ABSTRACT
Purpose: Shoulder pain and dysfunction may occur after surgery for head and neck cancer (HNC) as a result of damage to or resection of the spinal accessory nerve. Previous research found that 12 weeks of upper extremity progressive resistance exercise training (PRET) improved shoulder outcomes in survivors of HNC; the purpose of this study was to determine whether benefits persisted over the longer term. Methods: Survivors of HNC were assigned at random to PRET (n=27) or a standard therapeutic protocol (TP; n=25), with an opportunity for crossover in the TP group after 12 weeks. At 12-month follow-up, participants were mailed a questionnaire that assessed quality of life (QOL), shoulder outcomes, and exercise behaviour. Results: Of the 52 participants enrolled in the study, 44 were eligible at 12-month follow-up, and 37 (71%) completed the questionnaires. Overall, self-reported outcomes were largely sustained over the follow-up period. After 12 months, regardless of original group allocation, participants who continued resistance exercise training during the follow-up period reported better neck dissection–related functioning (p=0.021) and better QOL (p=0.011) than those who did not. Conclusions: Benefits of PRET were sustained at 12-month follow-up. Ongoing participation in resistance exercise training may prove valuable as a supportive care intervention for survivors of HNC.
Key Words: exercise therapy, follow-up studies, head and neck neoplasms, quality of life, shoulder pain
RÉSUMÉ
Objet : Une douleur à l'épaule et une dysfonction peuvent faire leur apparition après une intervention chirurgicale pour un cancer de la tête et du cou (CTC) parce que le nerf spinal accessoire a été endommagé ou réséqué. Des recherches antérieures ont révélé que 12 semaines d'exercice contre résistance progressive (ERP) des membres supérieurs amélioraient le résultat pour l'épaule chez les survivants d'un CTC. Cette étude visait à déterminer si les bienfaits persistaient à long terme. Méthodes : Les survivants d'un CTC ont été répartis au hasard pour suivre un programme d'ERP (n=27) ou un protocole thérapeutique habituel (PT; n=25) et ont pu passer au groupe PT après 12 semaines. Au suivi à 12 mois, on a envoyé par la poste aux participants un questionnaire d'évaluation de la qualité de vie (QDV), des résultats pour l'épaule et du comportement lié à l'exercice. Résultats : Sur les 52 participants inscrits à l'étude, 44 étaient admissibles au suivi à 12 mois et 37 (71 %) ont répondu aux questionnaires. Dans l'ensemble, les résultats autodéclarés ont été maintenus en grande partie au cours de la période de suivi. Après 12 mois, sans égard à leur affectation au groupe original, les participants qui ont poursuivi leur entraînement par l'exercice à résistance au cours de la période de suivi ont signalé un meilleur fonctionnement lié à la dissection subie au cou (p=0,021) et une meilleure QDV (p=0,011) que ceux qui ne l'ont pas fait. Conclusions : Les bienfaits de l'ERP persistaient au suivi à 12 mois. La participation continue à un programme d'exercice contre résistance peut se révéler utile comme soins de soutien pour les survivants d'un CTC.
Mots clés : douleur à l'épaule, études de suivi, néoplasmes de la tête et du cou, qualité de vie, spécialité de la physiothérapie, thérapie par l'exercice
Surgical treatment of head and neck cancer (HNC) typically includes dissection of lymph nodes in the neck, both for tumour staging and to treat lymph node metastases.1,2 Shoulder pain and dysfunction may occur after a neck dissection procedure as a result of temporary damage to or resection of the spinal accessory nerve (SAN).3,4 In the traditional radical neck dissection, the SAN is resected or “sacrificed” en bloc with the sternocleidomastoid muscle, resulting in permanent trapezius muscle paresis.5–7 Procedures such as the modified neck dissection and the selective neck dissection typically preserve the SAN and are used, when oncologically feasible, in an attempt to preserve trapezius muscle function.8 Temporary or permanent damage to the SAN may still occur, however, as a result of trauma to the nerve during the neck dissection procedure.9,10 Once developed, shoulder-related symptoms have been found to persist rather than improve even in cases of SAN recovery, and little is known about effective management of this condition over the long term.2,11
The results from our randomized controlled trial of upper extremity (UE) progressive resistance exercise training (PRET) demonstrated significant improvements in patient-rated shoulder pain and disability, UE strength and endurance, and range of motion in post-surgical HNC survivors.12 All other outcomes, including fatigue, neck dissection impairment, and quality of life (QOL), favoured the PRET group at the end of the 12-week intervention but did not reach statistical significance.12
In this article, we report the 12-month follow-up data from our randomized crossover trial comparing PRET with a standard therapeutic protocol (TP) in post-surgical HNC survivors. Our study is one of the first to provide follow-up data on a resistance exercise intervention for shoulder pain and dysfunction after neck dissection in HNC survivors.13–15 Because the study design allowed crossover for participants originally assigned to the TP group, we did not anticipate continued group differences at 12-month follow-up based on randomization. We were interested to determine, however, whether any benefits from the 12-week PRET intervention persisted over the longer term.
Methods
Setting and participants
Because the study methods have previously been reported,12 here we briefly review the methods, with additional information pertaining to the 12-month follow-up assessment.
