Abstract
BACKGROUND
The receptor tyrosine kinase Axl has been reported to be overexpressed in a variety of human cancers. Although previous studies have identified the role of Axl in the transformation, proliferation, survival, and invasion in cancers, the expression and functions of Axl in pancreatic cancer have not been studied in detail.
METHODS
The expression of Axl protein in 12 pancreatic cancer cell lines and 54 patient samples of stage II pancreatic ductal adenocarcinoma (PDA) and their paired non-neoplastic pancreatic tissue samples were examined. Using univariate and multivariate analysis, Axl expression was correlated with survival and other clinicopathologic features. To examine Axl functions in PDA, the effects of Axl knockdown on the invasion ability and radiation-induced apoptosis in PDA cell lines were measured.
RESULTS
Axl was overexpressed in 38 of 54 (70%) stage II PDA samples and 9 of 12 (75%) PDA cell lines. Axl overexpression was associated with higher frequencies of distant metastasis and poor overall and recurrence-free survivals (P = .03 and P = .04, respectively) independent of tumor size and stage or lymph node status in patients with stage II PDA. Knockdown of Axl expression in PDA cells abolished Gas6-mediated Akt activation, decreased invasion, and increased radiation-induced PARP cleavage and the percentage of apoptosis.
CONCLUSIONS
This study showed that Gas6 and Axl are frequently overexpressed in PDA cells and are associated with a poor prognosis in patients with stage II PDA. Axl promotes the invasion and survival of PDA cells. Therefore, targeting the Axl signaling pathway may represent a new approach to the treatment of PDA.
Keywords: receptor tyrosine kinase, Gas6, Axl, Akt, pancreatic cancer, prognosis, invasion
Pancreatic cancer is the fourth leading cause of cancer death in the United States, preceded only by lung, colon, and breast cancers.1 Despite the available treatment modalities for pancreatic ductal adenocarcinoma (PDA), including chemotherapy, radiotherapy, surgery, or a combination of these modalities, PDA has the worst prognosis of all major malignancies, with 5-year survival of less than 5%.2 Even resectable PDA at an early stage frequently recurs either with metastatic disease in the liver, lung, or peritoneal cavity or with local recurrence in the pancreatic surgery bed. Therefore, functional studies of genetic alterations involved in its aggressive growth and metastasis are important to develop new treatment modalities for pancreatic cancer.
Increased expression of the epidermal growth factor (EGF) family of mitogenic peptides and their corresponding receptor tyrosine kinases (RTKs) related to the EGF receptor is a common feature of PDA.3 The binding of growth factors to RTKs promotes dimerization and autophosphorylation in its cytoplasmic domain and the subsequent activation of downstream signal pathways that control a variety of cellular processes, such as proliferation, differentiation, migration, and survival.4,5 Unlike other RTKs, which are activated by growth factors, Axl is a unique RTK in that it is activated by growth arrest specific factor 6 (Gas6), a member of the vitamin K–dependent proteins.6,7 Axl protein has an extracellular domain resembling cell adhesion molecules, which consists of 2 immunoglobin-like domains and 2 fibronectin III-like motifs, and contains a typical intracellular kinase domain of an RTK.6,7 Using an EGF-Axl chimeric receptor construct consisting of the extracellular and transmembrane domains of EGFR and the Axl kinase domain, Fridell et al showed that overexpression of this chimeric protein is sufficient to induce Axl’s transforming activity and tumor formation in nude mice, suggesting that Gas6-independent mechanisms of Axl functions may also exist.8
Axl was originally identified as a transforming gene in chronic myelogenous leukemia and has been reported to be overexpressed in many types of human malignancies, including diffuse glioma, melanoma, osteosarcoma, and carcinomas of the lung, colon, prostate, breast, ovary, esophagus, stomach, and kidney.9–20 Previous studies have shown that Axl signaling promotes tumor cell proliferation, migration, and invasion in these tumors. Both Axl and Gas6 were overexpressed in glioma cell lines and human glioma tissue samples and are associated with a poor prognosis in patients with glioblastoma multiforme.9 Inhibition of Axl signaling by a dominant-negative Axl mutant suppresses tumorigenesis, migration, and invasion and resulted in long-term survival of mice after intracerebral implantation of glioma cells compared with glioma cells transfected with wild-type Axl.11 Axl was also overexpressed in metastatic prostatic carcinoma cell line compared with normal prostatic epithelial cells and other prostatic carcinoma cell lines.21 In addition, Axl has been shown to be a key regulator involved in multiple steps of angiogenesis, including endothelial cell migration, proliferation, and tube formation in vitro.22 Knockdown of Axl expression not only impaired the formation of functional blood vessels in vivo but also reduced the growth of MDA-MB-231 breast carcinoma cells in a xenograft mouse model.22 These findings indicate that Axl is critical for tumorigenesis, invasion, and angiogenesis. However, the expression and functions of Axl in PDA have not been well studied. In this study, we examined the expression of Gas6 and Axl proteins in 12 PDA cell lines, 54 pancreaticoduodenectomy specimens of stage II PDA, and their paired non-neoplastic pancreatic ductal epithelial cells. Using univariate and multivariate analysis, we correlated the expression of Axl with survival and other clinicopathologic features in patients with stage II PDA. To further examine the function of Axl in PDA, we used shRNA to knock down Axl expression in PDA cell lines and measured the effect of Axl knockdown on radiation-induced apoptosis and invasion ability. Our data showed that Axl and Gas6 are frequently overexpressed both in PDA cell lines and human PDA samples and play an important role in anti-apoptosis and invasion in pancreatic cancer. Therefore, targeting Axl signaling pathway may represent a new approach for the treatment of PDA.
MATERIALS AND METHODS
Tissue Microarrays and Immunohistochemical Analysis for Axl Expression
The tissue microarrays used in this study were constructed using formalin-fixed, paraffin-embedded archival tissue blocks from 54 patients who had undergone pancreaticoduodenectomy at The University of Texas M. D. Anderson Cancer Center between 1990 and 2005 and had not received any preoperative chemotherapy and/or radiation therapy. Patients who received preoperative chemotherapy and/or radiation and who died from postoperative complications were excluded. For each patient, 2 cores of tumor and 2 cores of paired non-neoplastic pancreatic tissue were sampled from representative areas using a 1.0-mm punch. The tissue microarrays were constructed using a tissue microarrayer (Beecher Instruments, Sun Prairie, WI) as described previously.23 This study was approved by the institutional review board of the M. D. Anderson Cancer Center.
