Abstract
Neurological symptoms are very common in children with life-limiting conditions and are challenging in terms of burden of illness. Moreover, neurological symptoms can significantly impact the child’s quality of life and contribute to distress among parents, families, caregivers and health care providers. Knowing how to manage and alleviated these symptoms is essential for providing good palliative care. In the present article, the more common neurological symptoms of agitation/irritability, spasticity and dystonia will be reviewed. The aim of the present brief review is to provide a basic approach to both the identification and treatment of these neurological symptoms. A medication table is provided for quick reference. A brief commentary and guidance for the management of pain are also incorporated, with reference to further literature sources.
Keywords: Agitation, Dystonia, Irritability, Paediatrics, Palliative care, Spasticity
Abstract
Les symptômes neurologiques sont très courants chez les enfants ayant des problèmes limitant l’espérance de vie, et le fardeau de la maladie est difficile à prendre en charge. De plus, les symptômes neurologiques peuvent avoir des effets marqués sur la qualité de vie de l’enfant et contribuer à la détresse des parents, des familles, des soignants et des dispensateurs de soins. Il est essentiel de savoir comment prendre en charge et soulager ses symptômes pour offrir de bons soins palliatifs. Dans le présent article, les symptômes neurologiques plus courants d’agitation ou d’irritabilité, de spasticité et de dystonie sont abordés. L’analyse qui y est présentée vise à proposer une conduite de base pour déterminer et traiter ces symptômes neurologiques. Un tableau de médicaments facilite la consultation. Un bref commentaire et des conseils sur la prise en charge de la douleur sont également proposés, de même que des références vers d’autres publications.
CASE PRESENTATION
A 22-month-old boy is referred to you for episodic body arching and screaming. The episodes occur daily and cluster, lasting for 5 min to 20 min at a time, with little relief in between. He has not slept well for weeks. His complex medical history includes severe neonatal hypoxic-ischemic encephalopathy, cortical visual impairment, seizures and gastroesophageal reflux; the latter two conditions are being pharmacologically treated. Pain assessment has been performed using the Non-Communicating Children Pain Checklist – Revised (NCCPC-R [1]) to monitor pain intensity, and thorough investigations have ruled out hidden sources of pain (Figure 1). Pain medications, including gabapentin, have been tried to treat the episodes, without significant improvement. During the episodes, he is in significant distress. His entire body arches, he is diaphoretic and cries inconsolably. His vital signs are within normal ranges except for a heart rate of 160 beats/min; he is so irritable that the physical examination is nearly impossible. His parents are distressed; they hope that you can identify a cause for his discomfort and determine a treatment approach that will restore quality of life. How would you assess and treat this young child?
INTRODUCTION
Symptom burden can be high in children with life-limiting conditions, especially those with neurodegenerative conditions and impairment of the central nervous system (2,3). Neurological symptoms are difficult in terms of quality of life for the child, and also contribute significantly to distress among parents, families and health care providers (2,3). Knowing how to manage and alleviated these symptoms is essential for providing good palliative care.
Neurological symptoms are varied and include seizures, agitation/irritability, spasticity/muscle spasms, and movement disorders such as dystonia, chorea and myoclonus. The underlying etiology for these symptoms is generally multifactorial, and overlap of many symptoms at once is common. Beyond identifying the specific symptom at hand, it is also essential to undertake a comprehensive and meticulous exploration of the differential diagnosis to clarify the underlying etiology. That said, exploring the numerous possible etiologies for each neurological symptom is beyond the scope of the present article. Rather, the present article will provide a basic approach for certain neurological symptoms and then focus on management. It will specifically focus on agitation/irritability, spasticity and dystonia.
The present article is aimed toward use by general practitioners and paediatricians. It should be noted that many of these clinical scenarios are highly complex, and involvement of paediatric palliative care specialists and neurologists should be considered. Further consultation with neurosurgery, physiotherapy, occupational therapy, dentistry and psychiatry/psychology may also be considered. Given the challenging nature of these neurological symptoms, working with a collaborative multidisciplinary team is recommended.