The original study was a single-centre study conducted at the Cross Cancer Institute and University of Alberta in Edmonton, Canada, and approved by the Health Research Ethics Board of the University of Alberta and the Research Ethics Committee of the Alberta Cancer Board. All participants were diagnosed with carcinoma in the head and neck region that had been managed by definitive surgical resection. Eligibility criteria also included (1) surgical treatment that included undergoing a lymph node dissection in the neck; (2) shoulder dysfunction as a result of SAN damage (clinical criteria: reduction in active shoulder abduction, trapezius atrophy, and shoulder droop, confirmed by nerve conduction, needle electromyography, or both as indicated); (3) Karnofsky Performance Status of 60% or less;16 (4) no evidence of residual cancer in the neck and no distant metastasis; (5) completion of adjuvant HNC treatment; and (6) provision of written informed consent.
Participants were not eligible for the study if they presented with a history of shoulder or neck pathology unrelated to cancer treatment or with any comorbid medical illness or psychiatric illness that would prevent completion of treatment or interfere with follow-up.
Experimental design and recruitment
The study was a prospective randomized controlled trial with optional crossover of the TP group. Potential participants were recruited through the Alberta Cancer Registry and by their surgeon or oncologist at Otolaryngology HNC follow-up clinics at the University of Alberta Hospital and the Cross Cancer Institute.
Randomization
Eligible participants were stratified by tumour location and type of neck dissection and randomly assigned to the PRET or TP group after completion of baseline testing. An independent researcher generated the allocation sequence using a computer-generated code; the randomized allocation was enclosed in sequentially numbered and sealed (opaque) envelopes to conceal the order from the study personnel.
Interventions
Exercise interventions for both groups took place at the University of Alberta's Behavioural Medicine Fitness Centre. Participants were asked to exercise three times per week, with a minimum of two supervised sessions per week. Participants assigned to the TP group followed a standard TP representing usual care at our centre, which consisted of supervised active and passive ROM, stretching exercises, postural exercises, and progressive strengthening exercises with light weights (e.g., 1–4.5 kg) and elastic resistance bands (e.g., light to strong resistance). Participants performed 2 sets of 10–15 repetitions of 5–8 UE exercises (see Box 1). The specific therapeutic exercises performed were chosen with the goal of enhancing scapular stability and restoring or maintaining UE strength. Participants in this group had the option to participate in the PRET program after the 12-week standard care period.
Box 1.
Muscle Groups and Equipment for TP and PRET Groups
| Exercise movement | Muscle groups | TP protocol equipment | PRET protocol equipment |
|---|---|---|---|
| 1. Scapular retraction | Rhomboids | Scapular retraction: elastic band | Vertical row machine |
| Middle trapezius | |||
| 2. Scapular elevation | Levator scapulae | Shoulder elevation (shrug): elastic band | Shoulder shrug: pulley weights |
| Upper trapezius | |||
| 3. Elbow flexion | Biceps | Bicep curl with free weights; with scapula supported manually or against wall, free weights | Bicep curl machine |
| 4. Elbow extension | Triceps | Elbow extension in supine: free weights | Elbow extension machine |
| 5. Scapular protraction | Pectoralis major | Scapular protraction in supine: free weights | Vertical bench machine |
| Triceps | |||
| Anterior deltoid | |||
| Additional exercises prescribed if trapezius muscle recovery evident | |||
| 6. Shoulder horizontal abduction | Middle trapezius | Bent-over row or prone horizontal abduction with physio ball: free weights | As per TP protocol |
| Lower trapezius | |||
| Teres minor | |||
| Infraspinatus | |||
| Posterior deltoid | |||
| 7. Shoulder elevation | Deltoid | Shoulder press with plinth on incline to sitting to progress range of motion: free weights | As per TP protocol OR if full shoulder flexion range of motion: shoulder press |
| Pectoralis major | |||
| Triceps | |||
| Trapezius | |||
| 8. Shoulder abduction in plane of scapula | Trapezius | Shoulder abduction in prone; with external rotation—progress to standing: free weights | As per TP protocol |
| Deltoid | |||
| Biceps | |||
TP=therapeutic (standard care) protocol; PRET=progressive resistance exercise training.
Participants randomized to the PRET group followed the same protocol as the TP group, but the strengthening component of the protocol was based on a percentage of each participant's one-repetition maximum (1RM; the maximum weight lifted one time) at baseline testing. Participants completed the same 5–8 exercises and followed the same exercise prescription (i.e., 2 sets of 10–15 repetitions) as the TP group. The resistance level (intensity), however, was started at 25%–30% of the participant's 1RM strength and systematically progressed to 60%–70% of 1RM by the end of the intervention period.