Immunohistochemical staining for Axl was performed on 4-µm unstained sections from the tissue microarray blocks. To retrieve the antigenicity, the tissue sections were treated at 100°C in a steamer containing 10 mmol citrate buffer (pH, 6.0) for 35 minutes. The sections were then immersed in 3% hydrogen peroxidase for 20 minutes to block the endogenous peroxidase activity and were incubated in 2.5% blocking serum to reduce nonspecific binding for 30 minutes. The sections were then incubated with a rabbit polyclonal antibody against Axl (Abcam Inc., Cambridge, MA) at a 1:50 dilution at 4°C overnight, washed, and then incubated with secondary antibody at room temperature for 60 minutes. Standard avidin-biotin immunohistochemical analysis of the sections was done according to the manufacturer’s recommendations (Vector Laboratories, Burlingame, CA).
Measurement of Axl Expression Using Computer-Assisted Image Analysis
The immunohistochemically stained slides of PDA tissue microarray were scanned at 200× magnification with an Ariol 2.1 scanner and digital imaging instrument (Applied Imaging, San Jose, CA). To measure Axl expression in PDA cells and non-neoplastic pancreatic ductal epithelial cells, the tumor cells or non-neoplastic pancreatic ductal cells were selected and marked with a hand-draw tool. The stromal cells, pancreatic acinar cells, and islet cells were not included in the measurement of Axl expression. The staining results were scored quantitatively based on the combined scores of intensity and percentage of cytoplasmic staining in the selected cells using an Ariol image analysis system (Applied Imaging, San Jose, CA). Cases were categorized into 2 groups based on the 25th percentile of Axl labeling score in PDA specimens: Axl high (Axl labeling score >7.56) and Axl low (Axl labeling score ≤7.56).
Cell Culture
Human pancreatic cancer cell lines Panc-1, BxPC-3, ASPC-1, and Capan-1 were purchased from the American Type Culture Collection (Manassas, VA). The Panc-48, CFPAC-1, Panc-3, Panc-28, Capan-2, MIA PaCa-2, Hs766T, and L3.6pl pancreatic cancer cells were generously provided by Dr. Paul Chiao (The University of Texas M. D. Anderson Cancer Center). All cell lines were maintained either in Dulbecco’s modified Eagle’s medium (DMEM) or in RPMI-1640 medium supplemented with 10% fetal bovine serum in a humidified incubator containing 5% CO2 at 37°C. The human pancreatic ductal epithelial (HPDE) cell line, an immortalized normal pancreatic ductal cell line, was a generous gift from Dr. Ming-Sound Tsao (Ontario Cancer Institute, Toronto, ON, Canada).
Immunoblotting and Antibodies
Antibodies against Axl, Gas6, poly(ADP-ribose) polymerase (PARP), and actin were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against Akt and phospho-Akt (Ser473) were purchased from Cell Signaling Technology (Beverly, MA). Protein expression was analyzed by 10% SDS-PAGE, which was electroblotted onto polyvinylidene fluoride membranes (Novex, San Diego, CA), blocked in 5% skim milk in 1× tris-buffered saline, and probed with the following primary antibodies against Axl, Gas6, PARP, phospho-Akt, Akt, and actin. Proteins were detected using an enhanced chemiluminescence kit (Amersham-Pharmacia Biotech, Piscataway, NJ).
Silencing Axl Expression by shRNA in PDA Cell Lines
The lentivirus-based Axl shRNA expression plasmids, pLKO.1-Axl-shRNA, were purchased from Open Biosystems Co. (Huntsville, AL). The viral particles were produced and purified by cotransfecting pLKO.1-Axl-shRNA and the lentivirus packaging plasmids (psPAX2 and PMD2.G) into HEK293T cells. The stable clones of Axl knockdown generated from Panc-1 and Panc-28 cells were selected in the culture medium containing 1.0 µg/mL puromycine. The selected stable clones were screened by Western blotting using the parental cells as control.
Response to Radiation Treatment
The effect of Axl knockdown on the response of PDA cells to radiation was measured using the PARP cleavage assay and propidium iodide–fluorescence-activated cell sorting analysis (PI-FACS). Radiation treatment was administered using a cesium 137 irradiator (Model E-0103; US Nuclear Corp., Burbank, CA) at a dose of 20 Gy for Panc-28, the Panc-28 pLKO.1 vector control cells, and the Panc-28 Axl stable knockdown cells and 30 Gy for the Panc-1 cells, Panc-1 pLKO.1 vector control cells, and Panc-1 Axl stable knockdown cells. Forty-eight hours after radiation, cells were harvested. PARP cleavage was measured by Western blotting. For PI-FACS, the cells were trypsinized, washed with cold phosphate-buffered saline, and then incubated for 30 minutes in 500 µL of hypotonic solution (0.1% sodium citrate, 0.1% Triton X-100, 100 µg/mLRNase, and 50 µg/mL PI) and analyzed by flow cytometry (Beckman Coulter Inc., Brea, CA).
Treatment of PDA Cells with EGF and Gas6
Panc-28-pLKO.1, AS4.9, and AS4.10 were subjected to serum starvation for 16 hours, and the cells were then treated with either 50 ng/mL EGF or 400 ng/mL recombinant human Gas6 (R&D Systems Inc., Minneapolis, MN) for 5, 10, and 30 minutes. The cells were harvested, and the expression of phospho-Akt and total Akt was detected by Western blotting.
