced (22).
NFκB activity assay
For analysis of basal NFκB activity, cells were simultaneously infected with Lenti-NFκB-luc and a Lenti-Ubiquitin-Renilla-Luc (25µL of each viral supernatant/mL of medium) mixed with polybrene (4µg/mL medium) to develop stable cell lines expressing the NFκB reporter and the renilla luciferase control. Stable cells were cultured in a 96-well plate until cells were 70% confluent, and functional validation of the NFκB reporter activity was conducted in vitro using tumor necrosis factor-α (TNF-α; 10 ng/mL; Sigma) as a positive control. Cell lysates were prepared, and firefly and renilla luciferase activities were quantified according to the manufacturer’s instructions (Dual-Luciferase Reporter Assay System; Promega). Each luciferase signal was measured by using a luminometer (Lumistar). To control for different levels of infectivity and cell numbers, NFκB promoter activities were calculated from firefly signals divided by renilla signals. To examine the response to gemcitabine, stable transfected NFκB reporter cells (L3.6pl, BxPC-3, MPanc-96, and PANC-1, ~1 × 105 cells) were cultured in a 96-well plate overnight and stimulated with various concentrations of gemcitabine for 24 h. For these experiments, stimulated levels of firefly luciferase were compared with control levels in the absence of gemcitabine.
Electrophoretic mobility shift assay
Nuclear extracts were prepared from cells using the NE-PER nuclear and cytoplasmic extraction reagents kits according to the manufacturer’s directions (Pierce Biotech). For NFκB DNA binding, the 10,000 cpm of the 22-bp oligonucleotide 5′-AGTTGAGGGGACTTTCCCAGGC-3′ containing the NFκB consensus sequence that had been labeled with [-32P]ATP (10 mCi/mmol) by T4 polynucleotide kinase was added to 15 µg nuclear extract. The reaction was allowed to proceed for 30 min at room temperature. For cold competition experiments, unlabeled NFκB oligonucleotide or OCT1 oligonucleotide as nonspecific competitor gels were dried and directly exposed to a B-1 phosphorimaging screen and visualized with a GS-250 Molecular Imaging System (Bio-Rad). TATA binding protein (TBP) was used as a nuclear loading control (Abcam Inc.).
SDS-PAGE and Western blot analysis
For analysis of protein expression, cells were lysed with 100µL Triton X-100 lysis buffer in 6-well plates by incubation for 1 h at 4°C. Protein concentrations were measured by as per the manufacturer’s Bio-Rad reagent protocol (Bio-Rad). Proteins (50 µg) were separated by 10% SDS-PAGE and transferred to nitrocellulose. Membranes were blocked for 1 h at room temperature in 5% milk solution. NFκB p65/relA was detected by incubating the transferred membrane overnight at 4°C with rabbit polyclonal antibody (Santa Cruz Biotech; cat #D2204) at 1:250 dilution in 5% milk solution, then washing and incubating with the secondary antibody (Goat antirabbit IRDye 680) for 1 h at room temperature and the signal was detected by Odyssey IR imaging system (Li-Cor Biosciences).
Transfection of small interfering RNA
Cells in 6-well plates or 96-well plates were grown to 50% confluence and transfected with double-stranded siRNA for relA/p65 (Dharmacon Res Inc.) target sequence (Sense 5′ CCAUCAACUAUGAUGAGUU dTdT 3′, Antisense 3′ dGdTGGUAGUUGAUACUACUCAA 5′) or with a siRNA nonspecific control (Ambion, Austin, TX cat# 4611) in serum-free medium without antibiotic supplements using Hiperfect Transfectin Reagent (Qiagen, Inc.). Cells were incubated under these conditions for 48 h and silencing was then confirmed by reporter assays as well as Western blotting.
Liposomal preparation
Neutral liposomes were used for siRNA delivery similarly to what has been previously described (23). Briefly, siRNA for in vivo delivery was incorporated into neutral liposome 1,2-dioleoylsn-glycero-3-phosphatidylchiline (DOPC). DOPC and siRNA were mixed in the presence of excess tertiary butanol at a ratio of 1:10 (w/w) siRNA/DOPC. Tween 20 was added to the mixture in a ratio of 1:19 Tween 20: siRNA/DOPC. The mixture was vortexed, frozen in an acetone/dry ice bath, and lyophilized. Before in vivo administration, this preparation was hydrated with normal 0.9% saline at a concentration of 50 µg/mL to achieve the desired dose in 200 µL per injection.
Cell growth studies
Analysis of cell growth was used to determine sensitivity of the cell lines to gemcitabine in vitro. Cells (104 cells/well) were seeded on 96-well plates in 100 µL of complete culture medium, allowed to attach for 24 h, then transfected with siRNA mixtures. After 24 h, cells were treated with gemcitabine (2′,2′-difluorodeoxycytidine; Eli Lilly) at indicated concentrations in a total volume of 150 µL of culture medium. After 72 h, cell viability was assessed using MTS reagent (Promega) according to the manufacturer’s directions. The percentage of viable cells was defined as therapy group divided by control group, multiplied by 100%. Fluorescence-activated cell sorting analysis and cell counting were done in parallel and gave similar results as the MTS assay.
