Abstract
Background
This study aimed to determine the prevalence of masked hypertension (MHT) and its association with asymptomatic organ damage (AOD) in a low socioeconomic district of Ankara, Turkey.
Material/Methods
We retrospectively reviewed data obtained from the medical records of 712 patients with no known diagnosis of hypertension who presented to a polyclinic due to symptoms related to elevated blood pressure (BP) and were screened for MHT. Essential hypertension (EHT) existed in 86 patients screened for AOD. The presence of AOD in patients diagnosed with MHT and EHT was recorded.
Results
Among the 712 patients, 206 were diagnosed with EHT. Among the remaining 506 patients, 73 were diagnosed with MHT. The patients with MHT had significantly higher left ventricular mass index, carotid intima-media thickness, and 24-h urinary microalbuminuria level (all indicators of AOD) than those with EHT.
Conclusions
A significantly higher percentage of patients with MHT had AOD, as compared to those with EHT, in a low socioeconomic district of Ankara. Based on this finding, patients who present with hypertensive symptoms but have a normal BP should be advised to measure their BP at home.
Keywords: Hypertension, Masked Hypertension, Socioeconomic Factors
Background
The term masked hypertension (MHT) was first used by Pickering in 1992 [1,2]. MHT occurs when the mean 24-h ambulatory blood pressure measurement (ABPM) ≥130/80 mm Hg or mean non-clinical (home or a pharmacy) periodic blood pressure measurement is ≥135/85 mm Hg despite normal (<140/90 mm Hg) polyclinic blood pressure [3]. The prevalence of MHT was observed to be 10–17% in general population-based studies [3]. MHT can lead to severe target organ damage if not diagnosed or treated in a timely fashion. A few recent studies have suggested that although MHT patients appear to be normotensive upon presentation to polyclinics, they are similar to patients with sustained hypertension (SHT) [4–6] in terms of asymptomatic organ damage (AOD) [left ventricular mass index (LVMI) >95 g/m2 for females and >115 g m2 for males, carotid intima media thickness (CIMT) >0.9 mm, microalbuminuria (urinary albumin [U-Alb] level 30–300 mg/dl, and grade 3 or 4 retinopathy] [3]. Another study on hypertensive patients receiving treatment reported that AOD was more common in the MHT group than in the SHT group [7].
Because MHT often remains undetected and is difficult to diagnose – even when patients are examined by a doctor – the prevalence of both MHT and AOD may be high among individuals of low socioeconomic status. To the best of our knowledge, the literature does not include any studies on MHT-associated AOD in a low socioeconomic population. As such, the present study aimed to determine the prevalence of MHT and its association with AOD in a low socioeconomic district of Ankara, Turkey.
Material and Methods
This retrospective study included patients who presented to Ankara Numune Education and Research Hospital, Internal Medicine Clinic, Ankara, Turkey, between April 2012 and May 2013. All the patients included in the study earned the minimum wage and lived in a low socioeconomic district, as defined by the Turkish Statistical Institute. The study protocol was approved by the Ankara Numune Education and Research Hospital Ethics Research Committee and was conducted in accordance with the Declaration of Helsinki.
We retrospectively reviewed the medical records of 712 patients aged >18 years, not previously diagnosed with hypertension, who presented to the polyclinic due to symptoms of elevated blood pressure and were screened for MHT. Patients with secondary hypertension, cerebrovascular events, coronary artery disease, congestive heart failure, chronic glomerulonephritis, nephrotic syndrome, renal failure, or diabetes mellitus were excluded from the study. The blood pressure measurement of patients admitted the polyclinic had been performed at initial admittance and control examination for 6 times in total, 3 each on 2 different days, with 5-minute intervals, following a rest for 5 minutes. After excluding the patients with high blood pressure at the polyclinic, the remaining patients were given verbal and written instructions for measuring their blood pressure at home. These patients were given a blood pressure measuring device and told to measure their blood pressure at home 12 times (4 times each day for 3 days).
Patients with systolic blood pressure (SBP) <140 mmHg and diastolic blood pressure (DBP) <90 mmHg according to mean polyclinic measurements, and SBP >135 mmHg and DBP >85 mmHg according to mean home measurements, were diagnosed as having MHT [8]. Blood pressure was measured using an Omron M3 (Omron Healthcare Co., Ltd., Japan) automated sphygmomanometer calibrated according to the 2002 and 2010 ESH-IP protocol (European Society of Hypertension International Protocol) [9]. Among the patients who received treatment (approximately 3–4 weeks) for MHT during their follow-up, 43 patients underwent echocardiography, funduscopic examination, carotid ultrasonography, and 24-h urinary protein measurement to determine the presence of AOD. The remaining 30 patients refused testing.
