Abstract
Background
In appropriately selected patients with severe carotid stenosis, carotid revascularization reduces ischemic stroke. Prior clinical research has focused on the efficacy and safety of carotid revascularization, but few investigators have considered readmission as a clinically important outcome.
Objectives
To examine frequency, timing, and diagnoses of 30-day readmission following carotid revascularization; to assess differences in 30-day readmission between patients undergoing carotid endarterectomy (CEA) and carotid artery stenting (CAS); to describe hospital variation in risk-standardized readmission rates (RSRR); and to examine whether hospital variation in procedural choice (CEA vs. CAS) was associated with differences in RSRRs.
Methods
We used Medicare fee-for-service administrative claims data to identify acute care hospitalizations for CEA and CAS from 2009–2011. We calculated crude 30-day all-cause hospital readmissions following carotid revascularization. To assess differences in readmission after CAS compared with CEA, we used Kaplan-Meier survival curves and fitted mixed-effect logistic regression. We estimated hospital RSRRs using hierarchical generalized logistic regression. We stratified hospitals into 5 groups by their proportional CAS use and compared hospital group median RSRRs.
Results
Of 180,059 revascularizations from 2,287 hospitals, CEA and CAS were performed in 81.5% and 18.5% of cases, respectively. The unadjusted 30-day readmission rate following carotid revascularization was 9.6%. Readmission risk after CAS was higher than after CEA. There was modest hospital-level variation in 30-day RSRRs (Median: 9.5%, Range: 7.5%–12.5%). Variation in proportional use of CAS was not associated with differences in hospital RSRR (range of median RSRR across hospital quartiles: 9.49%–9.55%, P 0.771).
Conclusions
Almost 10% of Medicare patients undergoing carotid revascularization were readmitted within 30-days of discharge. Compared with CEA, CAS was associated with higher readmission risk. However, hospitals’ RSRR did not differ by their proportional CAS use.
Keywords: Carotid artery stenosis, hospital readmission, carotid artery stenting, carotid endarterectomy
Introduction
Carotid revascularization is a commonly performed class of vascular interventions that in appropriately selected patients reduces the risk of ipsilateral stroke (1). Prior research has focused on the safety and efficacy of carotid revascularization, but few investigators have considered short term readmission as a clinically important outcome (2). However, the potential importance of readmissions following carotid revascularization has increased dramatically with the development of a measure of hospitals’ 30-day risk-standardized readmission rates (RSRR) following vascular procedures (3), with implications for potential financial penalties for hospitals with excess readmissions.
At present, many aspects of readmissions following carotid revascularization are poorly understood. Specifically, the timing and reasons for readmission as well as the extent of variation in hospitals’ 30-day readmission rates have not been described. Furthermore, like many vascular procedures, carotid revascularization can be accomplished through an open or endovascular approach (carotid endarterectomy [CEA] and carotid artery stenting [CAS], respectively). Although prior research has shown that patients undergoing CAS are at increased risk of readmission compared with patients undergoing CEA (2), it is not known whether differences in hospitals’ proportional use of CAS among all carotid revascularization procedures are associated with differences in hospital 30-day readmission rates.
To address these gaps in knowledge, we used administrative claims data to identify readmissions occurring within 30 days of hospitalization in which a carotid revascularization procedure was performed. We examined the timing and diagnoses associated with readmission and characterized variation in risk-standardized readmission rates (RSRRs) across hospitals. Furthermore, we assessed whether readmission rates varied by the choice of the revascularization strategy used (CAS vs. CEA) and whether hospitals’ 30-day RSRRs differed by their proportional use of CAS among all carotid revascularization procedures.
Methods
We used Medicare fee-for-service administrative claims data to identify hospitalizations with an associated carotid revascularization procedure performed between January 2009 and December 2011. We identified our patients using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes or Current Procedure Terminology (CPT) codes, previously reported by the Center for Medicare & Medicaid Service (CMS) vascular procedure readmission measure (3). As with this measure, our study sample consisted of patients who underwent either CEA (ICD-9-CM codes: 00.61, 00.63, 00.64, 39.72, 39.74; or CPT codes: 37215, 37216, 0075T) or CAS (ICD-9-CM codes: 38.02, 38.12, 38.32, 38.42 or CPT codes: 35201, 35005, 35231, 35301, 35701, 34001). Whether or not patients were symptomatic (i.e. had symptoms attributable to their carotid disease) was identified using the following ICD-9-CM codes previously reported in literature: 362.3(0–7), 362.84, 433.11, 433.31, 434.01, 434.91, 435.(0–3,8,9), and 781.4 (2,4,5). The primary outcome of interest was hospital readmission within 30-days of discharge from a hospitalization during which a carotid revascularization procedure had been performed. Among patients who had been readmitted, we examined the timing of the readmission by day after discharge (0–30). We categorized readmission diagnoses using the CMS condition category (CC) codes to group patients’ principal discharge diagnoses. Each of the 189 CMS CCs describes a disease entity or medical condition. However, because more than 90% of these CCs contributed less than 1% of all readmissions, we further consolidated the 189 CMS CCs into 30 modified CCs. The categorization of CCs is described in Supplementary Tables 1 & 2.