Assessment of primary and secondary endpoints
Patient-rated outcomes were assessed at baseline, post-intervention, and 12-month follow-up (12 months after the post-intervention assessment); objective outcomes were assessed at baseline and post-intervention only. Our primary outcome measure in the study was patient-rated shoulder pain and disability as assessed by the Shoulder Pain and Disability Index (SPADI);17,18 secondary outcomes included neck dissection impairment, fatigue, and QOL. We used the Neck Dissection Impairment Index (NDII) to assess treatment-specific QOL.19 The Functional Assessment of Cancer Therapy—General scale (FACT-G), the FACT-Anemia Scale (FACT-An), and the FACT-Fatigue subscale were used to assess health-related QOL, anemia-related symptoms, and symptoms of fatigue, respectively.20,21
Assessment of exercise during the 12-month follow-up
Exercise behaviour during the follow-up period was assessed using the Godin Leisure Time Exercise Questionnaire22,23 and questions on the type of shoulder-related exercise participants had been carrying out since completing the intervention: (a) no exercise, (b) low-intensity exercises only (i.e., ROM, stretching exercises, exercises with elastic resistance bands or light weights), or (c) resistance exercise training (RET; i.e., weight training) at a fitness facility. We compared participants who reported continuing RET at a fitness facility (RET group) with participants not continuing RET (NRE group).
Statistical analysis
We used dependent t-tests to examine changes from post-intervention to 12-month follow-up of the overall group for the patient-rated outcomes, and we used independent t-tests to examine between-groups differences for the two original groups from baseline to 12-month follow-up and to assess differences for self-reported resistance exercise behaviour (RET or NRE) during the follow-up period from post-intervention to 12-month follow-up. Adjusted analyses controlled for potential confounding variables related to the specific outcome, as well as participant and medical variables: initial value of the outcome (i.e., baseline or post-intervention value), age, gender, cancer stage, time since surgery, neck dissection type, and use of pain medication. Because our follow-up period was considered exploratory in nature, probability levels less than 0.05 (two-tailed) were accepted as significant.
Results
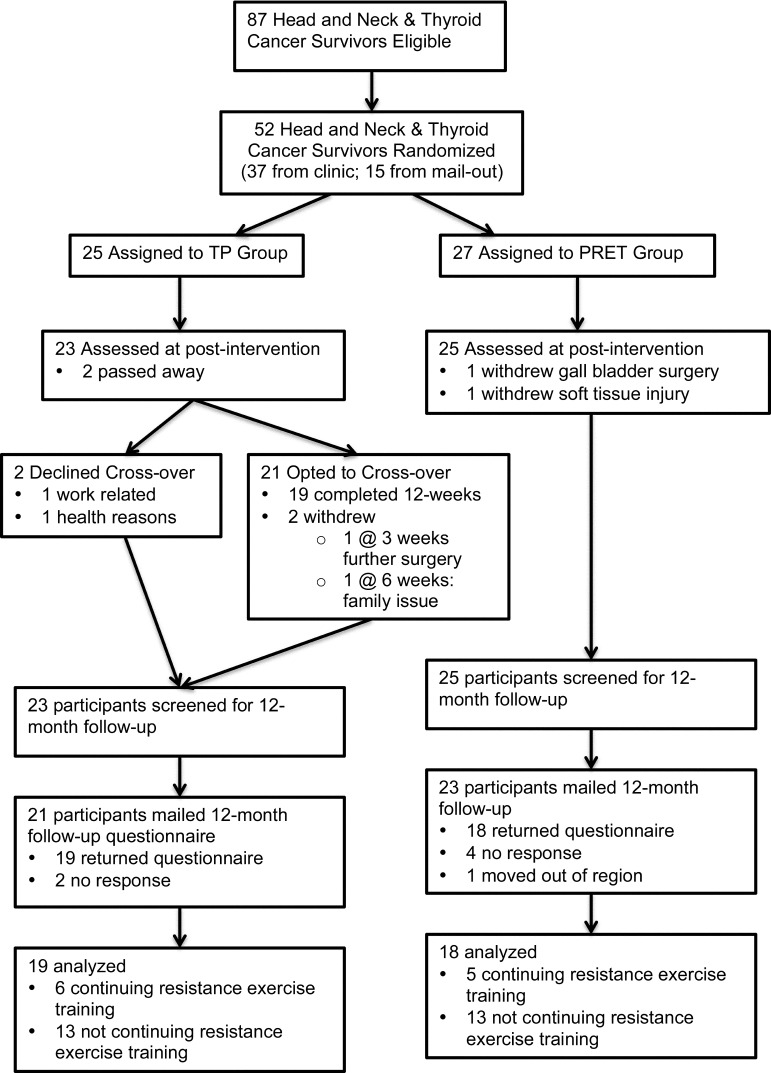
A total of 52 participants were recruited to the trial and randomized to either the TP group (n=25) or the PRET group (n=27). The groups were balanced at baseline. Age ranged from 32 to 76 years (mean=52 years); 67% were men, 62% had oral or oropharyngeal cancer, 58% had stage IV disease, 85% received adjuvant radiation therapy, 27% received adjuvant chemotherapy, 23% were regularly using narcotic medication for pain at baseline, and 15% reported meeting public health guidelines for physical activity. Of the 52 participants enrolled, 44 were deemed eligible for participation at 12-month follow-up, and 37 (71%) completed and returned the questionnaire (see Figure 1).
Figure 1.
Flow of study participants through trial and follow-up.