This study also included a control group composed of 86 patients diagnosed with EHT, had blood pressure <140/90 mmHg following anti-hypertensive therapy (approximately 3–4 weeks). All participants agreed to be tested and were screened for the presence of AOD. During MHT screening, EHT patients were matched to the MHT group by age, sex, and BMI.
Body mass index (BMI) was calculated as the ratio of dry weight in kilograms to height squared (in square meters).
Ambulatory blood pressure monitoring
The WatchBP 03 device (Microlife WatchBP AG, Switzerland) was used for 24-h ABPM. Patients were provided information about how to use the device. They were instructed to perform their normal daily activities, but to remain motionless during measurement recording. The devices were programmed to take SBP and DBP measurements for 24 h as follows: every 15 min between 07:00 and 23:00 (daytime) and every 20 min between 23:00 and 07:00 (night time). The method was considered reliable if >70% of measurements were valid.
Echocardiographic examination
Echocardiography was performed by an technician blinded to the patients’ clinical data, using a commercially available echocardiography machine (Vivid 7, GE-Vingmed Ultrasound AS, Horten, Norway) equipped with a 2.5-MHz phased array transducer. Complete 2D, color, pulsed, and continuous-wave Doppler examinations were performed, according to standard techniques. Left ventricular mass (LVM) was calculated from 2D echocardiographic measurements using the Devereux formula:
| [ 10]. |
The obtained LVM values were indexed to body surface area and are presented as the LVM index (LVMI) [11].
Carotid intima-media thickness measurement
Carotid intima-media thickness was measured by a radiologist blinded to the patients’ clinical status while the patients were in the supine position with their hand bent backwards, using a high-resolution B-mode ultrasound device (Logic 7, GE Med. Inc., USA), a linear probe on the right and left common carotid artery, and an automated system. Measurements were performed at 3 different points: the right and left common carotid artery, bifurcation, and the first 2 cm of the internal carotid artery. Longitudinal measurements were obtained from the distances defined as vessel lumen echogenicity and media-adventitia echogenicity [12]. Mean CIMT was calculated based on the average of 3 measurements from each carotid artery [13].
Funduscopic examination
For funduscopic examination, 1 drop of tropicamide 1% solution was dripped into each eye 15 min prior to examination using a biomicroscopy device (Topcon SL-3C, Topcon Corp., Japan). Retinopathy staging was in accordance with 2013 European Society of Hypertension and European Society of Cardiology Hypertension guidelines, as follows: Grade I: arteriolar narrowing – either focal or general in nature; grade II: arteriovenous nicking; grade III: retinal hemorrhages, microaneurysms, hard exudates, and cotton wool spots; grade IV: grade III signs plus papilledema and/or macular edema [3].
Biochemical parameters
The patients were provided written and verbal instructions on how to collect and store 24-h urine samples. The turbidimetric method was used to measure 24-h urinary protein and microalbumin; albumin was measured using the bromocresol green method; fasting blood sugar, creatinine, uric acid, total protein, total cholesterol, and triglyceride were measured via the colorimetric enzymatic method; and high-density lipoprotein (HDL) cholesterol was measured via the homogenous colorimetric enzymatic method using an Hitachi Modular P800 (Roche Diagnostics Corp. Indiana, USA) analyzer. Low-density lipoprotein (LDL) cholesterol was calculated according to the Friedewald method. The glomerular filtration rate (GFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation:
| [ 14]. |
Statistical analysis
All statistical analyses were performed using SPSS v.20.0 for Windows (IBM Corp., Armonk, NY). The Kolmogorov-Smirnov test was used for data with normal distribution. Numeric parameters with normal distribution are shown as mean ±SD, and those not normally distributed are shown as median (range). Categorical variables are expressed as number and percentage. Correlations between numeric parameters were analyzed using Pearson’s and Spearman’s correlation analyses. Categorical variables were compared via the chi-square test and Fisher’s exact chi-square test. Differences between groups in numeric parameters with normal distribution were analyzed using the t test for independent samples, and differences between groups in numeric parameters not normally distributed were analyzed using the Mann-Whitney U test. Stepwise multivariate robust regression analysis was used to identify factors that increase the risk of target AOD. The level of statistical significance was set at P<0.05.