Statistical Analyses
We used descriptive statistics to illustrate baseline characteristics. We compared differences in baseline characteristics between CEA and CAS patients using the Mantel-Haenszel chi-square test and Student’s T test, as appropriate. We calculated crude readmission rates following carotid revascularization procedures by dividing the number of patients readmitted within the 30-day period following carotid revascularization procedure by the total number of patients undergoing carotid revascularization. To examine the timing of readmission following carotid revascularization, we calculated the proportion of readmissions during each day over the 30-day period. We also identified the ten most common diagnoses responsible for readmission by modified CCs and presented their distribution over the following consecutive time periods after hospital discharge: 0–7 days, 8–15 days and 16–30 days, reflecting the time periods in which follow-up visits to ambulatory care providers frequently occur.
Procedure-specific readmission rates: CAS vs. CEA
To assess the difference in readmission rates between patients undergoing CEA versus CAS, we plotted Kaplan-Meier readmission-free survival curves and fitted a generalized estimating equation (GEE) logistic regression model. These models adjusted for patient and hospital characteristics. In these models, we considered all variables included in the models as fixed effects except the hospital variable, which was considered as a random effect to account for within hospital correlation.
To account for baseline differences between CEA and CAS, we used propensity score matching to create new matched data set (N =64,238) based on age, gender, race, symptomatic status and other comorbidities included in previous GEE model. After matching, the standardized difference between the two groups did not exceed 10% for any variables (See Supplementary Table 3), indicating successful matching in respect of the chosen matching variables. We then fit a GEE model using this propensity matched cohort.
Hospital-specific Risk Standardized Readmission Rates (RSRRs)
We estimated hospital-specific 30-day risk standardized readmission rates (RSRRs) using a hierarchical mixed-effect logistic regression model adjusting for demographics and co-morbidities. To account for within-hospital correlation, we estimated a random intercept for each hospital by including the hospital variable as a random effect variable. We estimated the hospital-specific RSRR by calculating the ratio of the “predicted” 30-day readmission rate within the hospital to the “expected” 30-day readmission rate within that hospital. We used this ratio to standardize an unadjusted national average of readmission rates following carotid revascularization procedures. We estimated the hospital “predicted” readmission rate using the random intercept specific to that hospital and the population mix within that hospital. We calculated the hospital “expected” readmission rate from an average hospital intercept, calculated from our sample of hospitals and the population mix of that specific hospital.
We also examined differences in hospital-specific RSRRs by the utilization of carotid revascularization strategy. In order to obtain a stable estimate of hospitals’ proportional use of CAS, we limited this analysis to hospitals that performed at least 25 carotid revascularization procedures. To examine whether hospital RSRR varied by the proportion of revascularization cases performed using CAS, we stratified hospitals into five groups by their proportional use of CAS among all carotid revascularization procedures (0%, 0–10%, 10–20%, 20–30%, >30%). We utilized descriptive statistics to summarize hospital-specific RSRRs within each hospital group. We then compared the medians of these five hospital groups using a Kruskal-Wallis test. The study was approved by the Human Investigation Committee of Yale University School of Medicine. We used SAS version 9.3 (SAS Institute, Cary, NC) for statistical analyses and defined statistical significance as two-tailed P <0.05.
Results
We identified 180,059 patients for 168,323 patients (93.2% patients with single carotid intervention and 6.8% with >1 carotid intervention) from 2,287 hospitals undergoing carotid revascularization procedures performed between January 2009 and December 2011. Of these procedures, 81.5% (146,831/180,059) underwent CEA and 18.5% (33,228/180,059) underwent CAS. The mean age of our cohort was 76.3 years and 93% of included patients were Caucasians (Table 1). Symptomatic carotid stenosis, diabetes, hypertension and congestive heart failure were present in 10%, 43%, 89%, and 20% of our study population, respectively. 43% of our patients had other peripheral vascular diseases and 18% had chronic kidney disease.
Table 1.