Of the 15 participants lost to follow-up between baseline testing and 12-month follow-up, 5 had died from their cancer, 3 were withdrawn from the study for medical reasons, and 7 did not respond to the questionnaire. Comparing baseline variables for participants who had completed and returned the 12-month follow-up (n=37) with those for participants lost to follow-up (n=15) revealed no clinically or statistically significant differences (power>80%) between the groups in terms of age, marital status, gender, education, income, employment, cancer stage, time since surgery, smoking and drinking status, or pain medication use; we also found no differences in shoulder pain and disability or neck dissection impairment. However, the 15 participants lost to follow-up and excluded from the analyses at 12 months had reported significantly lower baseline levels of QOL (FACT-G mean difference=−9.6; 95% CI, −19.2 to −0.03) and worse anemia-related symptoms (mean difference= −21.5; 95% CI, −37.2 to −5.7) than the 37 participants included in the 12-month follow-up analyses.
Within-group changes in outcomes from post-intervention to 12-month follow-up
From post-intervention to 12-month follow-up, we found no significant differences in shoulder pain and dysfunction, QOL, or fatigue; however, we found borderline significant improvements in neck dissection–related functioning as measured by the NDII (see Table 1).
Table 1.
Sustainability of Outcomes for Overall Group at 12-Month Follow-Up (N=37)
| Mean (SD) |
Within-group difference in mean change, post-intervention to 12-month follow-up (95% CI), p-value |
|||
|---|---|---|---|---|
| Outcome | Baseline | Post-intervention | 12-mo follow-up | |
| SPADI total score, % | 25.6 (20.7) | 16.1 (17.5) | 15.7 (21.6) | −0.4 (−4.25 to 2.30), 0.86 |
| NDII score, % | 55.8 (22.3) | 67.2 (22.1) | 73.1 (23.7) | +5.9 (−0.06 to 11.8), 0.052 |
| FACT-An + FACT-G score, 0–188 | 138.5 (24.7) | 145.8 (24.7) | 145.1 (27.4) | −0.68 (−7.56 to 6.21), 0.84 |
| FACT-G score, 0–108 | 80.8 (15.4) | 84.6 (14.9) | 82.9 (13.4) | −1.7 (−5.14 to 1.74), 0.32 |
| FACT-Fatigue score, 0–52 | 35.6 (8.5) | 38.2 (7.7) | 39.5 (10.5) | +1.3 (−1.64 to 4.23), 0.37 |
SPADI=Shoulder Pain and Disability Index; NDII=Neck Dissection Impairment Index; FACT-G=Functional Assessment of Cancer Therapy—General Scale; FACT-An=Functional Assessment of Cancer Therapy Anemia Scale; FACT-Fatigue=Functional Assessment of Cancer Therapy Fatigue subscale.
Effects of group assignment on patient-rated outcomes at 12-month follow-up
Although changes in patient-rated outcomes favoured the original PRET group at 12-month follow-up, we found no statistically significant differences between the randomized groups for any outcome (see Table 2).
Table 2.
Effects of Intervention by Randomized Group on Patient-Reported Outcomes at 12-Month Follow-up
| Mean (SD) |
Group difference in mean change (95% CI), p-value |
||||
|---|---|---|---|---|---|
| Outcome | Baseline | 12-mo follow-up | Change | Unadjusted | Adjusted* |
| SPADI total, % | |||||
| TP | 29.8 (22.4) | 20.6 (26.2) | −9.2 (18.0) | −1.4 (−14.4 to 11.6), 0.82 | −5.7 (−18.1 to 6.6), 0.35 |
| PRET | 21.1 (18.3) | 10.5 (13.4) | −10.6 (20.8) | ||
| NDII, % | |||||
| TP | 51.8 (23.1) | 69.1 (28.6) | +17.2 (16.6) | +0.1 (−12.1 to 12.3), 0.98 | +2.4 (−10.3 to 15.0), 0.70 |
| PRET | 60.0 (21.3) | 77.4 (17.1) | +17.3 (20.0) | ||
| FACT-An + FACT-G, 0–188 | |||||
| TP | 139.4 (25.7) | 144.4 (30.7) | +5.0 (14.9) | +3.4 (−11.9 to 18.8), 0.65 | +3.5 (−14.1 to 21.1), 0.69 |
| PRET | 137.4 (24.3) | 145.9 (24.4) | +8.4 (29.3) | ||
| FACT-G, 0–108 | |||||
| TP | 80.9 (16.1) | 82.2 (14.9) | +1.3 (8.7) | +1.8 (−6.1 to 9.8), 0.64 | +1.4 (−6.3 to 9.2), 0.72 |
| PRET | 80.6 (15.2) | 83.7 (12.0) | +3.1 (14.7) | ||
| FACT-Fatigue, 0–52 | |||||
| TP | 36.1 (8.8) | 39.3 (11.3) | 3.2 (7.3) | +1.56 (−5.1 to 8.2), 0.64 | +1.0 (−6.6 to 8.6), 0.79 |
| PRET | 35.0 (8.3) | 39.7 (10.0) | 4.7 (12.1) | ||
Adjusted for baseline value, age, gender, cancer stage, time since surgery, neck dissection type, and pain medication use.
