Abstract
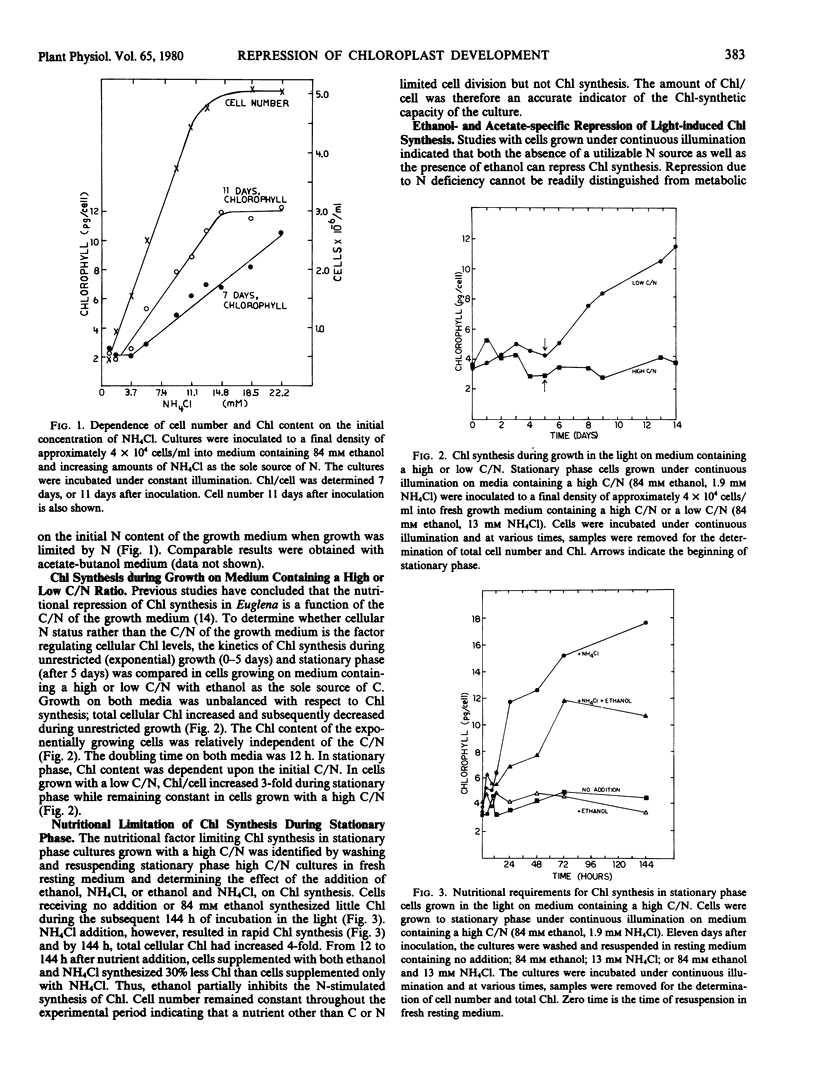
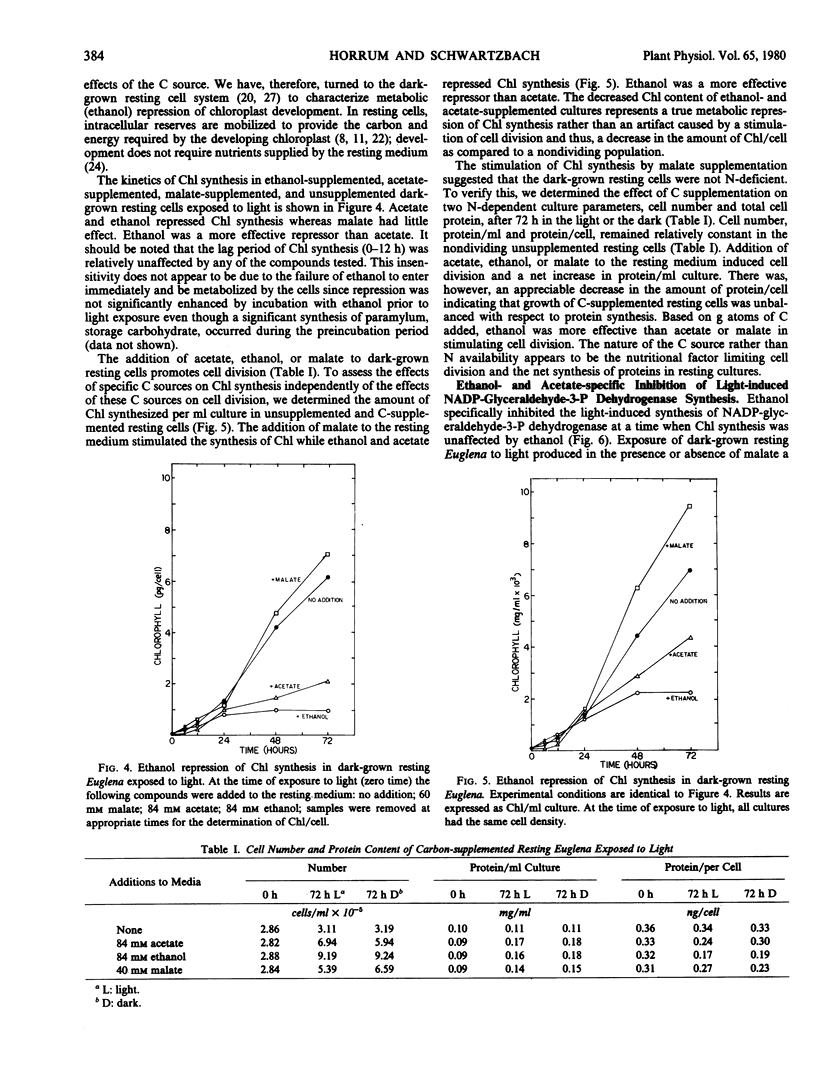
Nitrogen deficiency and the presence of specific organic carbon sources prevent chloroplast development in Euglena. In exponentially growing cultures, chlorophyll levels were low and independent of the nitrogen content of the growth medium. Chlorophyll levels increased in stationary phase and the amount of chlorophyll formed was proportional to the initial nitrogen content of the growth medium; the greater the concentration of nitrogen, the greater the amount of chlorophyll synthesized during stationary phase. Washing experiments demonstrated that the major nutritional factor inhibiting chlorophyll synthesis in stationary phase cultures grown on medium containing a high carbon to nitrogen ratio was the absence of nitrogen rather than the presence of utilizable organic carbon.