In Vitro Chemoinvasion Assay
Chemoinvasion was measured using 24-well BioCoat Matrigel invasion chambers (Becton Dickinson Labware, Bedford, MA) as described previously.24 Briefly, the lower compartment contained 0.6 mL of DMEM medium with 5.0% fetal bovine serum as chemoattractants or serum-free DMEM medium as a control. In the upper compartment, 2.5 × 104 cells/well were placed in triplicate wells and incubated for 24 hours at 37°C in a humidified incubator with 5% CO2. Then the cells that passed through the filter into the lower wells were stained with Giemsa (Fisher Scientific, Orangeburg, NY) and counted under a microscope in 5 predetermined fields. All assays were repeated at least 3 times. The differences in the invasion rates between the parental PDA cells, vector control cells, and the PDA cells with stable Axl knockdown were analyzed by 2-tailed Student t tests.
Statistical Analysis
The Fisher exact test was used to compare categorical data. Overall survival and recurrence-free survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used to evaluate the statistical significance of differences. The patients’ follow-up information through August 2008 was extracted from the prospectively maintained institutional pancreatic cancer database managed in the Department of Surgical Oncology and, if necessary, updated by review of the US Social Security Index. The recurrence information was updated every time a patient came to the clinic/institution for a follow-up visit. Recurrence-free survival was calculated as the time from the date of surgery to the date of first recurrence after surgery in patients with recurrence or to the date of last follow-up in patients without recurrence. Overall survival was calculated as the time from the date of diagnosis to the date of death or to the date of last follow-up if death did not occur. The prognostic significance of clinical and pathologic characteristics was determined using univariate Cox regression analysis. Cox proportional hazards models were fitted for multivariate analysis. After interactions between variables were examined, a backward stepwise procedure was used to derive the best-fitting model. Statistical analysis was performed using Statistical Package for Social Sciences software (for Windows 12.0, SPSS Inc., Chicago, IL). We used a 2-sided significance level of P < .05 for all statistical analyses.
RESULTS
Overexpression of Axl Protein Was Associated With Poor Prognosis in Patients With Stage II PDA
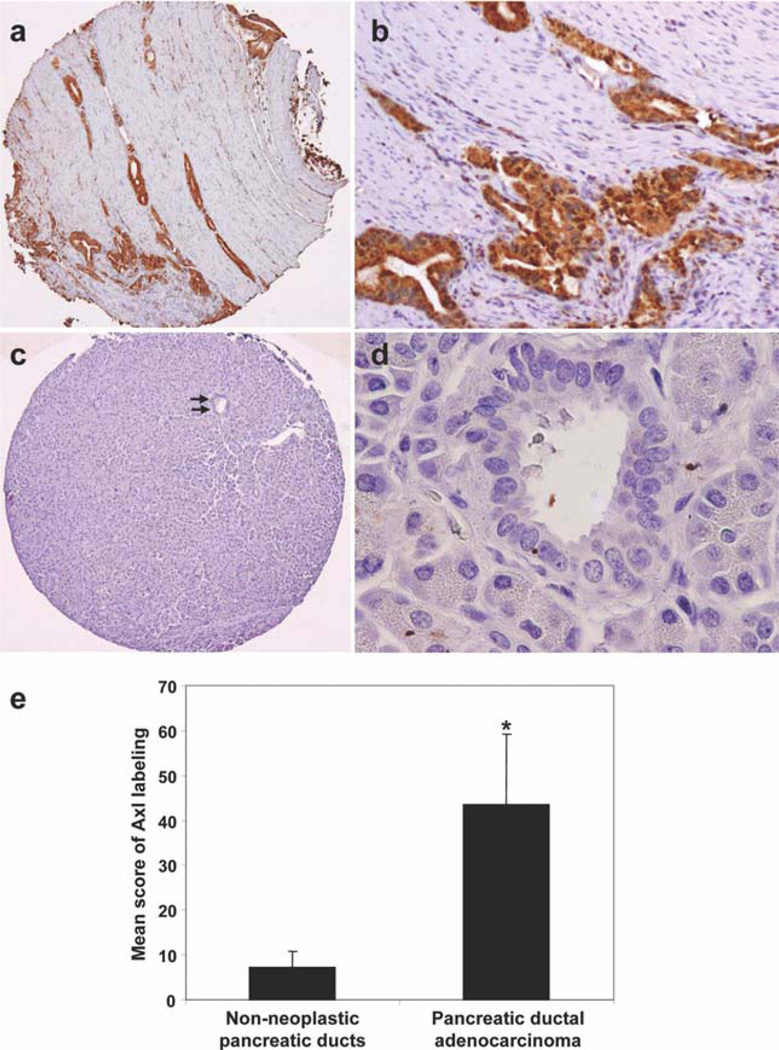
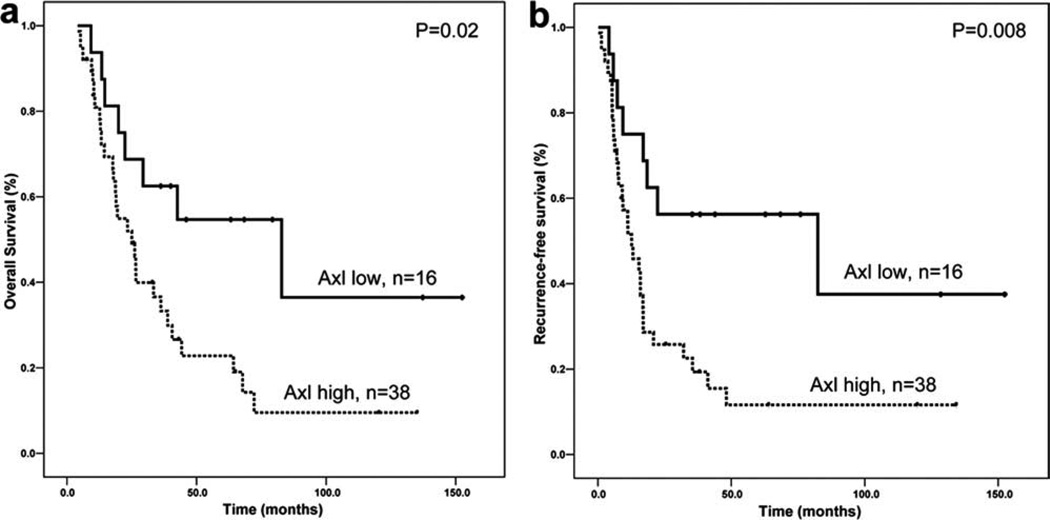
In Axl-positive PDA, the tumor cells showed diffuse cytoplasmic staining for Axl (Fig. 1a,b). Thirty-eight of 54 stage II PDAs (70%) were Axl high. Among 50 of these 54 cases, paired non-neoplastic pancreatic ductal tissue samples were available, 11 of which (22%) were Axl high. The mean Axl labeling score was 43.6 in PDA samples compared with 7.2 in non-neoplastic pancreatic ductal tissue samples (Fig. 1e). Axl expression was significantly higher in the stage II PDAs than in the paired non-neoplastic pancreatic duct samples (P = .0001). Clinicopathologic correlations of Axl expression