Study sample baseline characteristics
| Variable | Total Cohort (N=180,059 procedures) | CAS (N=33,228 procedures) | CEA (N=146,831 procedures) | P value* |
|---|---|---|---|---|
| Age, mean (standard deviation) | 76.3 (6.4) | 76.1 (6.6) | 76.3 (6.3) | <0.001 |
| Age > 80 years, % | 29.4 | 29.1 | 29.5 | 0.139 |
| Male, % | 56.4 | 53.2 | 57.1 | <0.001 |
| White, % | 93.2 | 90.2 | 93.9 | <0.001 |
| Comorbidities | ||||
| Symptomatic carotid stenosis, % | 9.7 | 13.7 | 8.8 | <0.001 |
| Diabetes, % | 43.3 | 40.9 | 43.9 | <0.001 |
| Diabetes with peripheral complications, % | 6.9 | 6.8 | 6.9 | 0.322 |
| Hypertension, % | 89.3 | 85.7 | 90.1 | <0.001 |
| Hypertensive complications, % | 14.0 | 15.2 | 13.7 | <0.001 |
| Congestive heart failure, % | 19.7 | 23.9 | 18.7 | <0.001 |
| Coronary atherosclerosis, % | 63.4 | 62.3 | 63.6 | <0.001 |
| Acute coronary syndrome, % | 8.5 | 10.2 | 8.1 | <0.001 |
| Precerebral arterial occlusion, % | 86.6 | 71.5 | 90.0 | <0.001 |
| Cerebral atherosclerosis, % | 10.7 | 19.1 | 8.8 | <0.001 |
| Unspecified cerebrovascular disease, % | 3.8 | 3.8 | 3.8 | 0.813 |
| Vascular disease, % | 43.1 | 45.2 | 42.6 | <0.001 |
| Vascular disease with complications, % | 5.7 | 6.3 | 5.6 | <0.001 |
| Renal failure, % | 17.6 | 19.0 | 17.3 | <0.001 |
| End stage renal disease, % | 1.0 | 1.2 | 1.0 | <0.001 |
t-test for continuous variables or chi-square test for categorical variables
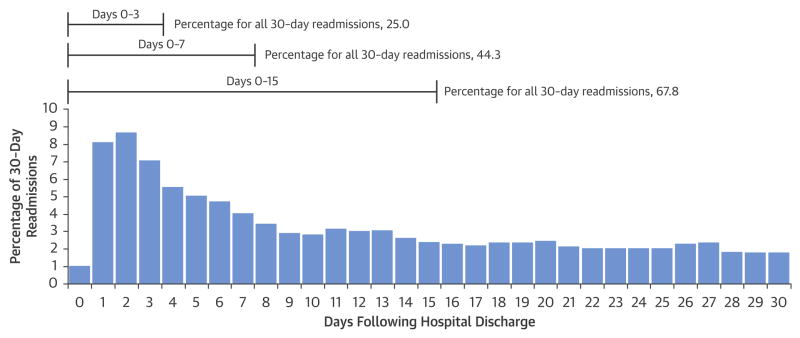
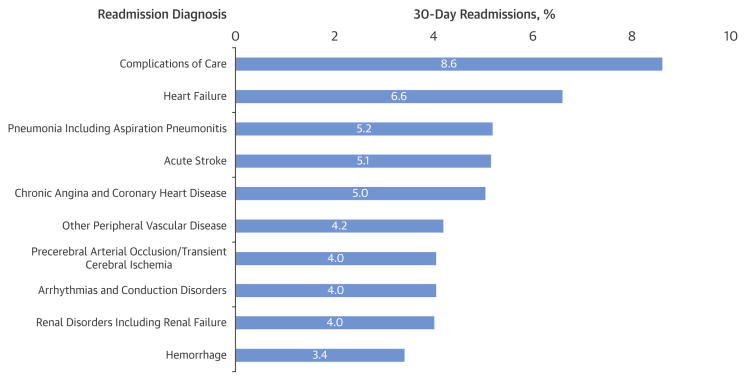
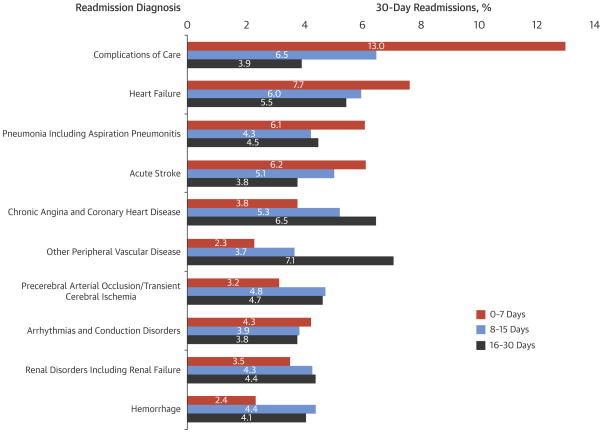
The unadjusted 30-day readmission rate following carotid revascularization was 9.6% (17,202/180,059). One quarter of all readmissions occurred within 3 days of hospital discharge, 44.3% occurred within the first 7 days of discharge, and 67.8% occurred within the first 14 days of discharge (Figure 1). The unadjusted 30-day mortality rate following carotid revascularization was 1.2% (2,247/180,059). The reasons for readmission following carotid revascularization procedures were broad (Figure 2), with the ten most common readmission diagnoses contributing only 50.0% of all 30-day readmissions. The most common reasons for readmission were complications of care (8.6%), heart failure (6.6%) and pneumonia (including aspiration pneumonitis) (5.2%). The most common complications of care included hematoma formation (2.3%), iatrogenic stroke/intracranial hemorrhage, (0.8%), and non-CNS bleeding (0.5%). Additionally, cerebral complications including ischemic stroke, TIA and cerebral hemorrhage were collectively responsible for 10.7% of readmissions. Some readmission diagnoses varied over time (Figure 3). For instance, complications of care, heart failure and acute stroke were more often encountered during the first week following hospitalization. In contrast, other peripheral vascular diseases, chronic angina and coronary artery disease were more common in the third and fourth week following hospitalization.