The light-induced synthesis of chlorophyll and of NADP-glyceraldehyde-3-phosphate dehydrogenase was inhibited when acetate or ethanol was added at the time of exposure of dark-grown resting cells to light. Malate addition, however, stimulated chlorophyll and enzyme synthesis. Both cell number and total cell protein increased after ethanol, acetate, or malate addition, indicating that the resting cells were not nitrogen-deficient. Ethanol and acetate specifically repress light-induced chlorophyll synthesis. NADP-glyceraldehyde-3-phosphate dehydrogenase synthesis was inhibited at a time, the first 24 hours of light exposure, when chlorophyll synthesis was unaffected by carbon addition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bovarnick J. G., Chang S. W., Schiff J. A., Schwartzbach S. D. Events surrounding the early development of Euglena chloroplasts: experiments with streptomycin in non-dividing cells. J Gen Microbiol. 1974 Jul;83(0):51–62. doi: 10.1099/00221287-83-1-51. [DOI] [PubMed] [Google Scholar]
- Bovarnick J. G., Schiff J. A., Freedman Z., Egan J. M. Events surrounding the early development of Euglena chloroplasts: cellular origins of chloroplast enzymes in euglena. J Gen Microbiol. 1974 Jul;83(0):63–71. doi: 10.1099/00221287-83-1-63. [DOI] [PubMed] [Google Scholar]
- Cohen D., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. Photoregulation of the transcription of chloroplastic and cytoplasmic ribosomal RNAs. Arch Biochem Biophys. 1976 Nov;177(1):201–216. doi: 10.1016/0003-9861(76)90430-6. [DOI] [PubMed] [Google Scholar]
- Dwyer M. R., Smillie R. M. A light-induced beta-1,3-glucan breakdown associated with the differentiation of chloroplasts in Euglena gracilis. Biochim Biophys Acta. 1970 Sep 1;216(2):392–401. doi: 10.1016/0005-2728(70)90231-8. [DOI] [PubMed] [Google Scholar]
- Egan J. M., Dorsky D., Schiff J. A. Events Surrounding the Early Development of Euglena Chloroplasts: VI. Action Spectra for the Formation of Chlorophyll, Lag Elimination in Chlorophyll Synthesis, and Appearance of TPN-dependent Triose Phosphate Dehydrogenase and Alkaline DNase Activities. Plant Physiol. 1975 Aug;56(2):318–323. doi: 10.1104/pp.56.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves L. B., Jr, Trelease R. N., Grill A., Becker W. M. Localization of glyoxylate cycle enzymes in glyoxysomes in Euglena. J Protozool. 1972 Aug;19(3):527–532. doi: 10.1111/j.1550-7408.1972.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Kirk J. T. Control of chloroplast formation in Euglena gracilis. Antagonism between carbon and nitrogen sources. Biochem J. 1969 Jun;113(1):195–205. doi: 10.1042/bj1130195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowinsky A. W., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. I. Induction by preillumination. Plant Physiol. 1970 Mar;45(3):339–347. doi: 10.1104/pp.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levedahl B. H., Wilson B. W. Succinate utilization by Euglena: comparison of growth on low levels of succinate, acetate and ethanol. Exp Cell Res. 1965 Aug;39(1):242–248. doi: 10.1016/0014-4827(65)90026-1. [DOI] [PubMed] [Google Scholar]
- Pohl P., Wagner H. Control of fatty acid and lipid biosynthesis in Euglena gracilis by ammonia, light and DCMU. Z Naturforsch B. 1972 Jan;27(1):53–61. doi: 10.1515/znb-1972-0111. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Draffan A. G. Light-induced Enzyme Formation in a Chlorophyll-less Mutant of Euglena gracilis. Plant Physiol. 1978 Nov;62(5):678–682. doi: 10.1104/pp.62.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbach S. D., Schiff J. A., Goldstein N. H. Events surrounding the early development of euglena chloroplasts: v. Control of paramylum degradation. Plant Physiol. 1975 Aug;56(2):313–317. doi: 10.1104/pp.56.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Stern A. I., Schiff J. A., Epstein H. T. Studies of Chloroplast Development in Euglena. V. Pigment Biosynthesis, Photosynthetic Oxygen Evolution and Carbon Dioxide Fixation during Chloroplast Development. Plant Physiol. 1964 Mar;39(2):220–226. doi: 10.1104/pp.39.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]