Results
The data set included records for 712 patients (288 males and 424 females) with a mean age of 57.1±9.6 years. In all, 206 (28.9%) patients were considered hypertensive based on blood pressure >140/90 mmHg; among the remaining 506 patients (164 males and 342 females) with a mean age of 57.1±9.6 years, 73 (10.2%) were diagnosed as MHT based on mean polyclinic measured SBP <140 mmHg and DBP <90 mmHg, and mean home-measured SBP >135 mmHg and DBP >85 mmHg. In total, 433 (60.8%) patients were considered normotensive based on mean polyclinic-measured blood pressure <140/90 mmHg and mean home-measured blood pressure <135/85 mmHg.
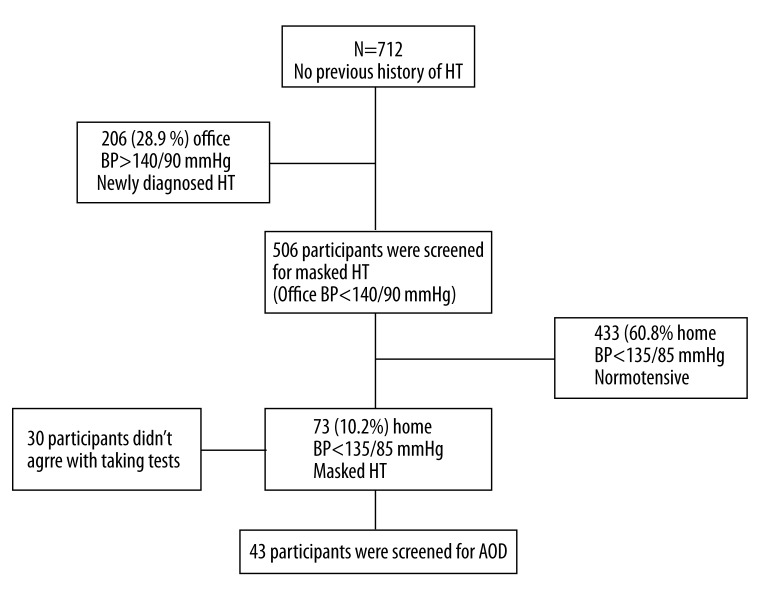
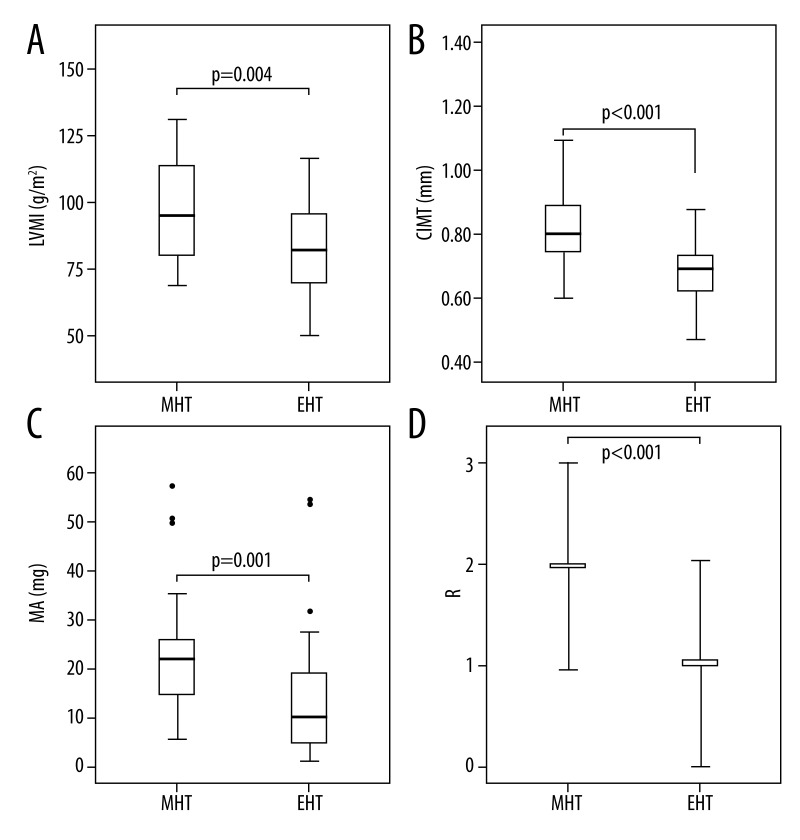
Of the 73 patients diagnosed with MHT, 30 were not examined for AOD (Figure 1). Demographic characteristics of the remaining 43 (23 males and 20 females, mean age of 52.6±9.4 years) MHT patients and the EHT group (86 patients, 46 males and 40 females, mean age of 49.9±10.7 years), and the indicators for AOD are presented in Table 1. The MHT and EHT groups were similar in terms of age, sex, BMI, and mean 24-h blood pressure, whereas the duration of hypertension was significantly longer in the EHT group than in the MHT group (3.3±0.9 years vs. 2.0±0.6 years, P<0.001). The AOD indicators CIMT (0.91±0.2 mm vs. 0.70±0.1 mm) and LVMI (95.7±18.2 g/m2 vs. 84.6±20.9 g/m2), and the mean microalbuminuria level (22 mg/d vs. 10.25 mg/d) and retinopathy stage were significantly higher (P<0.05) in the MHT group than in the EHT (Figure 2).