Analysis of NFκB in vivo
Male athymic nude mice (nu/nu) were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center and housed in specific pathogen-free conditions. They were cared for in accordance with guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the USPHS “Policy of Human Care and Use of Laboratory Animals,” and all studies were approved and supervised by the University of Texas M. D. Anderson Cancer Center Institutional Animal Care and Use Committee. Stable NFκB reporter-bearing cells were grown to 80% confluence, harvested by trypsinization, washed twice in PBS, and 50 µL of cell suspensions (1 × 106 cells) were used for intrapancreatic injection into 4-wk-old male nude mice to make an orthotopic pancreatic tumor model. For analysis of the effects of gemcitabine, after 3 wk the mice were injected with gemcitabine (50 mg/kg or 200 mg/kg i.p. injection) and bioluminescence imaging was done after 0, 6, 24, 48, and 72 h of gemcitabine treatment. Data were calculated as percentage of the NFκB signals in each individual mouse before i.p. injection of gemcitabine, and experiments were done at least thrice. For in vivo analysis of the effectiveness of liposomal delivery of siRNA, after 1 wk the mice were injected with d-luciferin (150 mg/kg body weight, i.p. injection), and basal NFκB activity was determined using the IVIS system (Xenogen Corp.). Subsequently, mice were injected with siRNAp65 or the control (siRNAc) coupled liposome complexes (10 µg/kg body weight, i.p.), and NFκB luciferase activity was reanalyzed after 48 h.
Analysis of tumor development
For monitoring of cancer cell growth in vivo, we used Lenti-luc-expressing cells and confirmed that there was a linear relationship between cell numbers (0–10 × 105 cells/well in a 24-well plate) and the light emission after adding luciferin (150 µg/mL) measured using the IVIS system. Cells carrying the Lenti-luc reporter gene were grown to 80% confluence, harvested by incubation with trypsin-EDTA, washed twice in PBS, and resuspended to a final concentration of 2.5 × 106 cells/mL (MPanc-96) or 5.0 × 106 cells/mL (BxPC-3) for 100 µL intrapancreatic injections (n = 20 mice/cell line) in 4-wk-old male nude mice. Tumors were allowed to become established for 1 wk, then bioluminescent imaging was done and the mice were grouped into four groups of five mice with equivalent mean tumor volumes between groups. The mice in group 1 were treated with liposomal relA siRNA (10 µg/mouse) and 24 h later the mice were treated with gemcitabine (100 mg/kg for Mpanc96 orthotopic model, 50mg/kg for BxPC3 orthotopic model) via i.p. injection (2 times/wk). The mice in group 2 were treated with liposomal relA siRNA and PBS (control) 24 h later via i.p. injection (2 times/wk). The mice in group 3 were treated with liposomal control siRNA (10 µg/mouse) and 24 h later the mice were treated with gemcitabine via i.p injection (2 times/wk). The mice in group 4 were treated with liposomal control siRNA and PBS (control) 24 h later via i.p. injection (2 times/wk). At the end of the 5 wk the animals were sacrificed and the pancreas were removed and weighed then processed for histology.
In vivo bioluminescence imaging
Bioluminescence imaging was conducted using a cryogenically cooled IVIS 100 imaging system coupled to a data acquisition computer running Living Image Software. Before imaging, the mice were placed in an acrylic chamber, anesthetized with 1.5% isofluorane-air mixture, and injected i.p. with 15 mg/mL of luciferin potassium salt in PBS at a dose of 150 mg/kg body weight. A digital gray scale image of each mouse was acquired, followed by acquisition and overlay of a pseudocolor image representing the spatial distribution of detected photons emerging from active luciferase within the mouse which was integrated for 5 min starting from 7 min after the injection of d-luciferin. Therapy was continued for 5 wk before the animals were sacrificed. The tissue was formalin-fixed, paraffin-embedded, and also frozen in −80°C in optimal cutting temperature compound.
Statistical analysis
All experiments were conducted in triplicate and carried out on three or more separate occasions. Data presented are means of the three or more independent experiments ± SE. Statistically significant differences were determined by two-tailed unpaired Student’s t test and were defined as *P < 0.05.
Results
Pancreatic cancer cell lines vary in resistance to gemcitabine
We examined the relative sensitivity of six commonly used pancreatic cell lines (PANC-1, MPanc-96, MIA PaCa-2, CFPAC-1, BxPC-3, and L3.6pl) to gemcitabine in vitro. Cells were treated with different concentrations of gemcitabine for 72 hours and the number of surviving cells was analyzed. Whereas the gemcitabine LD50 was >50 µmol/L for the PANC-1, MPanc-96, and MIA PaCa-2 cells, the LD50 was around 1µmol/L for BxPC-3, CFPAC-1, and L.3.6pl (Fig. 1). The same sensitivities were obtained when the effects of gemcitabine were analyzed on apoptosis using fluorescence-activated cell sorting (data not shown). These data supported the classification of the Panc-1, Mpanc96, and MIA PaCa-2 cell lines as gemcitabine-resistant and the BxPC3, CFPAC, and L3.6pl cell lines as gemcitabine-sensitive.
Fig. 1.
Pancreatic cancer cells have differing levels of native resistance to gemcitabine. Human pancreatic cancer cell lines PANC-1, MPanc-96, MIA PaCa-2, CFPAC-1, BxPC-3, and L3.6pl were treated with increasing concentrations of gemcitabine (0–100 µmol/L) for 72 h. The viabilities indicated on the y axis were determined by MTS assays and normalized to control. Data shown are means ± SE for n = 3 independent experiments.