GENERAL APPROACH
Several issues specific to the paediatric population need to be considered, the first being the variable age and stage of development. Not only will the expected symptoms differ according to the age and development of a child, but the child’s perception of these symptoms and the consequential affect on their quality of life may also vary (4). Furthermore, children with developmental delay and cognitive disability may express and experience symptoms differently (3,4). Second is the uncertainty of the evolution and prognosis for many of the rare neurodegenerative diseases encountered in paediatric palliative care (3). Third, it is important to remember that several neurological symptoms can coincide, as can other ‘non-neurological’ symptoms such as pain or signs of sepsis. Therefore, it can be very difficult to determine which symptom is the most bothersome and requires treatment. Pain is a notable example because it can both result from and exacerbate neurological symptoms. Although the present article does not focus specifically on pain, identifying and treating any hidden sources of pain should be a priority when caring for a child with challenging neurological symptoms (Figure 1). Finally, there is very little research documenting the incidence, presentation and management of neurological symptoms in paediatric palliative care (3–5). Therefore, one needs to rely on their clinical experience and the experience of their colleagues to help guide management. Often, a time-limited trial of medication is necessary to uncover what symptom is most problematic; frequent reassessments are important with consultation with specialists if the first-line treatments are unsuccessful. New or increased neurological symptoms may indicate a change or progression of a child’s condition; it is important to discuss any proposed intervention in the global context of care for the individual child.
Figure 1).
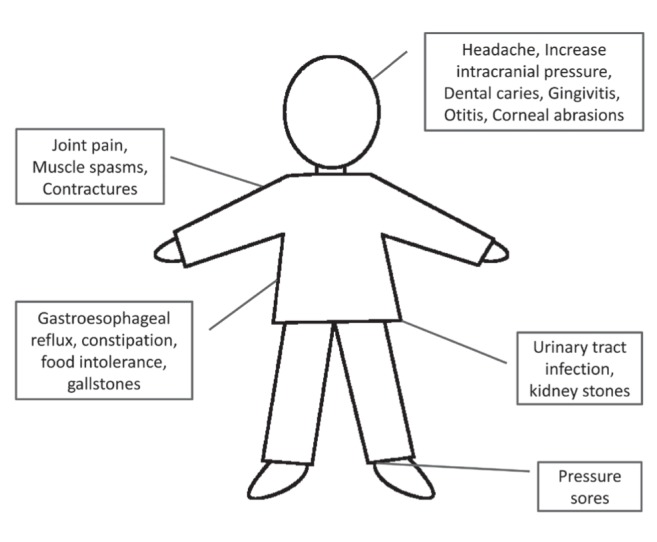
Sources of hidden pain in children with neurological impairment.
As a general approach to neurological symptomatology, we recommend that the first question to ask is, “What is this symptom?”. It is essential to obtain as detailed a history as possible. These days, technology can be of great support; asking caregivers to provide a video of an episode can help describe the complex symptomatology. Again, there may be more than one symptom at play, which is important to remember. Once the exact symptoms have been determined, the next question to ask is, “Are there provoking factors and, if so, what are they?”. Common provoking factors include pain, dyspnea, infections, constipation, gastroesophageal reflux and dehydration. Asking parents to keep a diary is a useful tool to identify potential triggers. Subsequently, a thorough medication review is necessary, often with the assistance of a pharmacist, to identify medications with potential adverse neurological effects. Once the precise symptoms have been identified, investigated and all provoking factors addressed, targeted treatment must be contemplated and implemented for persistent symptoms.
Agitation/irritability, spasticity and dystonia will now be reviewed in more detail (Tables 1 to 3); for each section, please refer to the attached table for pertinent medication details (Table 4).
TABLE 1.