Figure 1.
Study profile. AOD: Asymptomatic organ damage; BP: blood pressure; HT: hypertension.
Table 1.
Demographic characteristics of patients.
| Variables | Masked hypertension (n=43) | Essential hypertension (n=86) | p |
|---|---|---|---|
| Age (y) | 52.6±9.4 | 49.9±10.7 | >0.05 |
| Sex (male/female) | 23/20 | 46/40 | >0.05 |
| BMI (kg/m2) | 30.7±4.4 | 29.7±4.2 | >0.05 |
| Duration of hypertension (y) | 2.0±0.6 | 3.3±0.9 | <0.001 |
| Current smoking [n, (%)] | 7 (16.3) | 8 (9.3) | >0.05 |
| Habitual drinking [n, (%)] | 6 (14.0) | 8 (9.3) | >0.05 |
| GFR (ml/dk/1.73 m2) | 95.0±14.0 | 97.8±10.5 | >0.05 |
| Ambulatory BP (mmHg) | |||
| 24-h SBP | 120.6±11.3 | 124.5±15.6 | >0.05 |
| 24-h DBP | 76.4±8.4 | 77.9±10.2 | >0.05 |
| CIMT (mm) | 0.91±0.2 | 0.70±0.1 | <0.001 |
| LVMI (g/m2) | 95.7±18.2 | 84.6±20.9 | 0.004 |
| Microalbuminuria (mg/day) | 22 (5.8–200) | 10.25 (1.0–91.8) | 0.001 |
| Retinopathy stage | 2 (1–3) | 1 (0–3) | <0.001 |
Values are presented in mean ±SD, median (min-max) or percentage. 24-h SBP – 24 hours systolic blood pressure; 24-h DBP – 24 hours diastolic blood pressure, CIMT – carotid intima-media thickness, BMI – body mass index; GFR – glomerular filtration rate; LVMI – left ventricular mass index.
Figure 2.
Left ventricular mass index (LVMI) (A), carotid intima-media thickness (CIMT) (B), 24-h urine microalbuminuria (MA) (C), and retinopathy grade (R) (D) in the 2 study groups. * Outliers. MHT: masked hypertension; EHT: essential hypertension.
There was a positive correlation between the mean 24-h SBP and LVMI level in the MHT and EHT groups. Mean 24-h SBP was positively correlated with CIMT and the microalbuminuria level in the EHT group but there was no correlation in the MHT group. There was a positive correlation between retinopathy stage and mean 24-h SBP in the MHT group, but there was no correlation in the EHT group. There was no significant relationship between mean 24-h DBP and LVMI in the MHT group or EHT group. In the EHT group, mean 24-h DBP was positively correlated with CIMT and the microalbuminuria level, but in the MHT group there was a positive correlation only between 24-h DBP and the microalbuminuria level. Retinopathy stage was positively correlated with mean 24-h DBP in the MHT group, but not in the EHT group (Table 2).
Table 2.