in stage II PDAs are summarized in Table 1. According to World Health Organization classification standards, 8 cases (15%) were well-differentiated adenocarcinoma, 33 (61%) were moderately differentiated adenocarcinoma, and 13 (24%) were poorly differentiated adenocarcinoma. Of the 54 patients, 43 patients (80%) had R0 resection (defined as all surgical margins negative microscopically), 11 patients had R1 resection, and no patients had a grossly positive margin of resection. Forty-five patients (83%) underwent postoperative therapy because of recurrence or metastasis. Patients who had Axl-high PDA differed from those who had Axl-low PDA in the frequency of distant metastasis after pancreaticoduodenectomy (Table 1). Distant metastasis was present in 22 of 38 patients (58%) with Axl-high tumors compared with 4 of 16 patients (25%) with Axl-low PDA (P = .02). No association was found between Axl expression and other clinicopathologic features (Table 1). The patients whose tumors were Axl high had a worse prognosis. Median overall survival was 25.0 ± 4.3 months for patients who had Axl-high PDA compared with 82.9 ± 35.3 months for patients whose tumors were Axl low (P = .02, log-rank method; Fig. 2a). Median recurrence-free survival was 12.6 ± 3.5 and 82.4 ± 55.7 months for patients with Axl-high and Axl-low PDAs, respectively (P= .008, log-rank method; Fig. 2b). In multivariate analysis, high expression of Axl was associated with poor overall survival and recurrence-free survival (P = .03 and P = .04, respectively) independent of tumor size and lymph node status or stage (Table 2).
Figure 1.
Representative micrographs show (a and b) strong cytoplasmic staining for Axl in a moderately differentiated PDA and (c and d, arrows) no expression of Axl protein in normal pancreatic duct (original magnification, 20× for a and c, 200× for b, and 400× for d). (e) Axl expression in PDA samples and their paired non-neoplastic pancreatic ductal epithelial cells are shown. *P < .01.
Table 1.
Clinicopathologic Correlation of Axl Expression in Stage II PDAs
| Characteristics | N | Axl Low (n = 16) |
Axl High (n = 38) |
P |
|---|---|---|---|---|
| Age, y | .14 | |||
| <60 | 20 | 6 | 14 | |
| 60–70 | 22 | 9 | 13 | |
| >70 | 12 | 1 | 11 | |
| Sex | .13 | |||
| Female | 18 | 3 | 15 | |
| Male | 36 | 13 | 23 | |
| Tumor size, cm | .71 | |||
| ≤2.0 | 11 | 4 | 7 | |
| >2.0 | 43 | 12 | 31 | |
| Tumor differentiation | .24 | |||
| Well | 8 | 4 | 4 | |
| Moderate | 33 | 10 | 23 | |
| Poor | 13 | 2 | 11 | |
| Margin status | 1.00 | |||
| Negative | 43 | 13 | 30 | |
| Positive | 11 | 3 | 8 | |
| Lymph node status (stage) | .55 | |||
| Negative (IIA) | 20 | 7 | 13 | |
| Positive (IIB) | 34 | 9 | 25 | |
| Postoperative chemotherapy | .71 | |||
| No | 9 | 2 | 7 | |
| Yes | 45 | 14 | 31 | |
| Postoperative radiotherapy | .47 | |||
| No | 12 | 2 | 10 | |
| Yes | 42 | 14 | 28 | |
| Recurrence/distant metastasis | .02 | |||
| No | 14 | 8 | 6 | |
| Local | 14 | 4 | 10 | |
| Distant | 26 | 4 | 22 | |
Figure 2.
Kaplan-Meier curves for overall survival (a) and recurrence-free survival (b) by Axl expression in patients with stage II PDAs are shown.
Table 2.