SPADI=Shoulder Pain and Disability Index; TP=standard therapeutic protocol; PRET=progressive resistance exercise training; NDII=Neck Dissection Impairment Index; FACT-G=Functional Assessment of Cancer Therapy – General Scale; FACT-An=Functional Assessment of Cancer Therapy Anemia Scale; FACT-Fatigue=Functional Assessment of Cancer Therapy Fatigue subscale.
Exercise behaviour during follow-up
Of the 37 participants who provided 12-month follow-up data, 11 (30%) reported regularly performing UE RET at a fitness facility, 18 (49%) reported performing low-intensity exercises only (e.g., ROM, resistance bands, light weights), and 8 (22%) reported performing no UE exercise. For leisure-time physical activity, 8 of the 37 participants (22%) were meeting the public health guidelines of at least 150 minutes per week of physical activity (see Table 3). We found no significant differences between the originally assigned groups (PRET vs. TP) in terms of resistance exercise behaviour (p=0.54) or leisure-time physical activity (p=0.62) during the follow-up period, nor did we find significant between-group differences based on exercise behaviour during the follow-up period (RET vs. NRE groups) for any demographic or medical variables.
Table 3.
Self-Reported Exercise Behaviour at 12-Month Follow-Up, Overall and by Original Group Assignment
| Self-reported exercise | Overall (n=37), n (%) |
TP (n=19) , n (%) |
PRET (n=18) , n (%) |
|---|---|---|---|
| Shoulder exercises | |||
| No exercise or low-intensity exercise only | 26 (70) | 13 (35) | 13 (35) |
| Upper extremity resistance exercise | 11 (30) | 6 (16) | 5 (14) |
| Physical activity* | |||
| Not meeting guidelines at 12-month follow-up | 29 (78) | 15 (41) | 14 (38) |
| Meeting guidelines at 12-month follow-up | 8 (22) | 4 (11) | 4 (11) |
| Meeting guidelines at both baseline and 12-month follow-up | 4 (11) | 2 (5) | 2 (5) |
| Performing upper extremity resistance exercise and meeting physical activity guidelines at 12-month follow-up | 7 (19) | 3 (8) | 4 (11) |
Meeting physical activity guidelines≥150 minutes/week.
TP=standard therapeutic protocol; PRET=progressive resistance exercise training.
Resistance exercise during the follow-up period and patient-rated outcomes at 12-month follow-up
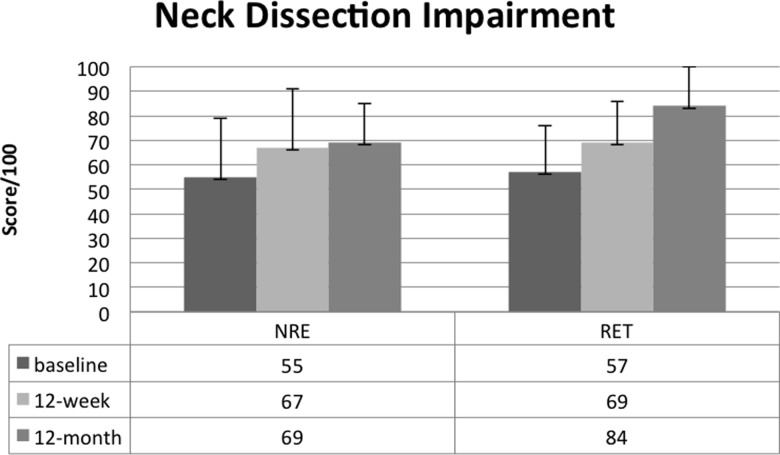
At 12-month follow-up, we found no significant differences between the RET and NRE groups for our primary outcome of shoulder pain and disability, but we found significant differences in favour of those continuing RET with respect to neck dissection–associated impairment (NDII adjusted mean difference=+12.9; 95% CI, 0.36–25.4; see Figure 2). Those who reported continued RET also reported significantly better QOL and performed borderline significantly better on the FACT-Fatigue subscale. Further details are provided in Table 4.
Figure 2.
Baseline, post-intervention, and follow-up results for the NDII by resistance exercise behaviour.
*Significant difference between groups at 12-month follow-up (adjusted); p=0.021.
NDII=Neck Dissection Impairment Index; RET=resistance exercise training.
Table 4.