Correlations between the masked hypertension and the essential hypertension.
| HT | Variables | 24-h SBP | 24-h DBP | HT TIME | |||
|---|---|---|---|---|---|---|---|
| R | P | R | p | r | p | ||
| Essential | BMI | 0.117 | 0.283 | 0.110 | 0.315 | −0.025 | 0.819 |
| 24-h SBP | – | – | 0.838 | <0.001 | 0.073 | 0.505 | |
| 24-h DBP | 0.838 | <0.001 | – | – | 0.042 | 0.700 | |
| Duration of HT | 0.073 | 0.505 | 0.042 | 0.700 | – | – | |
| GFR | 0.051 | 0.643 | 0.158 | 0.146 | −0.074 | 0.500 | |
| LVMI | 0.310 | 0.004 | 0.174 | 0.109 | 0.084 | 0.444 | |
| CIMT | 0.296 | 0.006 | 0.241 | 0.026 | 0.018 | 0.870 | |
| Retinopathy stage | 0.105 | 0.338 | 0.052 | 0.635 | −0.051 | 0.639 | |
| Microalbuminuria | 0.364 | 0.001 | 0.224 | 0.038 | −0.012 | 0.911 | |
| Masked | BMI | −0.131 | 0.403 | −0.065 | 0.681 | −0.229 | 0.139 |
| 24-h SBP | – | – | 0.777 | <0.001 | 0.245 | 0.113 | |
| 24-h DBP | 0.777 | <0.001 | – | – | 0.142 | 0.363 | |
| Duration of HT | 0.245 | 0.113 | 0.142 | 0.363 | – | – | |
| GFR | −0.035 | 0.826 | 0.105 | 0.503 | 0.086 | 0.585 | |
| LVMI | 0.328 | 0.032 | 0.249 | 0.108 | 0.074 | 0.637 | |
| CIMT | 0.247 | 0.110 | −0.025 | 0.874 | −0.150 | 0.336 | |
| Retinopathy stage | 0.390 | 0.010 | 0.300 | 0.030 | 0.389 | 0.010 | |
| Microalbuminuria | 0.200 | 0.199 | 0.320 | 0.036 | 0.064 | 0.682 | |
BMI – body mass index; 24-h SBP – 24 hours systolic blood pressure; 24-h DBP – 24 hours diastolic blood pressure; HT – hypertension; GFR – glomerular filtration rate; LVMI – left ventricular mass index; CIMT – carotid intima-media thickness.
In the EHT group, LVMI was positively correlated with age and mean 24-h SBP, and was negatively correlated with the creatinine level, but was not significantly correlated with any of the other parameters. In the MHT group, only LVMI was positively correlated with mean 24-h SBP. In the EHT group, CIMT was positively correlated with age, mean 24-h SBP, and mean 24-h DBP, and was negatively correlated with the albumin level, but was not significantly correlated with the other parameters. In the MHT group, age was positively correlated with the fasting blood sugar level and was negatively correlated with GFR, total cholesterol, and LDL levels. In the EHT group, the microalbuminuria level was positively correlated with mean 24-h SBP and the uric acid level, but was not significantly correlated with the other parameters. In the MHT group, there was a positive correlation between the microalbuminuria level and mean 24-h DBP (Table 3).
Table 3.
Correlations between the asymptomatic organ damage variables in patients with masked hypertension and essential hypertension.
| HT | Variables | LVMI | CIMT | 24-h Microalbuminuria | |||
|---|---|---|---|---|---|---|---|
| R | P | R | p | r | p | ||
| Essential | Age | 0.265 | 0.014 | 0.359 | 0.001 | −0.031 | 0.775 |
| Masked | −0.012 | 0.916 | 0.022 | 0.839 | 0.020 | 0.857 | |
| Body mass index | 0.069 | 0.526 | 0.064 | 0.560 | −0.017 | 0.875 | |