Univariate and Multivariate Analysis of Overall and Recurrence-Free Survival in Patients With Stage II Pancreatic Ductal Adenocarcinomas
| Parameters | N | Overall Survival | Recurrence-Free Survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Univariate analysis | |||||
| Age, y | |||||
| <60 (reference) | 20 | 1.00 | 1.00 | ||
| 60–70 | 22 | 1.39 (0.66–2.91) | .38 | 1.19 (0.59–2.41) | .63 |
| >70 | 12 | 1.46 (0.59–3.60) | .42 | 1.40 (0.58–3.38) | .45 |
| Sex | |||||
| Female (reference) | 18 | 1.00 | 1.00 | .36 | |
| Male | 36 | 0.84 (0.43–1.66) | .62 | 0.74 (0.38–1.42) | |
| Tumor size, cm | |||||
| ≤2.0 (reference) | 11 | 1.00 | 1.00 | ||
| >2.0 | 43 | 3.25 (1.15–9.24) | .03 | 3.82 (1.35–10.82) | .01 |
| Tumor differentiation | |||||
| Well (reference) | 8 | 1.00 | 1.00 | ||
| Moderate | 33 | 1.80 (0.68–4.76) | .23 | 1.92 (0.73–5.06) | .19 |
| Poor | 13 | 1.72 (0.57–5.16) | .34 | 1.81 (0.62–5.32) | .28 |
| Margin status | |||||
| Negative (reference) | 43 | 1.00 | 1.00 | ||
| Positive | 11 | 1.18 (0.51–2.72) | .72 | 0.83 (0.36–1.88) | .65 |
| Lymph node status (stage) | |||||
| Negative (IIA) | 20 | 1.00 | 1.00 | ||
| Positive (IIB) | 34 | 2.20 (1.04–4.62) | .04 | 3.31 (1.54–7.08) | .002 |
| Postoperative chemotherapy | |||||
| No (reference) | 9 | 1.00 | 1.00 | ||
| Yes | 45 | 1.92 (0.58–6.43) | .29 | 1.50 (0.53–4.27) | .45 |
| Axl expression | |||||
| Low (reference) | 18 | 1.00 | 1.00 | ||
| High | 36 | 2.48 (1.13–5.46) | .02 | 2.79 (1.27–6.13) | .01 |
| Multivariate analysis | |||||
| Tumor size (cm) | |||||
| ≤2.0 (reference) | 11 | 1.00 | |||
| >2.0 | 43 | 3.14 (1.10–8.93) | .03 | 2.87 (0.96–8.54) | .06 |
| Lymph node status (stage) | |||||
| Negative (IIA) | 20 | 1.00 | 1.00 | ||
| Positive (IIB) | 34 | 1.22 (0.53–2.81) | .65 | 2.12 (0.95–4.71) | .07 |
| Axl expression | |||||
| Axl low (reference) | 16 | 1.00 | 1.00 | ||
| Axl high | 38 | 2.40 (1.08–5.29) | .03 | 2.36 (1.06–5.28) | .04 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Gas6 and Axl Protein Was Overexpressed in Most Pancreatic Cancer Cell Lines
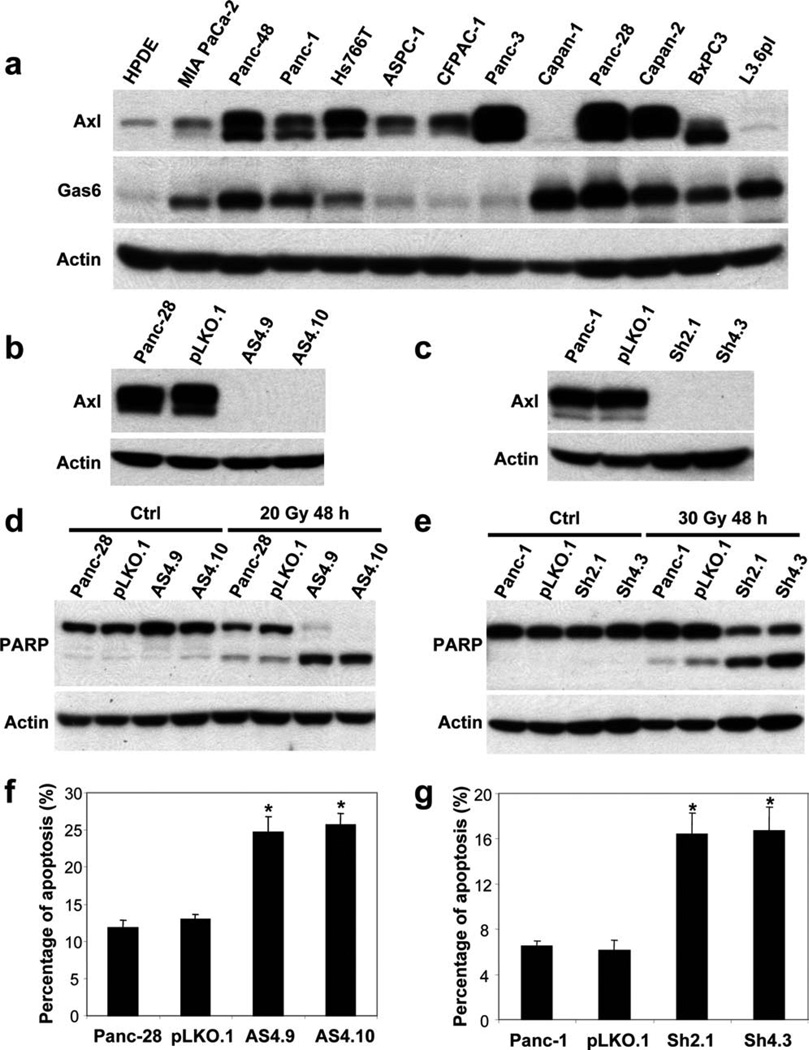
To further examine the expression of Axl in PDA, we examined Axl protein expression in 12 PDA cell lines and in HPDE cells, an immortalized normal pancreatic ductal cell line, by Western blotting. Compared with HPDE cells, Axl protein was overexpressed in 9 of 12 (75%) PDA cell lines (Fig. 3a). These results were consistent with our immunohistochemical findings that Axl was overexpressed in 70% of stage II PDA samples. To further examine the function of Axl in PDA, we examined the expression of Gas6 in the same set of PDA cell lines. We found that Gas6 was overexpressed in 9 of 12 (75%) PDA cell lines compared with that in HPDE cells (Fig. 3a).
Figure 3.
(a) Axl and Gas6 expression in PDA cell lines and HPDE cells, an immortalized normal pancreatic ductal cell line, is shown. (b and c) Effective knockdown of Axl protein expression by shRNA is shown. Axl silencing increased radiation-induced PARP cleavage (d and e) and the percentage of apoptosis by PI-FACS analyses in Panc-28 and Panc-1 cells (f and g). *P < .01.
Axl Silencing in Pancreatic Cancer Cells and Response to Radiation-Induced Apoptosis
The Gas6/Axl signaling pathway has been reported to promote cell survival in both neoplastic and non-neoplastic cells. To determine the functions of Axl, we used the shRNA approach to knockdown Axl expression in PDA cells. Axl shRNA could efficiently knockdown Axl protein expression in Panc-28 and Panc-1 cells (Fig. 3b,c). To determine whether Axl plays a role in apoptosis in PDA cells, we measured PARP cleavage and the percentage of apoptosis by PI-FACS analysis 48 hours after 20 Gy radiation in the Panc-28/Axl stable knockdown clones AS4.9 and AS4.10. Parental Panc-28 cells and Panc-28 vector control (pLKO.1) cells had very low levels of PARP cleavage. In contrast, AS4.9 and AS4.10 had markedly increased PARP cleavage after radiation (Fig. 3d). Similar results were obtained in Panc-1/Axl knockdown stable clones Sh2.1 and Sh4.3 after 30 Gy radiation. Both clones, Sh2.1 and Sh4.3, had significant increase in PARP cleavage (Fig. 3e). The percentages of apoptosis measured by PI-FACS were 24.7% and 25.7% for AS4.9 and AS4.10 cells, respectively, compared with 11.8% in Panc-28 parental cells and 13.0% in the vector control (P <.01, Fig. 3f). Similarly, Sh2.1 and Sh4.3 cells also showed increased apoptosis by PI-FACS compared with Panc-1 parental or vector control cells (Fig. 3g). These data suggested that Axl plays a role in anti-apoptosis and tumor survival in PDAs.