Figure 1. 30-day readmissions following hospitalizations for carotid revascularization procedures.
Patients undergoing carotid revascularization were disproportionately vulnerable to readmission in the first week after the hospitalization with 44.3% of all admissions occurring within one week of discharge. However, they remained at risk of readmission throughout the 30-day period.
Figure 2. Most common principal discharge diagnoses associated with readmissions following carotid interventions.
Patients were readmitted with a wide range of diagnoses with no single diagnosis contributing to more than 10% of readmissions. However, cerebral complications including ischemic stroke, TIA and cerebral hemorrhage were collectively responsible for 10.7% of readmissions.
Figure 3. The distribution of the most common diagnoses responsible for 30-day readmission over the time periods: 0–7 days, 8–15 days and 16–30 days.
Some readmission diagnoses varied over time. For instance, complications of care, heart failure and acute stroke were more often encountered during the first week following hospitalization. In contrast, other peripheral vascular diseases, chronic angina and coronary artery disease were more common in the third and fourth week following hospitalization.
Procedure-specific readmission rate: CAS vs. CEA
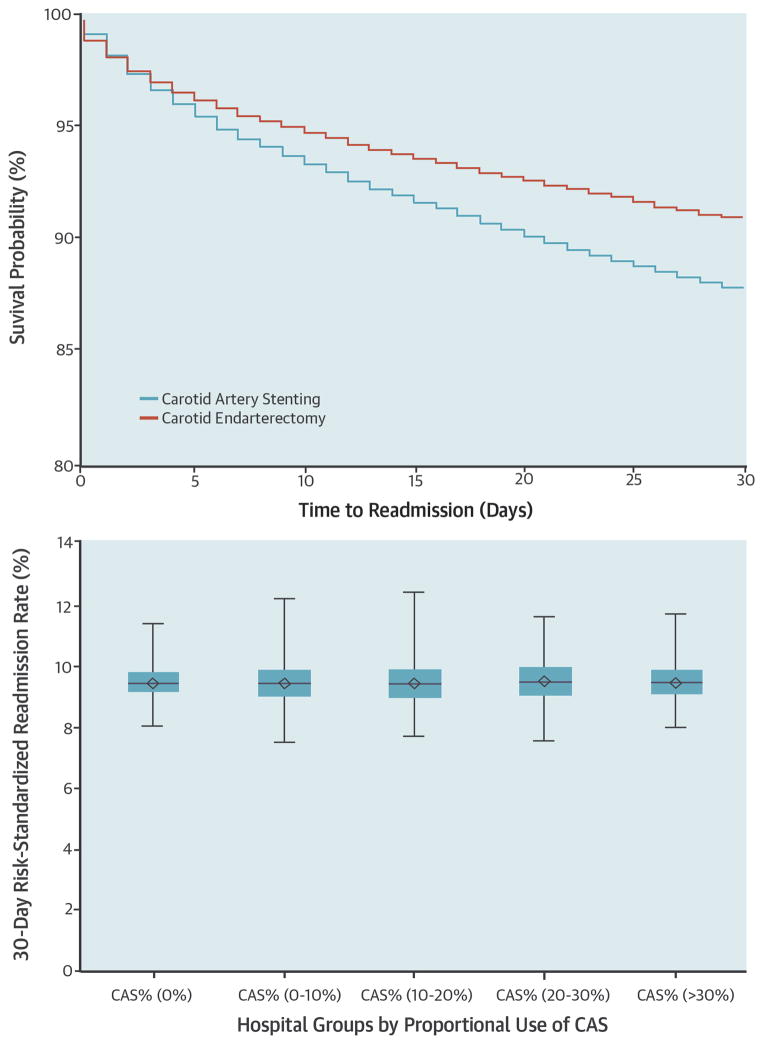
The characteristics of patients undergoing CEA and CAS procedures varied modestly (Table 1). The mean age of CAS and CEA patients was 76.1 years and 76.3 years, respectively. Male patients and Caucasian patients were more likely to undergo CEA than CAS. A higher proportion of patients undergoing CEA had diabetes, hypertension and coronary atherosclerosis (P< 0.001 for all). In contrast, a higher proportion of CAS patients had symptomatic carotid stenosis, congestive heart failure, coronary syndrome, other peripheral vascular diseases and chronic renal failure (P <0.001 for all). Crude 30-day readmission rates following CEA and CAS were 9.0% (13,222/146,831) and 12.0% (3,980/33,228), respectively. In multivariable analysis, the adjusted risk of readmission was significantly higher among patients undergoing CAS compared with patients undergoing CEA (Adjusted odds ratio: 1.13, 95% confidence interval: 1.08 – 1.18, P <0.001) (Central Illustration). Within the propensity matched subgroup, CAS patients also had significantly higher rate of readmission compared to patients undergoing CEA (Adjusted odds ratio: 1.18, 95% confidence interval: 1.07 – 1.23, P <0.001).