Approach to agitation/irritability
| Thought-provoking questions | Elements to consider |
|---|---|
| Is this agitation/irritability? |
|
| What are the provoking factors? |
|
| Are medications contributing? |
|
TABLE 3.
Approach to dystonia
| Thought-provoking questions | Elements to consider |
|---|---|
| Is this dystonia? |
|
| What are the provoking factors? |
|
| Are medications contributing? |
|
TABLE 4.
| Starting dose | Maximum dose | Plasma half-life, h | Most common side effects | Drug interactions | Contraindications | |
|---|---|---|---|---|---|---|
| Agitation | ||||||
|
| ||||||
| Gabapentin | 3–12 y: day 1: 5 mg/kg/dose PO at bedtime; day 2: 5 mg/kg/dose PO BID; day 3: 5 mg/kg/dose PO TID. After day 3: titrate every 2–3 days over 2–3 weeks as tolerated. ≥12 y: start at 100 mg TID, titrate to effect with increase of 300 mg/day. Can titrate faster or slower depending on tolerance and pain severity | Children: Usually 35–50 mg/kg/24 h divided in 3 doses. Adults: 3600 mg/day | 5–7 | Somnolence and fatigue and dizziness early on, tend to improve with time | Wait at least 2 h after antacids | Dose adjustment in renal disease |
| Clonidine | Day 1–3: 0.002 mg/kg PO at bedtime; day 4–6: 0.002 mg/kg PO BID; day 7–9: 0.002 mg/kg PO TID. Can titrate faster as tolerated | Max starting dose: 0.1 mg (50 kg) Max dose: 0.012 mg/kg/dose (0.6 mg, for 50 kg) TID |
5–25 | Decreased blood pressure, somnolence | Tricyclics (decrease effect), methylphenidate. Need to be careful with any medications lowering blood pressure or heart rate | Bradyarrhythmia, low blood pressure, galactose intolerance. Need to be careful with renal disease |
| Diazepam | 6 m and older: 0.12–0.8 mg/kg/24 h PO divided 3–4 times/day 0.04–0.3 mg/kg/dose IV every 2–4 h PR route also possible. Cannot be given SC | 0.6 mg/kg IV within an 8 h period | 24–48 | Drowsiness, ataxia, hypotonia, muscle flacidity, paradoxical reaction | Metabolized via Cytochrome P450 group of liver enzymes | Acute/severe pulmonary insufficiency, sleep apnea syndrome, severe liver disease, myasthenia gravis |
| Midazolam (continuous infusion IV/SC) | Neonatal: 1 μg/kg/min; 1 m–18 y: 50–300 μg/kg/h PO/buccal also possible | 6 m–5 y: 6 mg; 6–12 y: 10 mg | 2–5 | Drowsiness, paradoxical reaction | Other substrates of CYP3A4. | Careful with liver/renal disease |
| Lorazepam | Infants and children: 0.02–0.1 mg/kg/dose PO/SL/IV/SC every 4–8 h; | 2 mg/dose | 10–20 | Drowsiness, impaired psychomotor skills, lightheadedness, paradoxical reaction | Valproic acid, carbamazepine, rifampin | Mania |
| Haloperidol | Oral: <12 y: 10–20 μg/kg every 8–12 h; 12–18 y: 1.5 mg 3 times/day IV; 1 m to 12 y: 25–85 μg/kg/24 h; 12–18 y: 1.5–5 mg/24 h | 13–35 | Extrapyramidal effects, sedation | Carbamazepine (decrease plasma concentration of haloperidol) | Long QT | |
| Dimenhydrinate | Children over 2 years: 5 mg/kg/24 h PO/IV/PR divided q6h; 6–12 years: 25–50 mg/dose PO q6–8 h | 75 mg/24 h PO 150 mg/24 h PO 300 mg/24 h IV | Drowsiness, dizziness, paradoxical excitation (younger children) | Glaucoma | ||
| Methotrimeprazine | Data for SC – but has been used IV anecdotally. 1–12 y: 0.35–3 mg/kg/24 h; 12–18 y: 12.5–200 mg/24 h | 15–>30 | Drowsiness, postural hypotension, antimuscarinic effects | Parkinson, epilepsy (in some cases), hypothyroidisn, myasthenia gravis, antihypertensive medications | ||