Axl Silencing Abolished Gas6-Mediated Akt Activation in PDA Cells
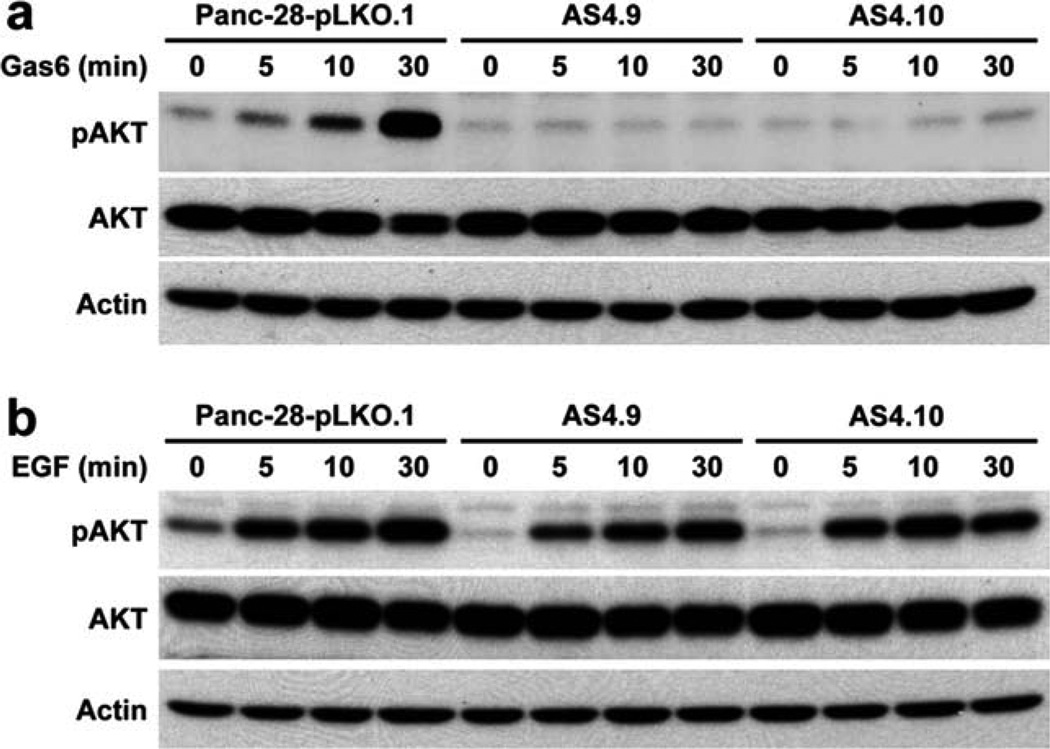
To examine the effect of Axl silencing on Gas6/Axl signaling in PDA cells, we examined the activation of Akt in Panc-28-pLKO.1 vector control cells and Panc-28 Axl stable knockdown clones AS4.9 and AS4.10 after treatment with 400 ng/mL recombinant human Gas6 for 5, 10, and 30 minutes. We found that Axl silencing by shRNA completely blocked Gas6-mediated Akt activation in Axl-knockdown clones AS4.9 and AS4.10 (Fig. 4a). On the other hand, Axl knockdown had no effect on EGF-mediated Akt activation in Axl-knockdown clones AS4.9 and AS4.10 (Fig. 4b). These data suggested that Gas6/Axl autocrine/paracrine signaling plays an important role in the survival of PDA cells.
Figure 4.
Axl silencing abolished Gas6-mediated Akt activation in Panc-28 cells. Panc-28-pLKO.1, AS4.9, and AS4.10 were treated with either 400 ng/mL recombinant human Gas6 (a) or 50 ng/mL EGF (b) for 5, 10, and 30 minutes after 16 hours of serum starvation. Phospho-Akt (pAkt) and total Akt were measured by Western blotting.