CENTRAL ILLUSTRATION. Readmissions after carotid artery revascularization in the Medicare population.
Risk of readmission was significantly higher among patients undergoing CAS compared with patients undergoing CEA (Upper Panel: CAS - blue line, CEA – red line). However, risk standardized readmission rate (RSRR) of hospitals that used CAS more frequently were comparable to those of hospitals that performed CAS less frequently (Lower Panel).
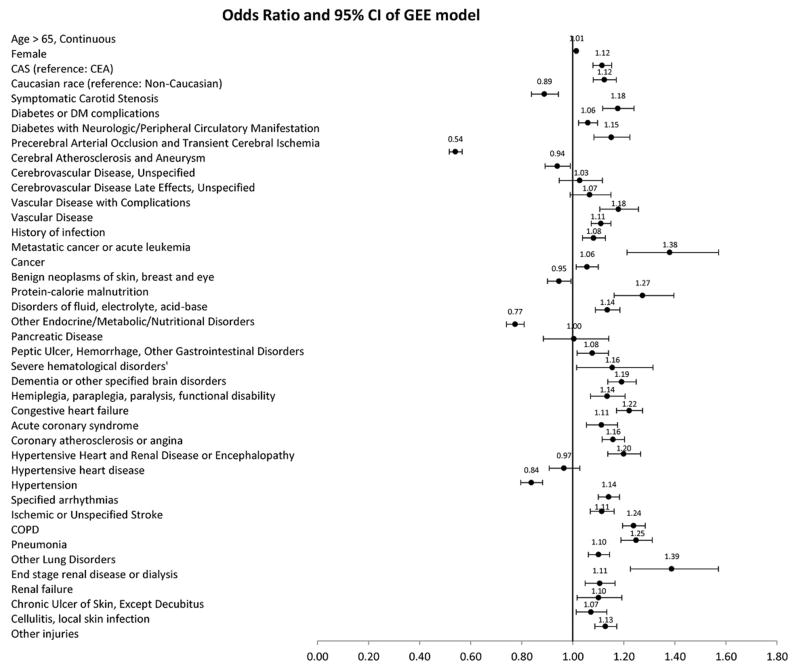
Symptomatic patients were significantly more likely to be readmitted compared with asymptomatic patients (Adjusted odds ratio: 1.18, 95% confidence interval: 1.12 – 1.24, P <0.001) (Figure 4). Other notable predictors of readmissions were age > 65 years (Adjusted odds ratio: 1.01, 95% confidence interval: 1.01 – 1.02, P <0.001), female gender (Adjusted odds ratio: 1.12, 95% confidence interval: 1.08 – 1.15, P <0.001) and Caucasian race (Adjusted odds ratio: 1.12, 95% confidence interval: 1.06 – 1.19, P <0.001).
Figure 4. Forest plot of odds ratios for risk factors associated with 30-day readmission.
A wide range of demographic characteristics and baseline comorbidities were associated with increased risk of readmission; most notably, age, female gender, Caucasian race, symptomatic carotid stenosis, diabetes, congestive heart failure, acute coronary syndrome and ischemic stroke.
In stratified analyses, compared to CEA, CAS had higher rates of readmission in both patients with symptomatic (Adjusted odds ratio: 1.24, 95% confidence interval: 1.12 – 1.37, P <0.001) and asymptomatic (Adjusted odds ratio: 1.10, 95% confidence interval: 1.05 – 1.14, P <0.001) carotid stenosis. An interaction term (symptomatic status*procedural approach) was not significant. Similarly, compared to CEA, CAS had higher rates of readmission among patients older than 80 years (Adjusted odds ratio: 1.12, 95% confidence interval: 1.04 – 1.20, P <0.001), younger than 80 years (Adjusted odds ratio: 1.12, 95% confidence interval: 1.07 – 1.18, P <0.001), male patients (Adjusted odds ratio: 1.13, 95% confidence interval: 1.07 – 1.19, P <0.001), female patients (Adjusted odds ratio: 1.13, 95% confidence interval: 1.07 – 1.20, P <0.001), Caucasian race (Adjusted odds ratio: 1.11, 95% confidence interval: 1.06 – 1.16, P <0.001) and Non-Caucasian race (Adjusted odds ratio: 1.25, 95% confidence interval: 1.10 – 1.42, P <0.001).