Associations between Resistance Exercise Behaviour and Patient-Reported Outcomes at 12-Month Follow-Up
| Mean (SD) |
Group difference in mean change (95% CI), p-value |
|||||
|---|---|---|---|---|---|---|
| Outcome | Post- intervention |
12-mo follow-up |
Change | Unadjusted | Adjusted* | MID |
| SPADI, % | ||||||
| NRE | 17.9 (19.6) | 18.3 (24.0) | +0.3 (12.7) | −2.5 (−12.8 to 7.8), 0.62 | −1.0 (−12.6 to 10.6), 0.85 | 10% absolute change13,14 |
| RET | 11.8 (10.9) | 9.6 (13.5) | −2.2 (17.2) | |||
| NDII, % | ||||||
| NRE | 66.5 (24.2) | 68.6 (25.5) | +2.1 (17.6) | +12.7 (0.2–25.1), 0.047 | +13.3 (2.2–24.5), 0.021 | Not established |
| RET | 68.8 (17.1) | 83.6 (15.6) | +14.8 (15.6) | |||
| FACT-An, 0–188 | ||||||
| NRE | 141.0 (25) | 138.5 (27.8) | −2.5 (21.5) | +6.3 (−8.9 to 21.4), 0.41 | +19.5 (2.4–36.7), 0.027 | 6 points17 |
| RET | 157.1 (20.9) | 160.8 (19.5) | +3.7 (18.6) | |||
| FACT-G, 0–108 | ||||||
| NRE | 81.8 (15.0) | 79.5 (13.8) | −2.3 (10.9) | +2.0 (−5.6 to 9.7), 0.59 | +10.1 (2.5–17.7), 0.011 | 4 points16,17 |
| RET | 91.2 (13.0) | 90.9 (8.4) | −0.3 (9.0) | |||
| FACT-Fatigue, 0–52 | ||||||
| NRE | 36.9 (7.9) | 37.4 (10.4) | +0.5 (9.0) | +2.8 (−3.6 to 9.2), 0.38 | +7.1 (−0.2 to 14.4), 0.055 | 3 points16,20 |
| RET | 41.2 (6.7) | 44.5 (9.4) | +3.3 (8.3) | |||
Adjusted for post-intervention value, age, gender, cancer stage, time since surgery, neck dissection type, and pain medication use.
MID=minimal important difference; SPADI=Shoulder Pain and Disability Index; NRE=not continuing resistance exercise; RET=resistance exercise training; NDII=Neck Dissection Impairment Index; FACT-An=Functional Assessment of Cancer Therapy Anemia Scale; FACT-G=Functional Assessment of Cancer Therapy—General Scale; FACT-Fatigue=Functional Assessment of Cancer Therapy Fatigue subscale.
Discussion
Our study is one of only a few to provide long-term follow-up data on the effects of a resistance exercise intervention in the HNC survivor population.13–15 A meta-analysis examining the effect of RET on QOL for all cancers reported a small clinical benefit from resistance exercise immediately post-intervention;14 because few of the included studies reported follow-up data, however, the authors were not able to draw conclusions on long-term effects.14 Our follow-up results show that benefits are largely sustained over the follow-up period and that further improvement may also occur in the subgroup of participants who continue regular RET.
In contrast to the post-intervention findings of our study, we found no significant differences at 12-month follow-up between the originally assigned PRET and TP groups for any outcome. Because most of the TP participants (84%) opted to take part in the PRET program at the crossover point, this finding is not surprising. Although both groups saw similar improvements, which suggests that the timing of the intervention was not a factor, we should note that 12 weeks of TP followed by 12 weeks of PRET (24-week intervention) did not result in significantly better patient-rated outcomes at 12-month follow-up than 12 weeks of PRET alone. One explanation for this finding may be that the lower intensity exercise protocol used with our TP group was not sufficiently demanding, in terms of physiologic overload, for many of the participants.24
Exploratory analyses
Exploratory analyses revealed that participants who continued RET during the follow-up period reported significantly better neck dissection–related functioning, better QOL, and lower levels of fatigue and anemia-related symptoms at 12-month follow-up than NRE participants. Mean NDII scores continued to improve over the 12-month follow-up period; participants who continued with RET had significantly better NDII scores at 12-month follow-up (see Figure 2). Because the NDII examines the impact of neck dissection on QOL and includes items related to work and recreational activities, this outcome measure may prove valuable to detect improvements in functioning over time specific to SAN injury.
Although preliminary, the QOL benefit we found at 12-month follow-up for participants who continued RET exceeded the minimal important difference on the respective FACT-G and FACT-An Scales.21,25 A recent prospective cohort study found that higher pretreatment health-related QOL was predictive of survival from HNC, independent of other known prognostic factors.26 Therefore, randomized controlled trials without post-intervention crossover are needed to better determine the direction of the cause-and-effect relationship between RET and QOL outcomes over the long term.
RET that includes both upper and lower extremity exercises may be particularly beneficial as a targeted intervention strategy for post-surgical HNC survivors to address the losses in lean body mass and declines in physical functioning that commonly occur with HNC treatment.27 A study examining an 18-week high-intensity RET program with a mixed cancer survivor group reported significant improvements in muscle strength, maximal oxygen consumption, and health-related QOL.28 Muscle strength was positively related to physical functioning both before and after the exercise intervention, leading the authors to recommend incorporating supervised RET in cancer rehabilitation programs.28 The high percentage of our TP participants who opted to participate in the PRET program, along with the high completion rate in our study, suggests interest in this type of exercise rehabilitation intervention among HNC survivors. To more clearly elucidate the benefits of resistance exercise, future studies should consider including objective measures to evaluate outcomes such as lean body mass.
Our trial was designed to examine the efficacy of a 12-week resistance exercise program on shoulder pain and dysfunction rather than to promote long-term exercise participation. Therefore, it is not surprising that, at follow-up, only 30% of participants reported continuing RET and only 22% of participants reported meeting public health guidelines for physical activity. A previous survey of HNC survivors found that only 8.5% of survivors were meeting public health guidelines for physical activity.29 Given the high morbidity associated with HNC and the potential benefit from exercise, research is needed to examine strategies that may facilitate and support participation in both therapeutic resistance exercise and general physical activity over the long term.