| Duration of HT | 0.078 | 0.476 | 0.003 | 0.980 | −0.012 | 0.911 | |
| Current smoking | 0.136 | 0.212 | 0.022 | 0.841 | 0.011 | 0.918 | |
| Habitual drinking | 0.056 | 0.607 | 0.146 | 0.181 | 0.073 | 0.502 | |
| GFR | 0.097 | 0.376 | −0.186 | 0.087 | 0.151 | 0.165 | |
| 24-h SBP | 0.310 | 0.004 | 0.400 | <0.001 | 0.253 | 0.019 | |
| 24-h DBP | 0.174 | 0.109 | 0.316 | 0.003 | 0.103 | 0.347 | |
| Fasting blood sugar | 0.167 | 0.124 | 0.111 | 0.308 | 0.147 | 0.177 | |
| Creatinine | −0.219 | 0.043 | 0.021 | 0.846 | −0.073 | 0.503 | |
| TPROT | −0.011 | 0.923 | −0.103 | 0.345 | −0.032 | 0.770 | |
| Albumin | −0.039 | 0.719 | −0.300 | 0.005 | 0.109 | 0.319 | |
| Uric Acid | 0.054 | 0.622 | −0.022 | 0.844 | 0.238 | 0.027 | |
| Triglycerides | −0.097 | 0.376 | −0.048 | 0.661 | 0.151 | 0.165 | |
| Cholesterol | −0.161 | 0.139 | −0.078 | 0.478 | 0.027 | 0.805 | |
| LDL | −0.113 | 0.298 | −0.103 | 0.348 | −0.034 | 0.753 | |
| HDL | −0.142 | 0.194 | 0.063 | 0.563 | −0.061 | 0.580 | |
| Masked | Age | 0.140 | 0.370 | 0.559 | <0.001 | −0.090 | 0.566 |
| Sex | 0.132 | 0.398 | 0.112 | 0.473 | −0.152 | 0.329 | |
| Body mass index | −0.070 | 0.657 | 0.014 | 0.348 | 0.061 | 0.699 | |
| Duration of HT | 0.067 | 0.668 | −0.147 | 0.348 | 0.064 | 0.682 | |
| Current smoking | 0.273 | 0.077 | 0.133 | 0.394 | 0.008 | 0.961 | |
| Habitual drinking | 0.130 | 0.406 | 0.056 | 0.719 | 0.038 | 0.809 | |
| GFR | −0.111 | 0.477 | −0.400 | 0.008 | −0.092 | 0.559 | |
| 24-h SBP | 0.328 | 0.032 | 0.188 | 0.228 | 0.254 | 0.100 | |
| 24-h DBP | 0.249 | 0.108 | 0.007 | 0.965 | 0.371 | 0.014 | |
| Glucose | 0.254 | 0.101 | 0.442 | 0.003 | −0.078 | 0.617 | |
| Creatinine | 0.066 | 0.676 | 0.149 | 0.341 | 0.056 | 0.720 | |
| TPROT | 0.001 | 0.993 | 0.138 | 0.376 | 0.075 | 0.631 | |
| Albumin | 0.002 | 0.988 | −0.035 | 0.823 | 0.132 | 0.397 | |
| Üric Acid | −0.033 | 0.834 | 0.187 | 0.230 | 0.008 | 0.961 | |
| Triglycerides | −0.059 | 0.705 | 0.052 | 0.742 | −0.031 | 0.843 | |
| Cholesterol | −0.124 | 0.427 | −0.300 | 0.031 | −0.110 | 0.484 | |
| LDL | −0.020 | 0.898 | −0.254 | 0.033 | −0.178 | 0.253 | |
| HDL | −0.082 | 0.599 | 0.020 | 0.898 | 0.253 | 0.102 | |
GFR – glomerular filtration rate; 24-h SBP – 24 hours systolic blood pressure; 24-h DBP – 24 hours diastolic blood pressure; TPROT – total protein; LDL – low density lipoprotein; HT – hypertension; HDL – high density lipoprotein.
Based on a stepwise regression model used for analyzing risk factors (age, sex, BMI, duration of hypertension, current smoking, habitual drinking, 24-h SBP, 24-h DBP, MHT, fasting blood sugar, GFR, total protein, albumin, uric acid, cholesterol, triglyceride, LDL, and HDL), we found that age, MHT, and mean 24-h SBP were predictors of LVMI. Age, MHT, mean 24-h SBP, fasting blood sugar level, and albumin level were observed to be predictors of CIMT. MHT, mean 24-h SBP, and uric acid level were predictors of microalbuminuria level (Table 4).
Table 4.
Independent predictors for asymptomatic organ damage by multivariable regression analysis.