Hospital-specific Risk Standardized Readmission Rates (RSRRs)
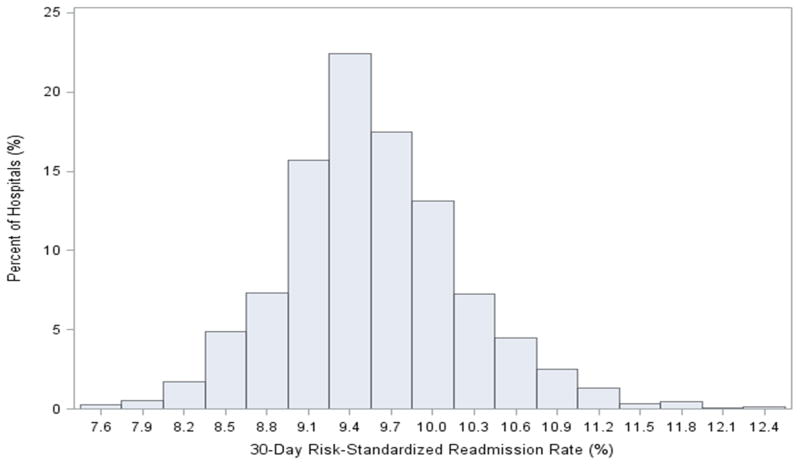
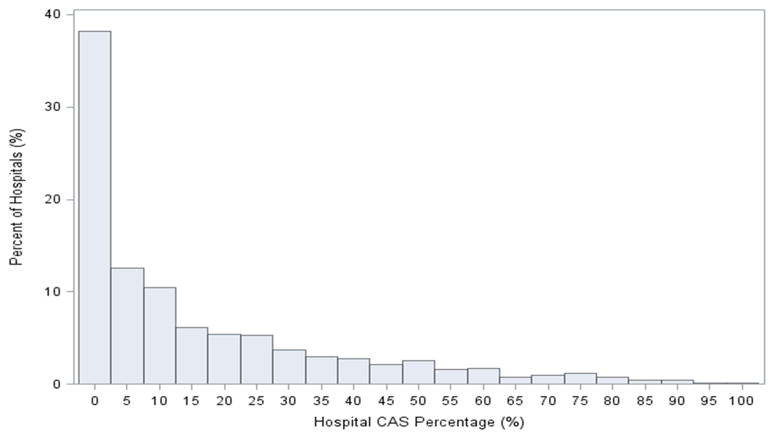
There was modest hospital-level variation in 30-day RSRRs (Figure 5). The median hospital 30-day RSRR was 9.5% with a range from 7.5% to 12.5% and interquartile range of 9.2% to 10.0%. The use of CAS varied significantly across hospitals (Figure 6). Of the 2,287 hospitals, 1,500 (65.6%) performed more than 25 carotid revascularization procedures and were included in our analysis of hospitals’ proportional use of CAS. Variation in proportional use of CAS was not associated with significant differences in hospital RSRR (Median RSRR: Group 1: 9.51%; Group 2: 9.49%; Group 3: 9.49%; Group 4: 9.52%; Group 5: 9.55%; P 0.771) (Central Illustration).
Figure 5. Distribution of 30-day hospital-specific risk standardized readmission rates (RSRR) following carotid revascularization procedures.
There was modest hospital-level variation in 30-day RSRRs (Median: 9.5%, Interquartile range: 9.2% – 10.0%).
Figure 6. Distribution of hospital proportional CAS use.
38% of the hospitals did not perform a CAS procedure, and among hospitals that performed both CEA and CAS, the proportional use of CAS varied widely.
Discussion
Among Medicare patients undergoing carotid revascularization, 9.6% were readmitted within 30 days of discharge (2,6–9). A high proportion of readmissions occurred within the first 7 days of hospital discharge, and readmissions were associated with a wide range of principal diagnoses. Furthermore, we observed that patients undergoing CAS were at higher risk of readmission compared with patients undergoing CEA. Yet, hospitals performing a higher proportion of revascularization via CAS did not have higher hospital 30-day RSRRs.
CMS has indicated its intent to publicly report hospitals’ 30-day readmission rates for patients undergoing vascular procedures, which includes carotid revascularization procedures (3). A measure of vascular readmissions has been submitted to the National Quality Forum for evaluation, and in the future, hospitals with higher than expected readmissions may be subject to payment penalties (3,10). In this context, our analyses of hospital RSRR have important implications. We have characterized both current national performance and the extent of hospital variation in readmissions following carotid revascularization. This information provides a benchmark to which hospitals can evaluate efforts to reduce readmission rates in this population.