Axl Silencing Decreased Invasion Ability of PDA Cells
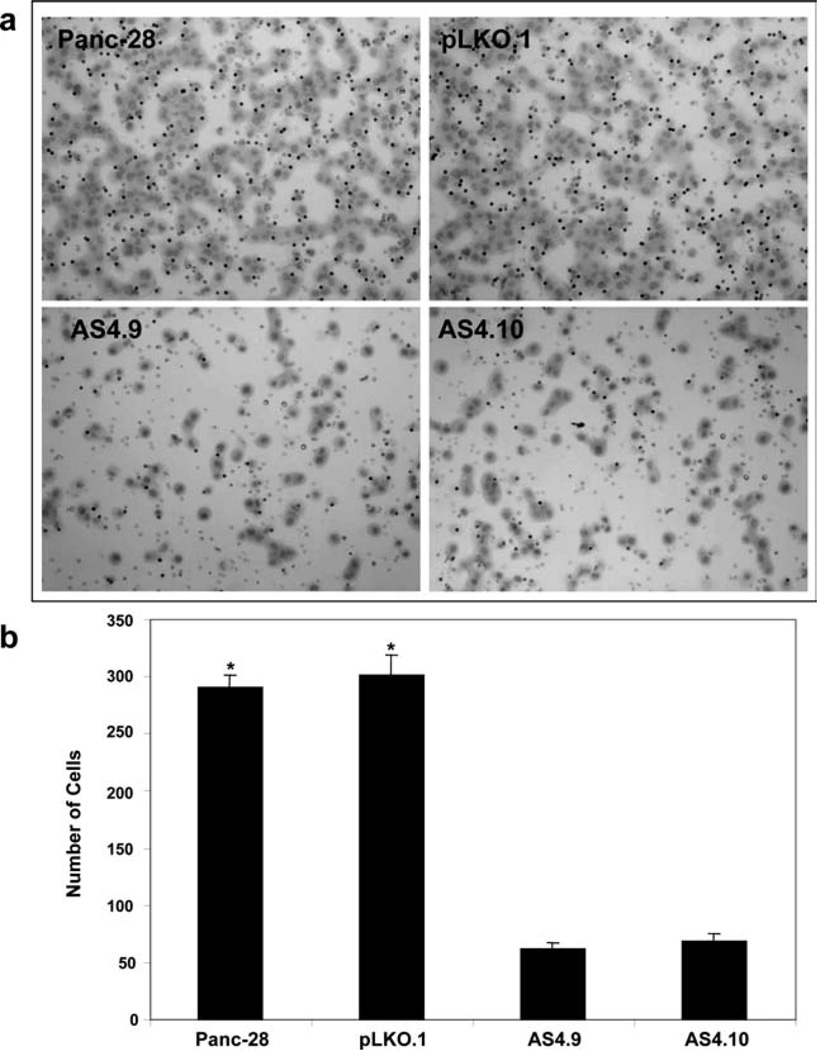
Because Axl overexpression was associated with increased frequencies of distant metastasis in patients with stage II PDAs, we tested whether Axl affected PDA invasion using a Matrigel in vitro invasion assay, with 5% fetal bovine serum used as a chemoattractant to stimulate cells to penetrate through the Matrigel and migrate through the filters. The invasion assays revealed that the knockdown of Axl expression in Panc-28 cells (AS4.9 and AS4.10 clones) markedly reduced the rates of invasion of Panc-28 cells compared with that in parental Panc-28 cells and vector control cells (P <.001; Fig. 5). Similar results were obtained in Sh2.1 and Sh4.3 Panc-1/Axl stable knockdown cells (data not shown). Thus, these data suggested that increased Axl expression in PDA cells may facilitate tumor cell invasion.
Figure 5.
Axl silencing by shRNA decreased invasion in Panc-28 cells as shown in vitro invasion assays. (a) The cells that invaded through Matrigel-coated trans-well inserts toward chemoattractant were stained with Giemsa (Photographs were taken at a magnification ×100). (b) The cells invading through the Matrigel were counted under a microscope in 5 predetermined fields at ×200. Each sample was assayed in triplicate, and assays were repeated at least twice. *P <.01.
DISCUSSION
In this study, we found that Gas6 and Axl were overexpressed in PDA cells. Axl overexpression was associated with a higher frequency of distant metastasis and poor overall and recurrence-free survival and was an independent prognostic factor for patients with stage II PDA. Knockdown of Axl expression in PDA cells abolished Gas6-mediated Akt activation and led to decreased invasion and increased radiation-induced apoptosis. Therefore, our data showed that Gas6/Axl autocrine/paracrine signaling plays an important role in invasion and antiapoptosis in pancreatic cancer.
Axl has been shown to be overexpressed in carcinomas of the lung, colon, prostate, breast, ovary, esophagus, stomach, and kidney and in other types of human malignancies, including diffuse glioma, melanoma, and osteosarcoma.9–20 Overexpression of Axl and Gas6 mRNA was associated with tumor progression and poor survival in patients with renal cell carcinoma.25 Our study was corroborated by the study of Koorstra et al, which showed that Axl protein was overexpressed in 55% of pancreatic cancers.26 However, the expression of Axl protein in benign pancreatic ductal samples was not reported in their study. We demonstrated that the expression of Axl protein was significantly higher in PDA samples than in the paired non-neoplastic pancreatic ducts. In our study, this was further confirmed by demonstrating that Axl was overexpressed in 75% of PDA cell lines compared with HPDE cells. Both studies showed that overexpression of Axl protein correlated with poor overall survival. However, we found that Axl overexpression correlated not only with poor overall survival, but also poor recurrence-free survival in patients with stage II PDAs. In addition, we performed multivariate analysis and showed that Axl overexpression was a prognostic factor for overall and recurrence-free survival independent of lymph node status (stage) and tumor size in patients with stage II PDAs. These findings are important because the majority of patients with surgically resectable PDAs have stage II disease, and little is known about the prognostic markers in this group of patients.
Axl knockdown reduces the growth of lung and breast cancer in orthotopic mice models.27 Similarly, down-regulation of Axl using a monoclonal antibody attenuates the growth of non–small cell lung carcinoma xenografts by reducing cell proliferation and inducing apoptosis.27 More recently, overexpression of Axl has been shown to be involved in the acquired resistance to lapatinib in Her2-positive, estrogen receptor-positive breast cancer cells28 and to play an essential role in breast cancer metastasis and survival associated with epithelial-to-mesenchymal transition.29 To further examine the function of Axl in pancreatic cancer, we found that knockdown of Axl abolished Gas6-mediated Akt activation and led to a significant decrease in invasion and increase in radiation-induced apoptosis in PDA cells. These results not only suggested that Gas6/Axl signaling plays an important role in tumor cell invasion and survival in pancreatic cancer, but also supported our finding that Axl overexpression correlated with distant metastasis after pancreaticoduodenectomy in patients with stage II PDA. Consistent with our results, Axl has been shown to be involved in invasion and tumor cell survival in human glioma, breast cancer, lung cancer, and malignant melanoma.11,20,26,30 Knockdown of Axl or inhibition of Axl phosphorylation using a Src/Abl inhibitor, bosutinib (SKI-606), significantly reduced the invasion and motility of breast cancer cells.20 One possible mechanism of Axl-mediated tumor cell invasion is that it is a result of the up-regulation of matrix metalloproteinase 9 at the transcriptional level, which involves the activation of NFκB, the Ras/Raf extracellular signaling-regulated kinase cascade, and the chromotin remodeling factor, brahma-related gene-1.31 Using a human pancreatic tumor xenograft model, Messersmith et al recently showed that 3 of 15 patient tumors were sensitive to bosutinib, which has been shown to inhibit Axl phosphorylation, cell motility, and invasion.32 Therefore, the ability to inhibit Axl and its downstream signaling pathways may offer new potential avenues for the development of novel therapeutic strategies for pancreatic cancer.