| Chloral hydrate | 25–50 mg/kg/24 h PO/PR divided q6–8 h | 500 mg/dose | 8 | Gastric irritation, vomitting; sedation, respiratory depression | Warfarin | Severe renal/hepatic dysfunction |
| Spasticity | ||||||
|
| ||||||
| Diazepam | See above | |||||
| Tizanidine | 18 m–7 y: 1 mg/day 7–12 y: 2 mg/day; ≥12 y: 2 mg/day | 36 mg/day (for ≥12 y) | 2.5 | Drowsiness, weakness, dry mouth, hypotension, dizziness | Dygoxin or hypotensive drugs, | Long QT, Careful with liver/renal disease |
| Baclofen | 1–10y: 0.3 mgkg/day in 4 doses; 10–18y: 5 mg 3 times/day | Max initial dose: 2.5 mg/max 100 mg/day (at maintenant) | 3.5 | Sedation, drowsiness, hypotonia, nausea, urinary frequency or incontinence, dysuria | Peptic ulceration, severe psychiatric disorders, epilepsy, respiratory impairment, diabetes mellitus, urinary retention, careful with liver/renal disease | |
| Dantrolene | 0.5 mg/kg/dose PO once to twice daily | 400 mg/24 h | Approximately 9 | Drowsiness, dizziness, muscle weakness, diarrhea | Careful with other hepatotoxic drugs | Active hepatic disease |
| Dystonia | ||||||
|
| ||||||
| Diazepam | See above | |||||
| Trihexyphenidyl | Average initial dose 0.095 mg/kg/day Adults: 2–5 mg 1–3 times/day | Anticholinergic effects, drowsiness, dizziness | Anticholinergics, cholinesterase inhibitors, potassium chloride | Glaucoma, myasthenia gravis | ||
| Benztropine | 0.02–0.05 mg/kg/dose IV/PO daily-twice a day | 1–4 mg/dose PO/IV daily – twice a day | Anhidrose, hyperthermia, illeus, gastroesophagial reflux, dry pulmonary secretions | Anticholinergics, cholinesterase inhibitors, potassium chloride | Glaucoma, pyloric or duodenal obstruction, or myasthenia gravis | |
| Nitrazepam | 0.3–1 mg/kg/24 h PO divided in 3 doses/day | 30 | Drowsiness, impaired psychomotor skills, hypotonia, bronchial hypersecretion | Difficulty managing oral secretions, careful with liver/renal disease | ||
| Tetrabenazine | No established paediatric dosing. Adults: 12.5–25 mg 2–3 times/day | Hypotension | CYP2D6 Inhibitors, levodopa, antidepressants and monoamine oxidase inhibitors, reserpine | Long QT, depression, careful with liver impairment | ||
| Carbidopa-levodopa | No established paediatric dosing. Adults: one tablet of 100/25 3 times a day | 1500 mg levodopa per day | 1.4 | Dyskinesias, nausea, mental changes | Antipsychotics, dopamine-depleting agents, iron, methoclopramide, isoniazid | Antihypertension medications (risk of postural hypotension) |
The initial dosages may differ according to the specific situation. Always verify dosages with a pharmacist if uncertain. Most medications mentioned should not be stopped abruptly. BID Twice daily; IV Intravenous; m Months of age; PO Per os (orally, nasogastric tube or g-tube); PR Per rectum; TID Three times daily; SC Subcunaneous; SL Sublingual; y Years of age
AGITATION/IRRITABILITY
According to the Oxford Dictionary, agitation is a state of anxiety or nervous excitement (6). Medically, agitation or irritability is used to describe unpleasant psychological and physical arousal, often in circumstances in which the underlying etiology remains unclear. It refers to a complex set of symptoms and signs that are distressing to the patient, their family and caregivers. Agitation/irritability may consist of psychological symptoms (anxiety, anger, irritability), physical symptoms (restlessness, hypermotor activity, crying, angered speech, disturbed sleep patterns) and autonomic changes (tachycardia, tachypnea, increased blood pressure, diaphoresis) (7,8).