We found that although patients undergoing carotid revascularization were disproportionately vulnerable to readmission in the first week after the hospitalization, they remained at substantial risk of readmission throughout the 30-day period. Furthermore, patients were readmitted with a wide range of diagnoses, including cardiovascular, pulmonary, neurological and renal disorders, as well as complications of care – with no single diagnosis contributing to more than 10% of readmissions. Indeed the ten most common readmission diagnoses were collectively responsible for only half of readmissions. Yet, almost one third of readmission diagnoses were potentially due to procedural complications including cerebral events (10.7%), complication of care (8.6%), acute coronary syndrome (5.0%), and arrhythmias (4.0%). These may represent high yield areas for targeted efforts to reduce readmission. However, the broad range of diagnoses is consistent with the theory that patients experience a period of increased vulnerability to a variety of illnesses following hospital discharge (11,12). As such, interventions targeting a specific readmission diagnosis or time period may be relatively ineffective at reducing readmission rates. Instead, more general strategies leading to improvement in discharge planning, medication reconciliation and early follow up after discharge may better address underlying vulnerabilities in the transitional care process (13).
In our sample of Medicare patients, the readmission rate attributed to stroke and myocardial infarction was 0.5% (883/180,059) and 0.5% (861/180,059), respectively. These rates are lower than rates of similar events reported by comparable randomized controlled trials (Stroke: CREST: 3.2%, SAPPHIRE: 3.3%; Myocardial Infarction: CREST: 1.7%, SAPPHIRE: 4.2%) (1,14). However, direct comparison are challenging given the current study included only events occurring after hospital discharge, while the numbers reported by the clinical trials included events occurring during both the hospitalization and during follow-up. Our results would suggest that the majority of these complications occur during the hospitalization and are relatively rare following discharge.
Consistent with prior investigators, we found that CAS patients were more likely to be readmitted than CEA patients (2). The difference in the risk of readmission is likely driven in part by patient selection. Currently, CEA is the mainstay treatment of symptomatic and asymptomatic carotid artery stenosis, and CAS is typically reserved for patients with comorbidities or anatomy that put them at increased risk for adverse outcomes following CEA (1,15,16). Nevertheless, the risk of readmissions for CAS was higher than CEA even after accounting for observed differences in patient characteristics. This may reflect the limitations of administrative data, but additional research examining whether the excess risk could be mitigated is warranted. CAS is still a relatively new procedure, and the excess risk of readmission may reflect the learning curve associated with incorporating CAS into routine clinical practice. Within a hospital, CAS may be performed by physicians in different specialties with a range of experience and expertise, and researchers have shown that most physicians who perform CAS are low volume operators with variable use of recommended strategies such as the use of embolic protection (17,18). Therefore, there may be an opportunity to improve care by consolidating experience within a smaller team. Such an approach could have ancillary benefits with regards to reducing readmissions by facilitating a smooth transition of care from the inpatient to the outpatient setting.
In our study, we identified a wide range of demographic characteristics (e.g. advancing age, female gender, and Caucasian race) and baseline comorbidities (e.g. symptomatic carotid stenosis, diabetes, congestive heart failure, acute coronary syndrome and ischemic stroke) associated with increased risk of readmission. These factors may serve as important markers for identifying patients vulnerable to readmission.
In our sample of hospitals, a large proportion of the hospitals did not perform a CAS procedure, and among hospitals that performed both CEA and CAS, the proportional use of CAS varied widely. Of all hospitals that performed carotid revascularization procedures, only 135 (9%) performed more CAS than CEA. Importantly, although readmissions were more common after CAS than CEA, the RSRR of hospitals that used CAS more frequently were comparable to those of hospitals that performed CAS less frequently. This observation suggests that hospitals using CAS more frequently will not necessarily be disadvantaged if and when measures of vascular readmissions are publicly reported and included into payment programs.
Our study has certain limitations. First, we used administrative claims data to identify the sample population and associated comorbidities. Such databases are subject to coding errors, coding differences across providers and institutions, and changes in coding patterns over time (19,20). The accuracy of these coding systems to identify carotid revascularization has not been reported; however, their specificity and positive predictive values to identify comorbidities in cardiovascular patients are generally reasonable (21,22). Second, administrative claims data may not be suitable to identify staged revascularization procedures, as some physicians may elect to perform the carotid repair over two or more consecutive sessions. These planned readmissions were included in our model despite being unrelated to the provided care quality and consequently, may have inflated our readmission estimates. Third, administrative claims data does not provide detailed information about the procedure itself (11,23). Other sources of data such as clinical registries may provide insights into the association of intra-procedural practices and risk of subsequent readmission. Finally, our patient cohort was not randomized to the carotid revascularization strategy and as a result, residual confounds may be present in our observed association despite the use of propensity score matching.
Conclusions
One in 10 Medicare patients undergoing carotid revascularization are readmitted within 30 days. This current study is in large part foundational, raising awareness among clinicians, hospital administrators, and policy makers regarding this issue, and illustrating the extent to which readmission rates differ by the type of revascularization procedure.
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Almost 10% of Medicare patients undergoing carotid revascularization are readmitted to hospital within 30 days of discharge for a wide range of conditions, and most occur within 7 days after discharge. Carotid artery stenting is associated with higher 30-day readmission rates than endarterectomy.
Translational Outlook
Further investigation is warranted to develop strategies that reduce the need for hospital readmission following carotid revascularizations procedures.