| Variables | β±SE | 95% C.I. | p value |
|---|---|---|---|
| LV mass index | |||
| Age | 0.509±0.16 | [0.196–0.822] | 0.002 |
| Masked HT | 12.041±3.47 | [5.175–18.907] | 0.001 |
| 24-h SBP | 0.427±0.11 | [0.201–0.654] | <0.001 |
| Creatinine | −18.423±9.72 | [(−37.657) − (0.810)] | 0.060 |
| Current smoking | 0.938±5.10 | [(−709) − (19.477)] | 0.068 |
| R2=0.252, F=8.303, P<0.001 | |||
| CIMT | |||
| Age | 0.617±0.13 | [0.400–0.900] | <0.001 |
| Masked HT | 18.85±2.71 | [13.482–24.224] | <0.001 |
| 24-h SBP | 0.364±0.088 | [0.190–0.539] | <0.001 |
| Glucose | 0.340±0.162 | [0.019–0.661] | 0.038 |
| Albumin | −0.826±0.320 | [(−1.459) − (−0.193)] | 0.011 |
| R2=0.495, F=24.148, P<0.001 | |||
| 24-h microalbuminuria | |||
| Masked HT | 13.37±5.49 | [(2.498) − (24.234)] | 0.016 |
| 24-h SBP | 0.56±0.18 | [(0.207) − (0.914)] | 0.002 |
| Albumin | −1.22±0.64 | [(−2.495) − (0.047)] | 0.059 |
| Uric Acid | 4.06±1.81 | [(0.466) − (7.644)] | 0.027 |
| R2=0.168, F=6.240, P<0.001 | |||
24-h SBP – 24 hours systolic blood pressure; CIMT – carotis intima-media thickness; LV – left ventricular; HT – hypertension, β – coefficients; SE – standard error; C.I. – confidence intervals. The stepwise robust regression model included age, sex, body mass index, duration of hypertension, current smoking, habitual drinking, creatinine clearance, daytime systolic BP, daytime diastolic BP, masked hypertension, glucose, glomerular filtration rate, total protein, albumin, uric acid, cholesterol, triglyceride, LDL and HDL, as possible independent variable.
Discussion
The prevalence of MHT in the present study was 10.2%. Mean LVMI, CIMT, 24-h urinary microalbuminuria level, and retinopathy stage were significantly higher in the MHT group than those in the EHT group. To the best of our knowledge, the present study is the first to examine the relationship between MHT and AOD in Turkey. Although the prevalence of MHT in the present study is similar to that reported by other studies [5,15,16], we think that the prevalence was higher as compared to other districts of Ankara because this review was performed in a district where socioeconomic status was low, the workload was heavy, and the working stress was high.
Regarding subgroups of hypertensive patients and AOD, Liu et al. performed a cross-sectional study that included 359 patents with hypertension and reported that LVMI and CIMT were similar in the SHT and MHT patients, but higher than in normotensive patients [4]. Similarly, the Pressione Arteriose Monitorate E Loro Associazioni (PAMELA) study, which included 3200 hypertensive patients, reported that LVMI and CIMT were similar in the MHT and SHT groups [5]. Other studies reported that the prevalence of AOD in MHT patients is similar to that in SHT patients, but higher than that in controlled hypertension and white coat hypertension groups [17–20]. In contrast, a study performed that included 332 patients with hypertension reported that CIMT, LVMI, and urinary albumin were higher in the MHT group than in the SHT, white coat hypertension, and controlled hypertension groups [7]; however, to the best of our knowledge, the literature is devoid of any study on the relationship between normotensive MHT and EHT patients receiving therapy, and AOD.
Although the duration of hypertension was significantly shorter in the present study’s normotensive MHT group receiving treatment than in the EHT group, the levels of AOD indicators were higher in the MHT group, which could be interpreted in several ways. Firstly, most of the patients in the MHT group were engaged in strenuous manual labor and the resultant high level of oxidative stress might have triggered inflammation leading to target organ damage [21]. Secondly, it could be that the patients in the MHT group were diagnosed late due to either hypertension patterns or their social status, and as a result had high blood pressure for a long time. Lastly, it is possible that the MHT group included more patients with morning hypertension or nighttime hypertension, which are often associated with more negative effects of target organ damage [22] than the EHT group.
In 2 large-scale prospective studies, Clement (22) and Bobrie (23) reported that even though office-based blood pressure in patients treated for hypertension was kept under control, high ambulatory or home systolic blood pressure was suggested to be a strong predictor of cardiovascular morbidity. Another study that included subgroups of hypertensive patients, daytime SBP and MHT were reported to be independent indicators of LVMI, CIMT, and microalbuminuria [7]. Similarly, in the present study, MHT and 24-h SBP were observed to be predictors of such indicators of AOD as LVMI, CIMT, and microalbuminuria in all of the patients treated for hypertension.
The present study has several limitations. Accurate identification of AOD might have been hampered by the study’s retrospective design and small study population; as such, we think prospective research with larger study populations is needed to more effectively assess the effect of MHT on target organ damage. Another limitation of the present study is that the patients did not all use the same antihypertensive medication. Because the cardioprotective and renoprotective effects of the medications they did use varied, future studies should include patients using the same medication, or multivariate regression analysis should be employed to determine the effects of all medications used by hypertensive patients on MHT and AOD.