NFκB levels in pancreatic cancer cell lines
To test the hypothesis that NFκB basal levels could predict gemcitabine sensitivity, we initially evaluated NFκB DNA binding by electrophoretic mobility shift assay (EMSA). Nuclear extracts were prepared from the six pancreatic cancer cell lines and also from noncancer immortalized human pancreatic duct epithelial cells. Nuclear extracts from each of the cell lines cultured under basal conditions bound an NFκB consensus oligonucleotide (Fig. 2A). Specificity was indicated by the observation that this binding was blocked by excess unlabeled oligonucleotide. Highest levels of binding were observed with MPanc-96, CFPAC-1, L3.6pl, and BxPC-3 cells, and lowest DNA binding was observed with PANC-1, CFPAC-1, MIA PaCa-2, and human pancreatic duct epithelial cells. Thus, pancreatic cancer cells exhibited elevated NFκB, but there was no consistent pattern of NFκB DNA binding in the resistant versus sensitive cell lines.
Fig. 2.
Basal levels of NFκB do not correlate with gemcitabine sensitivity. A, NFκB nuclear binding was analyzed in human pancreatic cancer cell lines. Nuclear extracts from six pancreatic cancer cell lines and human pancreatic duct epithelial cells were prepared and EMSA using a labeled oligonucleotide (Oligo) containing a consensus κ B binding site was done. TBP was used as a loading control for quality and quantity of cell extracts. Negative control, 32P-labeled NFκB Oligo without HeLa nuclear extract; positive control, HeLa nuclear extract and 32P-labeled NFκB Oligo; specific competitor, HeLa nuclear extract with 32P-labeled NFκB Oligo plus unlabeled NFκB Oligo; nonspecific competitor, HeLa nuclear extract with 32P-labeled NFκB Oligo plus unlabeled Oct1 Oligo. B, NFκB transcriptional activity was estimated using an NFκB reporter assay. Pancreatic cancer cells were coinfected with an NFκB reporter expressing firefly luciferase and a control lentivirus expressing renilla luciferase, and stable populations were developed. Basal NFκB transcriptional activity determined as a ratio of firefly and Renilla luciferase signals and presented as a comparison with the level measured in human pancreatic duct epithelial cells. As a positive control, all cell lines were also challenged with TNF-α (10 ng/mL) for 6 h and the level of NFκB activity was determined. Data shown are means ± SE for three independent experiments.
Because nuclear levels of NFκB subunits do not directly correspond to NFκB transcriptional activity, we examined NFκB activity using a reporter assay. In preliminary studies conducted using transient transfection assays we observed that the stress of transient transfection itself activated NFκB and interfered with the analysis (data not shown). Therefore, to avoid transient transfection artifacts, we developed a lentiviral vector to stably express an NFκB reporter. This reporter virus was coinfected with a control lentivirus that expressed renilla-luciferase from an ubiquitin promoter as a control for infection efficiency. Using the ratio of firefly and renilla luciferase signals, we observed that basal levels of NFκB transcriptional activity could be observed in all of the cell lines, including the control human pancreatic duct epithelial cells (Fig. 2B). Furthermore, all cells responded to TNF-α with increased NFκB activity. NFκB activity measured using the reporter assay did not perfectly match the level of nuclear NFκB DNA binding observed in EMSA. Only three cancer cell lines, CFPAC-1, MIA PaCa-2, and PANC-1, had higher basal levels of NFκB activity than the human pancreatic duct epithelial cells. Thus, no consistent difference was noted between NFκB activities in gemcitabine-sensitive versus gemcitabine-resistant cells.
Gemcitabine treatment did not affect NFκB activity
Although basal NFκB activity did not reflect gemcitabine sensitivity, it remained possible that responses to stress may be different in sensitive versus resistant cells. Therefore, we wished to evaluate whether NFκB activity in response to treatment with gemcitabine could predict sensitivity. Two sensitive cells (L3.6pl and BxPC-3) and two resistant cells (MPanc-96 and PANC-1) stably expressing the NFκB reporter were stimulated with various concentrations (0.05–2 µmol/L) of gemcitabine for 24 hours in vitro and NFκB activities were measured using the reporter assay. Gemcitabine treatment (1 µmol/L) for 24 hours had no significant effect on NFκB activity in any of the cells analyzed (Fig. 3A). Because the results of in vitro treatments may not reflect the situation in vivo, we established mouse orthotopic pancreatic tumor models of the most sensitive (L3.6pl) and most resistant (PANC-1) cells stably expressing the NFκB reporter gene. Animals were treated with either a lower dose (50 mg/kg) or higher dose (200 mg/kg) of gemcitabine and NFκB activities were evaluated. Neither the more gemcitabine-sensitive tumor (L3.6pl) nor the more resistant tumor (PANC-1) showed any significant change in NFκB activity from 0 to 72 hours after gemcitabine injection (Fig. 3B).
Fig. 3.