Abbreviations and acronyms
- CAS
Carotid Artery Stenting
- CC
Condition Category
- CEA
Carotid Endarterectomy
- CMS
Center for Medicare and Medicaid Service
- CPT
Current Procedure Terminology
- CREST
Carotid Revascularization: Endarterectomy versus Stenting Trial
- GEE
Generalized Estimating Equation
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- RSRR
Risk Standardized Readmission Rate
- SAPPHIRE
Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Trial
Footnotes
Disclosures: Dr. Curtis receives support from the Centers of Medicare and Medicaid Services to develop and maintain performance measures that are used for public reporting. Other authors have no conflicts of interest to disclose. This work was supported by grant U01 HL105270-03 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute in Bethesda, Maryland.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brott TG, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galinanes EL, et al. Evaluation of Readmission Rates for Carotid Endarterectomy Versus Carotid Artery Stenting in the US Medicare Population. Vasc Endovascular Surg. 2014;48(3):217–23. doi: 10.1177/1538574413518120. [DOI] [PubMed] [Google Scholar]
- 3.Bernheim SG, Lori Li, Shu-Xia Li, et al. Hospital 30-Day All-Cause Risk-Standardized Readmission Rate (RSRR) following Vascular Procedures - Measure Methodology report. 2011. [Google Scholar]
- 4.Brinjikji W, et al. Racial and insurance based disparities in the treatment of carotid artery stenosis: a study of the Nationwide Inpatient Sample. J Neurointerv Surg. 2014 doi: 10.1136/neurintsurg-2014-011294. [DOI] [PubMed] [Google Scholar]
- 5.Giacovelli JK, et al. Outcomes of carotid stenting compared with endarterectomy are equivalent in asymptomatic patients and inferior in symptomatic patients. J Vasc Surg. 2010;52(4):906–13. 913, e1–4. doi: 10.1016/j.jvs.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Curran T, et al. Predictors of 30-Day Readmission and Postdischarge Mortality Following Carotid Endarterectomy in the ACS-NSQIP. Journal of Vascular Surgery. 2013;57(5):93s–94s. [Google Scholar]
- 7.Gupta PK, et al. Unplanned readmissions after vascular surgery. J Vasc Surg. 2014;59(2):473–82. doi: 10.1016/j.jvs.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy BS, SP, Fortmann SP, Stafford RS. Elective and isolated carotid endarterectomy: health disparities in utilization and outcomes, but not readmission. J Natl Med Assoc. 2007;99(5):480–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Rambachan A, et al. Reasons for Readmission After Carotid Endarterectomy. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 10.(MEDPAC), M.P.A.C. Report to the Congress: Promoting Greater Efficiency in Medicare. 2007. [Google Scholar]
- 11.Dharmarajan K, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309(4):355–63. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–2. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooke BS, et al. Developing strategies for predicting and preventing readmissions in vascular surgery. J Vasc Surg. 2012;56(2):556–62. doi: 10.1016/j.jvs.2012.03.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav JS. Carotid stenting in high-risk patients: design and rationale of the SAPPHIRE trial. Cleve Clin J Med. 2004;71(Suppl 1):S45–6. doi: 10.3949/ccjm.71.suppl_1.s45. [DOI] [PubMed] [Google Scholar]
- 15.Al-Damluji MS, et al. Carotid revascularization: a systematic review of the evidence. J Interv Cardiol. 2013;26(4):399–410. doi: 10.1111/joic.12037. [DOI] [PubMed] [Google Scholar]
- 16.Brott TG, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Vasc Med. 2011;16(1):35–77. doi: 10.1177/1358863X11399328. [DOI] [PubMed] [Google Scholar]
- 17.Nallamothu BK, et al. Operator experience and carotid stenting outcomes in Medicare beneficiaries. JAMA. 2011;306(12):1338–43. doi: 10.1001/jama.2011.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perler BA, et al. Influence of age and hospital volume on the results of carotid endarterectomy: a statewide analysis of 9918 cases. J Vasc Surg. 1998;27(1):25–31. doi: 10.1016/s0741-5214(98)70288-5. discussion 31–3. [DOI] [PubMed] [Google Scholar]
- 19.Eslami MH, et al. National trends in utilization and postprocedure outcomes for carotid artery revascularization 2005 to 2007. J Vasc Surg. 2011;53(2):307–15. doi: 10.1016/j.jvs.2010.08.080. [DOI] [PubMed] [Google Scholar]
- 20.Iezzoni LI, et al. Comorbidities, complications, and coding bias. Does the number of diagnosis codes matter in predicting in-hospital mortality? JAMA. 1992;267(16):2197–203. doi: 10.1001/jama.267.16.2197. [DOI] [PubMed] [Google Scholar]
- 21.Birman-Deych E, et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 22.Kiyota Y, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148(1):99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Calvillo-King L, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med. 2013;28(2):269–82. doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.