Once agitation/irritability is identified and explored, it is important to treat all underlying provoking factors. If agitation/irritability persists, it is then time for directed symptom management. The first approach is with nonpharmacological interventions such as environmental control (limiting visitors, maintaining a tranquil environment), soothing and calming practices (playing music, reading stories), and using available therapies (psychology, play therapy, music therapy) (8). The next step is pharmacological management. There is little direct evidence as to what medications work best for agitation/irritability in paediatrics, but many medications are available (4,7,8).
Agitation/irritability and neuropathic (central) pain can be difficult to delineate because they may represent identical symptomatology. For this reason, neuropathic pain medications should be considered and tried. Gabapentin is a safe first-line treatment due to few medication interactions and very few side effects, although minor temporary sedation is possible (3,9). Pregabalin, a similar medication, is not used as frequently in children because it is only available in tablet form. Many other medications can be used to control neuropathic pain; a complete review is beyond the scope of the present article. We refer the readers to references from Hauer (10) and Siden et al (11).
When dysautonomia is prominent during the episodes, clonidine, an alpha-2-adrenergic receptor agonist, needs to be considered for use on a regular basis and ‘as needed’ during crisis (10). Other possible agents include gabapentin, opioids and benzodiazepines, the latter two mostly on an ‘as needed’ basis during autonomic storms (3).
Benzodiazepines, through their action on the GABAA receptor, can be helpful in treating agitation/irritability for short periods of time; many patients will develop tolerance over time when used regularly. They are readily available in several forms, dosages and durations of action. In the 2008 WHO Essential Medicines List for Children in palliative care, benzodiazepines (diazepam and midazolam) were recommended as essential for management of anxiety and agitation/irritability (4). The main drawback to benzodiazepines is sedation. Increased salivary secretions, respiratory depression and paradoxical reactions (restlessness, confusion, aggressivity, self-injurious behaviours) are also possible, although rare.
Antipsychotic medications are also used in the treatment of agitation/irritability; in fact, haloperidol is listed as an essential medication for treatment of agitation/irritability in the 2008 WHO Essential Medicines List for Children in palliative care (4). The main benefit of the antipsychotic medications is less sedation. The main drawback with these medications is the risk of extrapyramidal symptoms and, for some, a decreased seizure threshold. Most are also not available in suspension, and very little anecdotal data exist about their use buccally (using the intravenous formulation). We recommend consulting a paediatric neurologist or psychiatrist to help manage this class of medication.
In extreme situations when first- and second-line medications are not relieving the agitation/irritability, using a more sedative agent, at least temporarily, may be appropriate. Several medications may be tried including chloral hydrate, dimenhydrinate and methotrimeprazine (7,8). Collaborating with a paediatric palliative care specialist is strongly recommended in those situations.
SPASTICITY
Spasticity is a resistance in muscle tone that increases in a velocity-dependent manner and is associated with hyper-reflexia (12). Spasticity is usually considered to be nonpainful, but it often coexists with intermittent muscle spasms, and subsequently pain and discomfort (3). Spasticity can also lead to considerable difficultly in obtaining functional and comfortable postures for ambulation, sitting and sleeping (13). Over time, spasticity can lead to the development of contractures and deformity, which can feed back into issues with pain, muscle spams and difficult posture (13). Spasticity leads to difficulty with the passive movement needed for caregiving such as toileting, washing, dressing, etc. This can cause pain and discomfort for both the child and their family during activities of daily care (13).