Gemcitabine treatment does not influence NFκB activity in pancreatic cancer cells in vitro or in vivo. A, two gemcitabine sensitive (L3.6pl and BxPC-3) and two gemcitabine resistant (MPan-96 and PANC-1) cell lines were treated with indicated concentrations of gemcitabine for 24 h in vitro and activity of an NFκB reporter was analyzed. B, the most gemcitabine-sensitive cell line (L3.6pl) and the most resistant (PANC-1) were implanted orthotopically in nude mice, allowed to form tumors, and then the level of NFκB reporter activity was measured before and 24, 48, 72 h after treatment with either a low dose (50 mg/kg) or a high dose (200 mg/kg) of gemcitabine. All measurements are shown as a percentage of basal and are means ± SE for three independent experiments.
In vitro knockdown of p65/relA influenced sensitive but not resistant cells
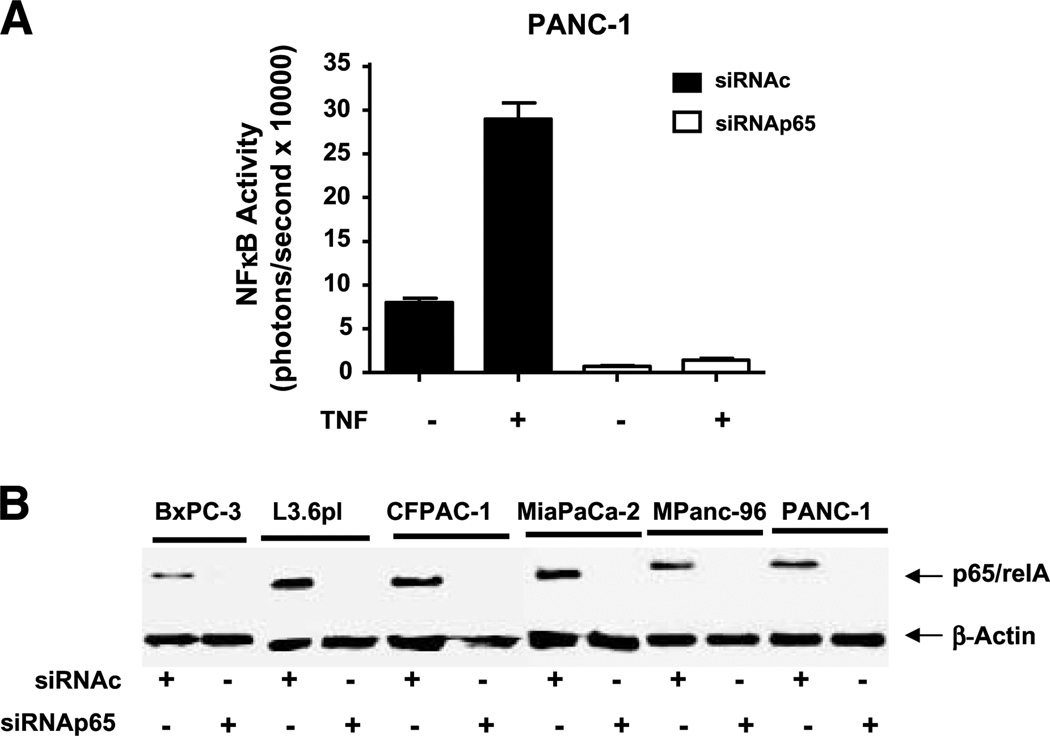
To test the hypothesis that inhibition of NFκB would increase the sensitivity of pancreatic cancer cell lines to gemcitabine-mediated apoptosis, we used small interfering RNA (siRNA) to knock down p65/relA. To validate the effectiveness of this approach, we examined basal and TNF-α–stimulated NFκB-dependent luciferase activity in PANC-1 cells transfected with either control siRNA (siRNAc) or p65/relA siRNA (siRNAp65). After transfection with siRNAp65, but not siRNAc, the NFκB-dependent signal was dramatically reduced from its basal level and the ability of TNFα to increase NFκB transcriptional activity was completely blocked (Fig. 4A). As further confirmation of the silencing, Western blotting of the p65/relA subunit in the six cell lines transfected with siRNAp65, but not siRNAc, also showed complete silencing (Fig. 4B).
Fig. 4.
siRNA silencing of p65/rel A was highly effective in vitro. A, to examine the effectiveness of p65/relA silencing on NFκB activity in the cancer cells, PANC-1 cells expressing NFκB luciferase gene were transfected with control siRNA and relA siRNA. After 72 h, cells were treated with or without TNF-α (10 ng/mL) and bioluminescent imaging was used to estimate NFκB reporter activity. B, Western blotting was conducted 72 h after treatment with control siRNA or siRNA against p65/relA in the six cell lines. Full-length gels are presented in Supplementary Fig. S1. Columns, mean for three experiments; bars, SE. *, P < 0.05, versus control.
Next, we transiently silenced expression of p65/relA in each of the six pancreatic cell lines and examined the effects alone and with LD50 concentrations of gemcitabine (Fig. 5). Silencing of p65/relA alone significantly reduced cell numbers in vitro of all gemcitabine-sensitive pancreatic cell lines (BxPC-3, L3.6pl, CFPAC-1). Furthermore, in gemcitabine-sensitive cell lines p65/relA silencing also increased the effectiveness of gemcitabine treatment. In contrast, p65/relA silencing did not affect cell numbers of the gemcitabine-resistant cell lines (MPanc-96, PANC-1, MIAPaCa-2). Nor did silencing of p65/relA increase the effectiveness of gemcitabine killing of the gemcitabine-resistant cell lines.