In conclusion, our study demonstrated that Gas6 and Axl are overexpressed in PDA cells. High level of Axl expression is associated with a higher frequency of distant metastasis after pancreaticoduodenectomy and poor prognosis and is an independent prognostic marker for stage II PDA. In addition, we showed that Gas6/Axl signaling plays an important role in the invasion and survival of PDA cells. Therefore, targeting Axl signaling pathway may represent a novel target for the treatment of pancreatic cancer.
Acknowledgments
We thank Dr. Yue Chen for her valuable technical support and Drs. Jiansheng Wang and Xiaoyi Duan for critically reviewing the article and providing valuable comments.
CONFLICT OF INTEREST DISCLOSURES
Supported by the AACR—Pancreatic Cancer Action Network Career Development Award in Pancreatic Cancer Research and Institutional Research Grant from The University of Texas M. D. Anderson Cancer Center.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri BA, Huang L, Berger D, et al. Molecular pathology of primary and metastatic ductal pancreatic lesions: analyses of mutations and expression of the p53, mdm-2, and p21/WAF-1 genes in sporadic and familial lesions. Cancer. 1997;79:700–716. [PubMed] [Google Scholar]
- 4.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 5.Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 6.Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki T, Knyazev PG, Clout NJ, et al. Structural basis for Gas6-Axl signalling. EMBO J. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridell YW, Jin Y, Quilliam LA, et al. Differential activation of the Ras/extracellular-signal-regulated protein kinase pathway is responsible for the biological consequences induced by the Axl receptor tyrosine kinase. Mol Cell Biol. 1996;16:135–145. doi: 10.1128/mcb.16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutterer M, Knyazev P, Abate A, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 10.O’Bryan JP, Frye RA, Cogswell PC, et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11:5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vajkoczy P, Knyazev P, Kunkel A, et al. Dominant-negative inhibition of the Axl receptor tyrosine kinase suppresses brain tumor cell growth and invasion and prolongs survival. Proc Natl Acad Sci U S A. 2006;103:5799–5804. doi: 10.1073/pnas.0510923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung BI, Malkowicz SB, Nguyen TB, Libertino JA, McGarvey TW. Expression of the proto-oncogene Axl in renal cell carcinoma. DNA Cell Biol. 2003;22:533–540. doi: 10.1089/10445490360708946. [DOI] [PubMed] [Google Scholar]
- 13.Craven RJ, Xu LH, Weiner TM, et al. Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer. 1995;60:791–797. doi: 10.1002/ijc.2910600611. [DOI] [PubMed] [Google Scholar]
- 14.Meric F, Lee WP, Sahin A, Zhang H, Kung HJ, Hung MC. Expression profile of tyrosine kinases in breast cancer. Clin Cancer Res. 2002;8:361–367. [PubMed] [Google Scholar]
- 15.Nakano T, Tani M, Ishibashi Y, et al. Biological properties and gene expression associated with metastatic potential of human osteosarcoma. Clin Exp Metastasis. 2003;20:665–674. doi: 10.1023/a:1027355610603. [DOI] [PubMed] [Google Scholar]
- 16.Nemoto T, Ohashi K, Akashi T, Johnson JD, Hirokawa K. Overexpression of protein tyrosine kinases in human esophageal cancer. Pathobiology. 1997;65:195–203. doi: 10.1159/000164123. [DOI] [PubMed] [Google Scholar]
- 17.Shieh YS, Lai CY, Kao YR, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7:1058–1064. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ginkel PR, Gee RL, Shearer RL, et al. Expression of the receptor tyrosine kinase Axl promotes ocular melanoma cell survival. Cancer Res. 2004;64:128–134. doi: 10.1158/0008-5472.can-03-0245. [DOI] [PubMed] [Google Scholar]
- 19.Wu CW, Li AF, Chi CW, et al. Clinical significance of AXL kinase family in gastric cancer. Anticancer Res. 2002;22:1071–1078. [PubMed] [Google Scholar]
- 20.Zhang YX, Knyazev PG, Cheburkin YV, et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008;68:1905–1915. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 21.Jacob AN, Kalapurakal J, Davidson WR, et al. A receptor tyrosine kinase, UFO/Axl, and other genes isolated by a modified differential display PCR are overexpressed in metastatic prostatic carcinoma cell line DU145. Cancer Detect Prev. 1999;23:325–332. doi: 10.1046/j.1525-1500.1999.99034.x. [DOI] [PubMed] [Google Scholar]
- 22.Holland SJ, Powell MJ, Franci C, et al. Multiple roles for the receptor tyrosine kinase axl in tumor formation. Cancer Res. 2005;65:9294–9303. doi: 10.1158/0008-5472.CAN-05-0993. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Wang H, Shen W, et al. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315–4321. [PubMed] [Google Scholar]
- 25.Gustafsson A, Martuszewska D, Johansson M, et al. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res. 2009;15:4742–4749. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- 26.Koorstra JB, Karikari CA, Feldmann G, et al. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol Ther. 2009;8:618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Ye X, Tan C, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–3455. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Greger J, Shi H, et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009;69:6871–6878. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 29.Gjerdrum C, Tiron C, Hoiby T, et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc Natl Acad Sci U S A. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee WP, Liao Y, Robinson D, Kung HJ, Liu ET, Hung MC. Axl-gas6 interaction counteracts E1A-mediated cell growth suppression and proapoptotic activity. Mol Cell Biol. 1999;19:8075–8082. doi: 10.1128/mcb.19.12.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW. Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene. 2008;27:4044–4055. doi: 10.1038/onc.2008.57. [DOI] [PubMed] [Google Scholar]
- 32.Messersmith WA, Rajeshkumar NV, Tan AC, et al. Efficacy and pharmacodynamic effects of bosutinib (SKI-606), a Src/Abl inhibitor, in freshly generated human pancreas cancer xenografts. Mol Cancer Ther. 2009;8:1484–1493. doi: 10.1158/1535-7163.MCT-09-0075. [DOI] [PubMed] [Google Scholar]