Once spasticity is identified and explored, it is important to treat all underlying provoking factors. Specific treatment for the spasticity should be considered and implemented as required, balancing benefits and side effects. The treatment options include nonpharmacological therapeutics, medications and surgical interventions. Before implementing spasticity treatment, it is important to confirm that the spasticity does not have a functional component. This is most often considered in the setting of an ambulatory child in which the increased tone may be counteracting underlying weakness (8,13). At times, spasticity also supports the seated position of the child, so this needs to be carefully considered.
Nonpharmacological treatment options for spasticity include physiotherapy, adjustments to their environment and activities of daily living by occupational therapy, as well as efforts provided by caregivers such as stretching exercises and massage (14). Pharmacological treatments generally include either enteral medication for generalized spasticity or injection medications for alleviation of more localized spasticity (13–15). Common enteral medications include benzodiazepines (diazepam), alpha-2 adrenergic agonists (tizanidine), GABA-receptor agonists (baclofen) and muscular calcium-blockers (dantrolene) (13–15). According to the American Academy of Neurology practice guideline, there are level B data for diazepam as a short-term treatment (caution regarding toxicity), level C data for tizanidine which may be considered, and insufficient data for the others (13). That said, clinical experience indicates that baclofen is often used as a first-line treatment in many centres. Botulinum toxin A is administered by injection for localized spasticity (13–15). For this use, botulinum toxin A is considered to be safe and efficacious, with level A data (13). Phenol is another injection medication, but the evidence for its use is insufficient according to the American Academy of Neurology practice parameter (13,15).
Neurosurgical and orthopedic interventions are available for the management of spasticity, such as muscle lengthening, tendon release, intrathecal baclofen, selective dorsal rhizotomy, etc (14,16,17). These procedures are typically not initiated during end-of-life, but may be of benefit in specific circumstances during the broader palliative care context (management of life-limiting conditions).
DYSTONIA
Dystonia is a hyperkinetic involuntary movement disorder in which sustained or intermittent contraction of both agonist and antagonist muscles results in repetitive movements, twisting and unnatural positioning of the body (12). Dystonia commonly affects the limbs, but it can also involve the muscles of the trunk, cervical region and even the facial (oral) musculature. For the purpose of the present article, the most important classification is between generalized dystonia and focal dystonia, a distinction that determines the treatment approach (enteral medication versus localized injection). Dystonia can be extremely painful and lead to significant difficulties for both self-care and caregiving by others. Thus, dystonia can be particularly difficult in terms of its impact on the child’s activities of daily living and quality of life.
Once underlying provoking factors and medication have been appropriately addressed, the next step is pharmacological treatment. It is important to remember that the benefit may be incomplete or accompanied by significant and intolerable side effects; thus, medicating for dystonia is only recommended if the symptom is truly leading to significant pain and/or burden on the child’s quality of life.
Pharmacological treatment includes both enteral and injection routes. Among enteral medications, anticholinergic treatment is the most common (19,20). The efficacy and tolerability of anticholinergic medications (trihexyphenidyl and benztropine) for dystonia in children is poorly documented and, therefore, physicians must proceed with caution, starting with small dosages and increasing slowly as needed, balancing benefit and side effects. It is essential to understand the potential side effects of anticholinergic and monitor appropriately; this includes reduced diaphoresis (increased body temperature), dilated pupils, decreased bodily fluids (dry mouth, dry eyes), erythema of the skin and confusion/delirium (20). Other enteral medications that can be considered for dystonia (with less evidence) are benzodiazepines (diazepam, nitrazepam), GABA-receptor agonists (baclofen) and dopamine-depleting agents (tetrabenazine) (19–21). Drugs ordinarily reserved for dopamine-responsive dystonia (carbidopa-levedopa) may also be considered and trialed in specific situations, in consultation with a neurologist (19,20). Finally, botulinum toxin A injection is the treatment of choice for most types of focal dystonia (19,20). Clinical experience indicates that anticholinergic medications and tetrabenazine are first-line treatments; that said, dystonia is a complex symptom and often very delicate to manage appropriately. Therefore, consultation with a neurologist and/or a formal referral to neurology is recommended before initiating treatment for dystonia.