Fig. 5.
Silencing of p65/relA is effective against gemcitabine-sensitive but not resistant cells. A, effects of gemcitabine on cell viability of pancreatic cancer cell lines after silencing of relA/p65. Human pancreatic cancer cell lines BxPC-3, L3.6pl, CFPAC-1, MPanc-96, PANC-1, and MIA PaCa-2 were treated with different concentrations of gemcitabine for 48 h. The cell numbers indicated on the y axis were determined by MTS assays and normalized to control as described in Materials and Methods. B, cell death assay for measuring apoptosis induced by siRNAp65, gemcitabine, and combination. Cells were plated at an equal density and the sub-G1 faction was assessed by fluorescence-activated cell sorting analysis after propidium iodide staining in BxPC-3 and Mpanc-96 cells. Data are means ± SE for n = 3–5 experiments.
Knockdown of p65/relA reduced in vivo tumor growth in sensitive but not resistant orthotopic tumors
To determine whether the results of the in vitro studies were relevant to the in vivo situation, we used neutral liposomes to deliver the siRNAp65 to pancreatic orthotopic xenografts. To validate the approach, we first examined NFκB activity of orthotopically transplanted MPanc-96 cells stably expressing an NFκB luciferase reporter. After 1 week, NFκB luciferase activity was determined before (0 time point) and 48 hours after a single injection of siRNAp65 or siRNAc liposomal complex (10 µg/mouse by i.p injection). Liposomal siRNAp65 administration reduced NFκB activity in the tumors by 90% versus the controls (Fig. 6A).
Fig. 6.
Liposomal delivery of siRNA against p65/relA effectively inhibits NFκB activity and it also effectively inhibits growth of gemcitabine-sensitive pancreatic cancer cells but not gemcitabine-resistant pancreatic cancer cells in vivo. A, tumors were formed in nude mice with MPanc-96 cells stably expressing the NFκB reporter. The mice were treated with or without neutral liposomes bearing siRNAp65 and the effects on bioluminescence imaging indicated significant reduction. B, tumors were formed in nude mice with MPanc-96/BxPC-3 cells stably expressing a constitutively regulated luciferase for analysis of tumor formation. Bioluminescent imaging was done weekly to assess tumor growth. C, after 5 wk the weights of the pancreata including the primary tumors were quantified. Columns, mean for five animals; bars, SE. *, P < 0.05, versus control.
We then used this approach to analyze the effects of the liposomal delivery of siRNAs in orthotopic models of one gemcitabine resistant cell line (MPanc-96) and one gemcitabine sensitive cell line (BxPC-3). Mice with established orthotopic tumors were randomized (n = 5 mice/group) into four treatment groups, each with approximately equal mean tumor size. Groups of mice were treated with siRNAp65 liposomal complex with or without gemcitabine, or siRNAc liposomal complex with or without gemcitabine. Treatments were continued for 5 weeks and the tumor burden was analyzed by weekly bioluminescence imaging. In the gemcitabine-resistant MPanc-96 orthotopic model, neither siRNAp65 alone, gemcitabine alone, nor the combination influenced the time-course of tumor development (Fig. 6B). Likewise, none of the treatments had any significant effect on tumor weight at the end of the experiment (Fig. 6C). In contrast, in the gemcitabine-sensitive BxPC-3 orthotopic model, gemcitabine alone, even at a lower dose than used in the MPanc-96 model, had a significant inhibitory effect. Furthermore, treatment with siRNAp65 alone dramatically reduced tumor growth; in fact, no tumor could be found in those animals after 5 weeks. For this reason, it was not possible to determine whether siRNAp65 increased the effectiveness of gemcitabine in this model (Fig. 6B, C).
Discussion
Most pancreatic cancer patients receive little benefit from current adjuvant therapies largely because most pancreatic cancer cells are resistant to chemotherapeutic agents. NFκB is thought to be important in cancer cell resistance to apoptosis and thus contribute to chemoresistance. More than 10 years ago it was noted that pancreatic cancer cells possess high levels of NFκB (7) and the level of p65/relA observed in tissue sections has been reported to correlate with poor survival in patients who received resections (24). Furthermore, many studies have suggested that inhibition of NFκB using a wide variety of inhibitors sensitizes pancreatic cancer to cytotoxic treatments (25–27). Therefore, we hypothesized that NFκB activity levels would predict chemoresistance. Furthermore, we predicted that inhibition of NFκB activity using a siRNA directed at the most common transcriptionally active subunit, p65/rel, would reduce chemoresistance. However, our data did not confirm these hypotheses and indicated rather that the role and regulation of NFκB in pancreatic cancer is more complex than previously recognized. Of particular significance, our results on the effects of NFκB inhibition on sensitivity to gemcitabine indicated that natively sensitive but not resistant cells were affected. This suggests that clinical trials based on inhibition of NFκB in pancreatic cancer may not be uniformly successful. This study differs in several specifics compared with previous studies including the technique of analysis of NFκB activity, the means of NFκB inhibition, the use of a large number of cell lines that were well defined in terms of native gemcitabine sensitivity, and the focus on gemcitabine as the cytotoxic.