Limitations
The main limitation of this investigation was our inability to evaluate the long-term benefits of PRET relative to TP because of the optional crossover design of the original study. Another limitation is that participants lost to follow-up (n=15) were excluded from the 12-month follow-up analyses; because of the small sample size and the observational design of the follow-up period for the trial, we did not use statistical techniques to account for such missing data, and these participants may have had QOL outcomes not accounted for in the results reported here. Our exploratory findings, therefore, may represent an overly positive interpretation of the effect of resistance exercise among survivors of HNC.
Additional limitations of this report include the small sample size, reliance on patient-rated outcomes and self-reports of exercise during follow-up, lack of objective outcomes, the exploratory nature of the analyses, and the possibility of chance findings as a result of multiple testing.
Conclusions
Patient-reported outcomes were largely sustained over the 12-month follow-up period. Continuing UE RET may produce further improvements in both neck dissection–related and QOL outcomes in post-surgical HNC survivors. Future research is needed to determine strategies to facilitate ongoing participation in RET. In light of these preliminary findings, research with a larger sample, focusing on behavioural strategies to enhance long-term exercise adoption and without post-intervention crossover, is warranted.
Key Messages
What is already known on this topic
Surgery, including neck dissection procedures, used in treating HNC may result in long-term shoulder morbidity. Evidence exists that PRET produces short-term benefits for shoulder pain and dysfunction.
What this study adds
Patient-reported outcomes after a 12-week PRET program were largely sustained at 12-month follow-up. Long-term participation in RET may be an effective strategy to optimize QOL and reduce impairment related to neck dissection for post-surgical HNC survivors.
Physiotherapy Canada 2015; 67(1);85–93; doi:10.3138/ptc.2014-13O
References
- 1.Nori S, Soo KC, Green RF, et al. Utilization of intraoperative electroneurography to understand the innervation of the trapezius muscle. Muscle Nerve. 1997;20(3):279–85. doi: 10.1002/(SICI)1097-4598(199703)20:3<279::AID-MUS3>3.0.CO;2-8. http://dx.doi.org/10.1002/(SICI)1097-4598(199703)20:3<279::AID-MUS3>3.0.CO;2-8. Medline:9052805. [DOI] [PubMed] [Google Scholar]
- 2.Bradley PJ, Ferlito A, Silver CE, et al. Neck treatment and shoulder morbidity: still a challenge. Head Neck. 2011;33(7):1060–7. doi: 10.1002/hed.21495. http://dx.doi.org/10.1002/hed.21495. Medline:20960564. [DOI] [PubMed] [Google Scholar]
- 3.Nahum AM, Mullally W, Marmor L. A syndrome resulting from radical neck dissection. Arch Otolaryngol. 1961;74(4):424–8. doi: 10.1001/archotol.1961.00740030433011. http://dx.doi.org/10.1001/archotol.1961.00740030433011. Medline:14477989. [DOI] [PubMed] [Google Scholar]
- 4.Terrell JE, Welsh DE, Bradford CR, et al. Pain, quality of life, and spinal accessory nerve status after neck dissection. Laryngoscope. 2000;110(4):620–6. doi: 10.1097/00005537-200004000-00016. http://dx.doi.org/10.1097/00005537-200004000-00016. Medline:10764008. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman JL, Myer CM, III, Aseff JN, et al. Rehabilitation following radical neck dissection. Laryngoscope. 1983;93(8):1083–5. doi: 10.1288/00005537-198308000-00020. Medline:6877017. [DOI] [PubMed] [Google Scholar]
- 6.Herring D, King AI, Connelly M. New rehabilitation concepts in management of radical neck dissection syndrome. Phys Ther. 1987;67(7):1095–9. doi: 10.1093/ptj/67.7.1095. [DOI] [PubMed] [Google Scholar]
- 7.Villanueva R, Ajmani C. The role of rehabilitation medicine in physical restoration of patients with head and neck cancer. Cancer Bull. 1977;29(2):46–54. [Google Scholar]
- 8.Hillel A, Patten C. Neck dissection: morbidity and rehabilitation. Cancer Treat Res. 1990;52:133–47. doi: 10.1007/978-1-4613-1499-8_9. http://dx.doi.org/10.1007/978-1-4613-1499-8_9. Medline:1976363. [DOI] [PubMed] [Google Scholar]
- 9.Gordon SL, Graham WP, III, Black JT, et al. Accessory nerve function after surgical procedures in the posterior triangle. Arch Surg. 1977;112(3):264–8. doi: 10.1001/archsurg.1977.01370030036005. http://dx.doi.org/10.1001/archsurg.1977.01370030036005. Medline:843216. [DOI] [PubMed] [Google Scholar]
- 10.Köybasioglu A, Tokcaer AB, Uslu S, et al. Accessory nerve function after modified radical and lateral neck dissections. Laryngoscope. 2000;110(1):73–7. doi: 10.1097/00005537-200001000-00014. http://dx.doi.org/10.1097/00005537-200001000-00014. Medline:10646719. [DOI] [PubMed] [Google Scholar]
