Abstract
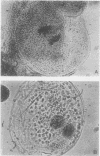
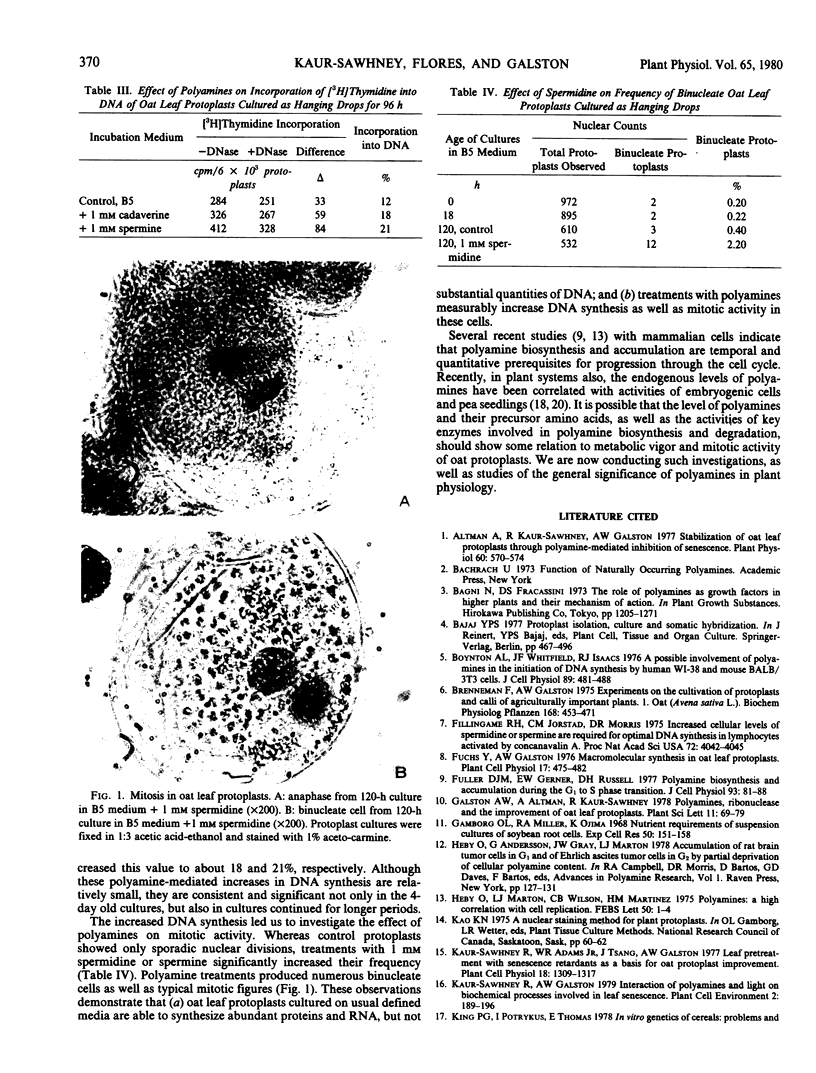
Freshly isolated protoplasts from leaves of oat seedlings (var. Victory) which do not divide when cultured on a wide range of media are capable of incorporating tritiated leucine, uridine, and thymidine into trichloroacetic acid-insoluble macromolecules. Over 70% of the leucine and uridine incorporated over an 18-hour period are found in protein and RNA, respectively, as shown by hydrolysis of the macromolecular products with a specific protease or RNase. In contrast, little or none of the tritiated thymidine is incorporated into macromolecules hydrolyzable by DNase over an 18- to 96-hour period. Incorporation of thymidine into trichloroacetic acid-insoluble material declines sharply with increasing time of culture after 18 hours. However, addition of diamines or polyamines to the medium not only prevents the decline, but actually increases net thymidine incorporation, including a fraction going into DNA. A significant increase in mitoses and binucleate protoplasts is also observed in 72- to 168-hour cultures.
The inability of oat leaf protoplasts to synthesize significant quantities of DNA suggests that they are arrested at the G1 phase of the cell cycle. Treatment with polyamines appears to promote both DNA synthesis and the inception of mitotic activity in oat protoplasts, as in numerous animal and microbial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J. A possible involvement polyamines in the initiation of DNA synthesis by human WI-38 and mouse BALB/3T3 cells. J Cell Physiol. 1976 Nov;89(3):481–488. doi: 10.1002/jcp.1040890313. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller D. J., Gerner E. W., Russell D. H. Polyamine biosynthesis and accumulation during the G1 to S phase transition. J Cell Physiol. 1977 Oct;93(1):81–88. doi: 10.1002/jcp.1040930111. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Wilson C. B., Martinez H. M. Polyamines: a high correlation with cell replication. FEBS Lett. 1975 Jan 15;50(1):1–4. doi: 10.1016/0014-5793(75)81026-x. [DOI] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: I. Changes in Intracellular Content and Rates of Synthesis. Plant Physiol. 1978 Sep;62(3):430–433. doi: 10.1104/pp.62.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak H. T., Paul D. Inhibition of spermidine and spermine synthesis leads to growth arrest of rat embryo fibroblasts in G1. J Cell Physiol. 1978 Feb;94(2):161–170. doi: 10.1002/jcp.1040940205. [DOI] [PubMed] [Google Scholar]
- Shepard J. F., Totten R. E. Mesophyll cell protoplasts of potato: isolation, proliferation, and plant regeneration. Plant Physiol. 1977 Aug;60(2):313–316. doi: 10.1104/pp.60.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkara P. S., Pargac M. B., Nishioka K., Rao P. N. Differential effects of inhibition of polyamine biosynthesis on cell cycle traverse and structure of the prematurely condensed chromosomes of normal and transformed cells. J Cell Physiol. 1979 Mar;98(3):451–457. doi: 10.1002/jcp.1040980303. [DOI] [PubMed] [Google Scholar]