Neurosurgical approaches, including deep-brain stimulation of the globus pallidum internum, are well established for treatment of dystonia; however, these would be considered to be extreme and invasive measures in a palliative setting (19,20,22).
CONCLUSIONS
Clinical scenarios, such as that described at the beginning of the present article, are not only distressing for patients and families, but they are also challenging for health care teams. Neurological symptoms often present in children that are medically complex, and these symptoms can be particularly difficult to assess and treat effectively. Distinguishing among different neurological symptoms requires a detailed history and careful verification of all potential triggering factors. Pain, either nociceptive or neuropathic, needs to be meticulously explored and treated. Management includes nonpharmacological therapeutics endeavors and medications, as well as consideration of surgical procedures in intractable situations. Collaborative and interdisciplinary teamwork is strongly recommended in the management of children with complex neurological symptomatology. As noted in the medication table, specific recommendations for paediatric dosing are too often lacking. Not only is further focused pediatric palliative care research needed, but publications on clinical experience are required as well. Local expertise, once captured and reviewed at a national level, becomes relevant and helpful to other clinicians.
TABLE 2.
Approach to spasticity
| Thought-provoking questions | Elements to consider |
|---|---|
| Is this spasticity? |
|
| What are the provoking factors? |
|
| Are medications contributing? |
|
REFERENCES
- 1.Breau LM, McGrath PJ, Camfield CS, Finley GA. Psychometric properties of the non-communicating children’s pain checklist-revised. Pain. 2002;99:349–57. doi: 10.1016/s0304-3959(02)00179-3. [DOI] [PubMed] [Google Scholar]
- 2.Rajapakse D, Comac M. Symptoms in life-threatening illness: Overview and assessment. In: Goldman A, Hain R, Liben S, editors. Oxford Textbook of Palliative Care for Children. New York: Oxford University Press; 2012. pp. 167–77. [Google Scholar]
- 3.Hauer JM, Faulkner KW. Neurological and neuromuscular conditions and symptoms. In: Goldman A, Hain R, Liben S, editors. Oxford Textbook of Palliative Care for Children. New York: Oxford University Press; 2012. pp. 295–308. [Google Scholar]
- 4.Aindow A, Brook L. Essential Medicines List for Children (EMLc); Palliative Care. World Health Organization; Jun, 2008. < www.who.int/selection_medicines/committees/subcommittee/2/palliative.pdf> (Accessed July 27, 2014). [Google Scholar]
- 5.Steele R, Siden H, Cadell S, et al. Charting the territory: Symptoms and functional assessment in children with progressive, non-curable conditions. Arch Dis Child. 2014;99:754–62. doi: 10.1136/archdischild-2013-305246. [DOI] [PubMed] [Google Scholar]
- 6.Oxford Dictionary On-line. <www.oxforddictionaries.com/definition/english/agitation> (Accessed July 27, 2014).