- 11.Watkins JP, Williams GB, Mascioli AA, et al. Shoulder function in patients undergoing selective neck dissection with or without radiation and chemotherapy. Head Neck. 2011;33(5):615–9. doi: 10.1002/hed.21503. http://dx.doi.org/10.1002/hed.21503. Medline:21484915. [DOI] [PubMed] [Google Scholar]
- 12.McNeely ML, Parliament MB, Seikaly H, et al. Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: a randomized controlled trial. Cancer. 2008;113(1):214–22. doi: 10.1002/cncr.23536. http://dx.doi.org/10.1002/cncr.23536. Medline:18457329. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho AP, Vital FM, Soares BG. Exercise interventions for shoulder dysfunction in patients treated for head and neck cancer. Cochrane Database Syst Rev. 2012;(4):CD008693. doi: 10.1002/14651858.CD008693.pub2. Medline:22513964. [DOI] [PubMed] [Google Scholar]
- 14.Cramp F, James A, Lambert J. The effects of resistance training on quality of life in cancer: a systematic literature review and meta-analysis. Support Care Cancer. 2010;18(11):1367–76. doi: 10.1007/s00520-010-0904-z. http://dx.doi.org/10.1007/s00520-010-0904-z. Medline:20502922. [DOI] [PubMed] [Google Scholar]
- 15.McGarvey AC, Chiarelli PE, Osmotherly PG, et al. Physiotherapy for accessory nerve shoulder dysfunction following neck dissection surgery: a literature review. Head Neck. 2011;33(2):274–80. doi: 10.1002/hed.21366. http://dx.doi.org/10.1002/hed.21366. Medline:20222043. [DOI] [PubMed] [Google Scholar]
- 16.Mor V, Laliberte L, Morris JN, et al. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–7. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. http://dx.doi.org/10.1002/1097-0142(19840501)53:9<2002::AID-CNCR2820530933>3.0.CO;2-W. Medline:6704925. [DOI] [PubMed] [Google Scholar]
- 17.Heald SL, Riddle DL, Lamb RL. The Shoulder Pain and Disability Index: the construct validity and responsiveness of a region-specific disability measure. Phys Ther. 1997;77(10):1079–89. doi: 10.1093/ptj/77.10.1079. Medline:9327822. [DOI] [PubMed] [Google Scholar]
- 18.Roach KE, Budiman-Mak E, Songsiridej N, et al. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4(4):143–9. http://dx.doi.org/10.1002/art.1790040403. Medline:11188601. [PubMed] [Google Scholar]
- 19.Taylor RJ, Chepeha JC, Teknos TN, et al. Development and validation of the neck dissection impairment index: a quality of life measure. Arch Otolaryngol Head Neck Surg. 2002;128(1):44–9. doi: 10.1001/archotol.128.1.44. http://dx.doi.org/10.1001/archotol.128.1.44. Medline:11784253. [DOI] [PubMed] [Google Scholar]
- 20.Cella D. The Functional Assessment of Cancer Therapy–Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3) Suppl 2:13–9. Medline:9253779. [PubMed] [Google Scholar]
- 21.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9. doi: 10.1200/JCO.1993.11.3.570. Medline:8445433. [DOI] [PubMed] [Google Scholar]
- 22.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77(5):359–62. Medline:3791117. [PubMed] [Google Scholar]
- 23.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–6. Medline:4053261. [PubMed] [Google Scholar]
- 24.Kisner C, Colby L. Resistance exercise for impaired muscle performance. In: Kisner C, Colby L, editors. Therapeutic exercise: foundations and techniques. Philadelphia: F. A. Davis Company; 2007. p. 839. [Google Scholar]
- 25.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57(9):898–910. doi: 10.1016/j.jclinepi.2004.01.012. http://dx.doi.org/10.1016/j.jclinepi.2004.01.012. Medline:15504633. [DOI] [PubMed] [Google Scholar]
- 26.Østhus AA, Aarstad AK, Olofsson J, et al. Prediction of survival by pretreatment health-related quality-of-life scores in a prospective cohort of patients with head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139(1):14–20. doi: 10.1001/jamaoto.2013.1056. http://dx.doi.org/10.1001/jamaoto.2013.1056. Medline:23329087. [DOI] [PubMed] [Google Scholar]
- 27.Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck. 2007;29(10):893–900. doi: 10.1002/hed.20607. http://dx.doi.org/10.1002/hed.20607. Medline:17405169. [DOI] [PubMed] [Google Scholar]
- 28.De Backer IC, Van Breda E, Vreugdenhil A, et al. High-intensity strength training improves quality of life in cancer survivors. Acta Oncol. 2007;46(8):1143–51. doi: 10.1080/02841860701418838. http://dx.doi.org/10.1080/02841860701418838. Medline:17851864. [DOI] [PubMed] [Google Scholar]
- 29.Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer. 2006;14(10):1012–9. doi: 10.1007/s00520-006-0044-7. http://dx.doi.org/10.1007/s00520-006-0044-7. Medline:16538497. [DOI] [PubMed] [Google Scholar]