Our initial goal was to determine whether NFκB levels could predict chemosensitivity to gemcitabine. High basal levels of NFκB in pancreatic cancer cells have previously been noted in a large number of studies by EMSA (8, 14, 25, 28–31). Some studies have suggested that the level of basal NFκB is correlated with resistance to chemotherapies (10, 14). We also observed levels of nuclear NFκB DNA binding that were higher in pancreatic cancer cells than in the nontumorigenic human pancreatic duct epithelial cell line. However, we found no correlation between the level of NFκB observed by EMSA and the gemcitabine sensitivity of the cell lines. It is not possible to conclude from the small sampling of cell lines in this study that no statistical correlation exists between NFκB activity and gemcitabine resistance. It is clear from these examples, however, that NFκB activity of a specific cell line is not enough information to determine gemcitabine resistance. One important issue that has not been well investigated in pancreatic cancer is that EMSA does not directly relate to NFκB transcriptional activity due to the large number of other regulatory mechanisms (5, 32). One previous study used an NFκB reporter in transient reporter assays to help address this issue (8). However, we found that transient transfection of reporters gave highly variable results due to the dramatic effects of transfection procedures themselves on NFκB activity. To avoid these complications, we used for the first time in pancreatic cancer studies lentiviral vectors to stably express the NFκB reporter in the cells. The results indicated that all of the cell lines had basal NFκB activity, even the nontumorigenic human pancreatic duct epithelial cells. This may reflect the basal level of cell stress involved in the cell culture procedures. There was no obvious relationship between DNA binding complexes observed in EMSA studies and NFκB transcriptional activity evaluated using the reporter. Only three of the six cancer cell lines showed NFκB activity levels significantly higher than that of the human pancreatic duct epithelial cells. There was no simple correlation between basal reporter activity and gemcitabine sensitivity, thus disproving our hypothesis.
We also investigated whether sensitive and resistant pancreatic cancer cells could be differentiated on the basis of the NFκB response to gemcitabine treatment. Using a stably expressed NFκB reporter, we did not observe any effect of gemcitabine on NFκB activity in vitro. Furthermore, this was the first study to analyze the effects of gemcitabine on pancreatic cancer cell NFκB activity in vivo, in which we also did not detect any effects. This is in contrast to a previous report that indicated that gemcitabine treatment stimulated NFκB in pancreatic cancer cells in vitro as measured using EMSA (10). One important difference between the current study and the previous study is the use of the stable reporter, which measures transcriptional activity, rather than EMSA, which measures DNA binding. Another difference between the studies was the dosage of gemcitabine used. For the in vitro experiments Arlt et al. (10) used a concentration of 20 µmol/L gemcitabine whereas our highest concentration was 2 µmol/L. Perhaps the lower concentration is less effective. In the in vivo study, however, we used a maximal dose of gemcitabine at 200 mg/kg, which is higher than the level used in patients clinically. Therefore, it seems unlikely that the concentration of gemcitabine explains the lack of effects on NFκB in the current study.
To evaluate the effects of inhibiting NFκB, we used siRNA silencing of the p65/relA subunit. This subunit is responsible for the activation of gene expression on NFκB activation by most known pathways and was identified as the important subunit in pancreatic cancer (7). However, the noncanonical pathway does not use p65/relA but rather relB, which would not be inhibited by p65/relA silencing. Silencing p65/relA would also not be expected to inhibit rel C-mediated gene expression. Thus, the pattern of inhibition expected after p65/relA silencing would be similar, but not identical, to that of overexpressing an IκB inhibitory subunit. It is important to be aware that either silencing or overexpression of NFκB subunits may have effects on a variety of other molecules and signaling pathways. However, these approaches are still more specific than the use of pharmacologic agents and natural products. Several previous studies have used drugs such as genestein (25, 31), sulfasalazine (10, 26), or curcumin (27, 30) as NFκB inhibitors. Each of these drugs is known to have many other targets besides NFκB, such that interpretation of the actions of these drugs is quite difficult. We observed that the siRNA against the NFκB p65/relA subunit very effectively reduced cellular levels of this subunit and blocked NFκB transcriptional activity in all of the pancreatic cancer cells. Therefore, differences in the effectiveness of siRNA-mediated silencing do not explain the observation that p65/relA silencing inhibited gemcitabine-sensitive but not resistant cells.
Recently there has been a growing interest in the use of in vivo delivery of siRNAs as a potential novel approach to cancer treatment. Therefore, we tested whether in vivo delivery of the p65/relA siRNA using neutral liposomes in a manner previously described (23) would affect orthotopic pancreatic tumors. We found that delivery of the siRNA by i.p. injection reduced the basal level of NFκB reporter activity. Furthermore, siRNA treatment had a dramatic inhibitory effect such that it completely eliminated tumors formed from the gemcitabine-sensitive BxPC-3 cells. It was not possible to see an additional effect of gemcitabine in those mice, because the siRNA treatment was so highly effective. In contrast, the same siRNA treatment had no discernable effect on tumor development when using gemcitabine-resistant MPanc-96 cells. These observations strongly suggest that therapies directed toward NFκB may not be universally successful but could be advantageous in certain patients.