- 7.Cummings MR, Miller BD. Pharmacologic management of behavioral instability in medically ill pediatric patients. Curr Opin Pediatr. 2004;16:516–22. doi: 10.1097/01.mop.0000139300.13152.20. [DOI] [PubMed] [Google Scholar]
- 8.Wusthoff CJ, Shellhaas RA, Licht DJ. Management of common neurologic symptoms in pediatric palliative care: Seizures, agitation, and spasticity. Pediatr Clin North Am. 2007;54:709–33. doi: 10.1016/j.pcl.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Hauer J, Wical B, Charnas L. Gabapentin successfully manages chronic unexplained irritability in children with severe neurologic impairment. Pediatrics. 2007;119:e519–22. doi: 10.1542/peds.2006-1609. [DOI] [PubMed] [Google Scholar]
- 10.Hauer J. Improving comfort in children with severe neurological impairment. Prog Palliat Care. 2012;20:349–56. [Google Scholar]
- 11.Siden HB, Carleton BC, Oberlander TF. Physician variability in treating pain and irritability of unknown origin in children with severe neurological impairment. Pain Res Manag. 2013;18:243–8. doi: 10.1155/2013/193937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ropper AH, Brown RH, editors. Adams and Victor’s Principles of Neurology. 8th edn. New York: McGraw-Hill; 2005. [Google Scholar]
- 13.Delgado MR, Hirtz D, Aisen M, et al. Practice Parameter: Pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review) Neurology. 2010;74:336–43. doi: 10.1212/WNL.0b013e3181cbcd2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duff MEA, Morton RE. Managing spasticity in children. Paediatr Child Health. 2007;17:463–6. [Google Scholar]
- 15.Chung CY, Chen CL, Wong AM. Pharmacotherapy of spasticity in children with cerebral palsy. J Formos Med Assoc. 2011;110:215–22. doi: 10.1016/S0929-6646(11)60033-8. [DOI] [PubMed] [Google Scholar]
- 16.Roberts A. Surgical management of spasticity. J Child Orthop. 2013;7:389–94. doi: 10.1007/s11832-013-0512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayanan UG. Management of children with ambulatory cerebral palsy: An evidence-based review. J Pediatr Orthop. 2012;32(Suppl 2):S172–81. doi: 10.1097/BPO.0b013e31825eb2a6. [DOI] [PubMed] [Google Scholar]
- 18.Skidmore F, Reich SG. Tardive dystonia. Curr Treat Options Neurol. 2005;7:231–6. doi: 10.1007/s11940-005-0016-0. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Alvarez E, Nardocci N. Update on pediatric dystonias: Etiology, epidemiology, and management. Degener Neurol Neuromuscul Dis. 2012;2:29–41. doi: 10.2147/DNND.S16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844–56. doi: 10.1016/S1474-4422(09)70183-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen JJ, Ondo WG, Dashtipour K, Swope DM. New Drug Review. Tetrabenazine for the treatment of hyperkinetic movement disorders: A review of the literature. Clin Ther. 2012;34:1487–502. doi: 10.1016/j.clinthera.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Kupsch A, Kuehn A, Klaffke S, et al. Deep brain stimulation in dystonia. J Neurol. 2003;250(Suppl 1):I/47–I/52. doi: 10.1007/s00415-003-1110-2. [DOI] [PubMed] [Google Scholar]
- 23.Hain R DW, Jassal SS. Paediatric Palliative Medicine. New York: Oxford University Press; 2010. [Google Scholar]
- 24.Twycross R, Wilcock A, Dean M, Kennedy B, editors. Palliative care Formulary: Canadian Edition. 1st edn. Nottingham: Palliativedrugs.com; 2012. [Google Scholar]
- 25.e-therapeutics.ca [Internet] Ottawa, Ontario: Canadian Pharmacists Association; < www.e-therapeutics.ca> (Accessed July 27, 2014). [Google Scholar]
- 26. Halifax, Nova Scotia: IWK Drug Information. Updated 2014. < www.iwk.nshealth.ca/page/iwk-drug-information> (Accessed July 27, 2014).
- 27.Carranza-del Rio J, Clegg NJ, Moore A, Delgado MR. Use of trihexyphenidyl in children with cerebral palsy. Pediatr Neurol. 2011;44:202–6. doi: 10.1016/j.pediatrneurol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Rose MA, Kam PCA. Gabapentin: Pharmacology and its use in pain management. Anaesthesia. 2002;57:451–62. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwantes S, O’Brien HW. Pediatric palliative care for children with complex chronic medical conditions. Pediatr Clin North Am. 2014;61:797–821. doi: 10.1016/j.pcl.2014.04.011. [DOI] [PubMed] [Google Scholar]