Conclusions
The present study determined that the prevalence of MHT in a low socioeconomic district of Ankara is similar to that reported by other population-based studies; however, AOD was significantly more common in the MHT group than in the EHT group. Based on these findings, we think that patients that present due to symptoms of hypertension that are normotensive at presentation should measure their blood pressure at home. In such MHT patients, AOD screening should be performed at the onset of treatment, followed by initiation of antihypertensive medication. Larger scale, longer term studies are needed to more clearly understand the effect of MHT on cardiovascular outcome.
Footnotes
Source of support: Departmental sources
Statement
The authors report there no conflicts of interest – financial or otherwise – related to the materials presented herein. The authors alone are responsible for the content and writing of this manuscript.
This study did not receive any financial support, such as grants, equipment, or drugs.
References
- 1.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40(6):795–96. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 2.Konstantopoulou AS, Papargyrio IK, Antonıadou ES, et al. Masked hypertension: a new entity under investigation. Hellenic J Cardiol. 2006;47(4):232–35. [PubMed] [Google Scholar]
- 3.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 4.Liu JE, Roman MJ, Pini R, et al. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131(8):564–72. doi: 10.7326/0003-4819-131-8-199910190-00003. [DOI] [PubMed] [Google Scholar]
- 5.Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study) Circulation. 2001;104(12):1385–92. doi: 10.1161/hc3701.096100. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos DP, Makris TK. Masked hypertension definition, impact, outcomes: a critical review. J Clin Hypertens (Greenwich) 2007;9(12):956–63. doi: 10.1111/j.1524-6175.2007.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomiyama M, Horio T, Yoshii M, et al. Masked hypertension and target organ damage in treated hypertensive patients. Am J Hypertens. 2006;19(9):880–86. doi: 10.1016/j.amjhyper.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Bacaksiz A, Erdogan E, Sonmez O, et al. Ambulatory blood pressure monitoring can unmask hypertension in patients with psoriasis vulgaris. Med Sci Monit. 2013;19:501–9. doi: 10.12659/MSM.889197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien E, Atkins N, Stergiou G, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15(1):23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55(4):613–18. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 11.Grabysa R, Wankowicz Z. Echocardiographic markers of left ventricular dysfunction among men with uncontrolled hypertension and stage 3 chronic kidney disease. Med Sci Monit. 2013;19:838–45. doi: 10.12659/MSM.889586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mieczkowska J, Mosiewicz J, Sak J, et al. Effects of cigarette smoking, metabolic syndrome and dehydroepiandrosterone deficiency on intima-media thickness and endothelial function in hypertensive postmenopausal women. Med Sci Monit. 2012;18(4):CR225–34. doi: 10.12659/MSM.882622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pignoli P, Tremoli E, Poli A, et al. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai Y, Tsuji I, Nagai K, et al. Ambulatory blood pressure monitoring in evaluating the prevalence of hypertension in adults in Ohasama, a rural Japanese community. Hypertens Res. 1996;19(3):207–12. doi: 10.1291/hypres.19.207. [DOI] [PubMed] [Google Scholar]
- 16.Belkic KL, Schnall PL, Landsbergis PA, et al. Hypertension at the workplace – an occult disease? The need for work site surveillance. Adv Psychosom Med. 2001;22:116–38. doi: 10.1159/000059280. [DOI] [PubMed] [Google Scholar]
- 17.Charvat J, Chlumsky J, Szabo M, et al. The association of masked hypertension in treated type 2 diabetic patients with carotid artery IMT. Diabetes Res Clin Pract. 2010;89(3):239–42. doi: 10.1016/j.diabres.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Yoon HJ, Ahn Y, Park JB, et al. Are metabolic risk factors and target organ damage more frequent in masked hypertension than in white coat hypertension? Clin Exp Hypertens. 2010;32(7):480–85. doi: 10.3109/10641963.2010.496517. [DOI] [PubMed] [Google Scholar]
- 19.Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21(4):393–99. doi: 10.1038/ajh.2008.15. [DOI] [PubMed] [Google Scholar]
- 20.Konstantopoulou AS, Konstantopoulou PS, Papargyriou IK, et al. Masked, white coat and sustained hypertension: comparison of target organ damage and psychometric parameters. J Hum Hypertens. 2010;24(3):151–57. doi: 10.1038/jhh.2009.55. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34(4):431–40. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 22.Kawano Y, Horio T, Matayoshi T, Kamide K. Masked hypertension: subtypes and target organ damage. Clin Exp Hypertens. 2008;30(3):289–96. doi: 10.1080/10641960802071026. [DOI] [PubMed] [Google Scholar]