Our data indicate that the benefits of inhibiting NFκB are limited to cell lines that are natively gemcitabine-sensitive. Most previous studies have focused on one or two cell lines and have not determined the native gemcitabine resistance of the cells. One study that was somewhat similar to this one also characterized the cells being used for gemcitabine sensitivity, but in that study the BxPC-3 cells were considered gemcitabine-resistant. In contrast, we found BxPC-3 cells to be among the most gemcitabine-sensitive. The most likely explanation for this difference is that their analysis of gemcitabine effectiveness was conducted within 24 hours of treatment. Gemcitabine functions as a toxin by influencing DNA synthesis during S-phase. We did not observe large effects within 24 hours of gemcitabine (data not shown); therefore, in the current study the effects of gemcitabine were examined after 72 hours to allow time for cell killing.
The current data suggest that the resistant pancreatic cancer cells do not rely on NFκB as a major survival pathway under either control or gemcitabine-treated conditions. In contrast, sensitive pancreatic cancer cells rely on NFκB for survival under basal conditions and during treatment with gemcitabine. Thus, the sensitivity of pancreatic cancer cells to gemcitabine and to p65/relA silencing was correlated. The mechanisms responsible for gemcitabine resistance are not completely understood, but are largely thought to involve molecules involved in drug metabolism (33). Other molecules have also been suggested to be important in gemcitabine resistance, such as p8 (34), HSP27 (35), S100A4 (36), and Bcl-2 (37, 38). More recently it has been noted that resistant cells have more of a mesenchymal phenotype, suggesting epithelial-to-mesenchymal transition as an important resistance mechanism (39). Our data support the concept that NFκB-independent mechanisms determine chemoresistance in pancreatic cancer. Further investigations will be necessary to delineate these important mechanisms.
In summary, inhibition of NFκB seems to be a useful treatment in pancreatic cancer cell lines that are natively sensitive to gemcitabine. In such cells, silencing of p65/relA itself reduced cell numbers and improved the effectiveness of gemcitabine. In highly resistant cell lines, however, silencing of p65/relA had no effect alone or in combination with gemcitabine. Because clinical studies indicate that only about 12% of patients with advanced pancreatic adenocarcinoma have either a partial or complete response to gemcitabine treatment (33), it seems apparent that targeting NFκB will have limited utility in battling this disease. Nevertheless, our studies suggest that in a subset of patients in which gemcitabine has effectiveness, the combination of inhibition of NFκB could be very useful. Unfortunately, it is not possible at the current time to predict which patients will respond to gemcitabine. Clearly future efforts should be directed to understanding the mechanisms of gemcitabine resistance and at developing biomarkers that can predict gemcitabine responsiveness.
Supplementary Material
Translational Relevance.
Nuclear factor κB (NFκB) is widely considered a useful target for cancer therapy. Most studies in this regard, however, have used NFκB inhibitors that are not entirely specific. Furthermore, most studies have investigated only a small number of cancer cell lines and have not taken into account the heterogeneity that exists among tumor cells. In the current study, the effects of the highly specific approach of silencing NFκB using small interfering RNA were investigated in several pancreatic cancer cells with either high or low levels of resistance to gemcitabine. The data indicated that inhibition of NFκB was highly effective in gemcitabine-sensitive pancreatic cancer cells but was without effect in gemcitabine-resistant cells. Thus, patients with gemcitabine-sensitive tumors would likely respond well to inhibition of NFκB alone or in combination with gemcitabine. However, only a small percentage of patients have tumors that are sensitive to gemcitabine. Therefore, it seems unlikely that NFκB inhibition will be widely useful in the treatment of pancreatic cancer. Clearly the development of biomarkers or diagnostic tests that could identify the subset of patients with gemcitabine-sensitive tumors would be valuable as this is the group that would be most likely to benefit from NFκB inhibition.
Acknowledgments
Grant support: Lockton Endowment, Lustgarten Foundation (G. Halder, Principal Investigator), Program Project Development Grant from the Ovarian Cancer Research Fund, Inc., and National Cancer Institute Pancreatic Cancer Specialized Program of Research Excellence grant P20-CA101936 (J.L. Abbruzzese, Principal Investigator).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.O’Reilly EM, Abou-Alfa GK. Cytotoxic therapy for advanced pancreatic adenocarcinoma. Semin Oncol. 2007;34:347–353. doi: 10.1053/j.seminoncol.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi C, Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat Rev. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 5.Neumann M, Naumann M. Beyond IκBs: alternative regulation of NF-κB activity. FASEB J. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 6.Rayet B, Gelinas C. Aberrant Rel/NFκb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 8.Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM. Mitogenic and antiapoptotic role of constitutive NF-κB/Rel activity in pancreatic cancer. Int J Cancer. 2003;105:735–746. doi: 10.1002/ijc.11081. [DOI] [PubMed] [Google Scholar]
- 9.Chandler NM, Canete JJ, Callery MP. Increased expression of NF-κB subunits in human pancreatic cancer cells. J Surg Res. 2004;118:9–14. doi: 10.1016/S0022-4804(03)00354-8. [DOI] [PubMed] [Google Scholar]
- 10.Arlt A, Gehrz A, Muerkoster S, et al. Role of NF-κB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- 11.Arlt A, Schafer H. NFκB-dependent chemoresistance in solid tumors. Int J Clin Pharmacol Ther. 2002;40:336–347. doi: 10.5414/cpp40336. [DOI] [PubMed] [Google Scholar]
- 12.Melisi D, Chiao PJ. NF-κ B as a target for cancer therapy. Exp Opin Ther Targets. 2007;11:133–144. doi: 10.1517/14728222.11.2.133. [DOI] [PubMed] [Google Scholar]
- 13.Sebens S, Arlt A, Schafer H. NF-κB as a molecular target in the therapy of pancreatic carcinoma. Recent Res Cancer Res Fortschritte der Krebsforschung. 2008;177:151–164. doi: 10.1007/978-3-540-71279-4_17. [DOI] [PubMed] [Google Scholar]
- 14.Arlt A, Vorndamm J, Breitenbroich M, et al. Inhibition of NF-κB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001;20:859–868. doi: 10.1038/sj.onc.1204168. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Odagiri H, Ikenaga SK, Maruyama M, Sasaki M. Chemosensitivity of human pancreatic carcinoma cells is enhanced by IκBα super-repressor. Cancer Sci. 2003;94:467–472. doi: 10.1111/j.1349-7006.2003.tb01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhnel F, Zender L, Paul Y, et al. NFκB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem. 2000;275:6421–6427. doi: 10.1074/jbc.275.9.6421. [DOI] [PubMed] [Google Scholar]
- 17.Reuther-Madrid JY, Kashatus D, Chen S, et al. The p65/RelA subunit of NF-κB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol Cell Biol. 2002;22:8175–8183. doi: 10.1128/MCB.22.23.8175-8183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortis F, Cardozo AK, Crispim D, Storling J, Mandrup-Poulsen T, Eizirik DL. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-κB activation. Mol Endocrinol (Baltimore MD) 2006;20:1867–1879. doi: 10.1210/me.2005-0268. [DOI] [PubMed] [Google Scholar]
- 19.Bian X, McAllister-Lucas LM, Shao F, et al. NF-κB activation mediates doxorubicin-induced cell death in N-type neuroblastoma cells. J Biol Chem. 2001;276:48921–48929. doi: 10.1074/jbc.M108674200. [DOI] [PubMed] [Google Scholar]
- 20.Dong QG, Sclabas GM, Fujioka S, et al. The function of multiple IκB: NF-κB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene. 2002;21:6510–6519. doi: 10.1038/sj.onc.1205848. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang H, Mou L, Luk C, et al. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157:1623–1631. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci U S A. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landen CN, Merritt WM, Mangala LS, et al. Intra-peritoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5:1708–1713. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- 24.Weichert W, Boehm M, Gekeler V, et al. High expression of RelA/p65 is associated with activation of nuclear factor-κB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer. 2007;97:523–530. doi: 10.1038/sj.bjc.6603878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee S, Zhang Y, Ali S, et al. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 26.Muerkoster S, Arlt A, Witt M, et al. Usage of the NF-κB inhibitor sulfasalazine as sensitizing agent in combined chemotherapy of pancreatic cancer. Int J Cancer. 2003;104:469–476. doi: 10.1002/ijc.10963. [DOI] [PubMed] [Google Scholar]
- 27.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 28.Bai J, Demirjian A, Sui J, Marasco W, Callery MP. Histone deacetylase inhibitor trichostatin A and proteasome inhibitor PS-341 synergistically induce apoptosis in pancreatic cancer cells. Biochem Biophys Res Commun. 2006;348:1245–1253. doi: 10.1016/j.bbrc.2006.07.185. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Ellis KL, Ali S, et al. Apoptosis-inducing effect of chemotherapeutic agents is potentiated by soy isoflavone genistein, a natural inhibitor of NF-κB in BxPC-3 pancreatic cancer cell line. Pancreas. 2004;28:e90–e95. doi: 10.1097/00006676-200405000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-κB and IκB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor κB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 32.Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 33.Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine) Drug Resist Updat. 2002;5:19–33. doi: 10.1016/s1368-7646(02)00002-x. [DOI] [PubMed] [Google Scholar]
- 34.Giroux V, Malicet C, Barthet M, et al. p8 is a new target of gemcitabine in pancreatic cancer cells. Clin Cancer Res. 2006;12:235–241. doi: 10.1158/1078-0432.CCR-05-1700. [DOI] [PubMed] [Google Scholar]
- 35.Mori-Iwamoto S, Kuramitsu Y, Ryozawa S, et al. Proteomics finding heat shock protein 27 as a biomarker for resistance of pancreatic cancer cells to gemcitabine. Int J Oncol. 2007;31:1345–1350. [PubMed] [Google Scholar]
- 36.Mahon PC, Baril P, Bhakta V, et al. S100A4 contributes to the suppression of BNIP3 expression, chemoresistance, and inhibition of apoptosis in pancreatic cancer. Cancer Res. 2007;67:6786–6795. doi: 10.1158/0008-5472.CAN-07-0440. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto K, Ocker M, Neureiter D, et al. Bcl-2-specific siRNAs restore gemcitabine sensitivity in human pancreatic cancer cells. J Cell Mol Med. 2007;11:349–361. doi: 10.1111/j.1582-4934.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bold RJ, Chandra J, McConkey DJ. Gemcitabine-induced programmed cell death (apoptosis) of human pancreatic carcinoma is determined by Bcl-2 content. Ann Surg Oncol. 1999;6:279–285. doi: 10.1007/s10434-999-0279-x. [DOI] [PubMed] [Google Scholar]
- 39.Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.