Abstract
Carotenes are naturally abundant unsaturated hydrocarbon pigments, and their fascinating physical and chemical properties have been studied intensively not only for better understanding of the roles in biological processes but also for the use in artificial chemical systems. However, their metal-binding ability has been virtually unexplored. Here we report that β-carotene has the ability to assemble and align ten metal atoms to afford decanuclear homo- and heterometal chain complexes. The metallo–carotenoid framework shows reversible metalation–demetalation reactivity with multiple metals, which allows us to control the size of metal chains as well as the heterobimetallic composition and arrangement of the carotene-supported metal chains.
 Carotenes are naturally abundant, widely studied unsaturated hydrocarbon pigments but their metal-binding ability has been virtually unexplored. Here, the authors demonstrate that they can be used to reversibly assemble and align homo- and hetero-metallic decanuclear chain complexes.
Carotenes are naturally abundant, widely studied unsaturated hydrocarbon pigments but their metal-binding ability has been virtually unexplored. Here, the authors demonstrate that they can be used to reversibly assemble and align homo- and hetero-metallic decanuclear chain complexes.
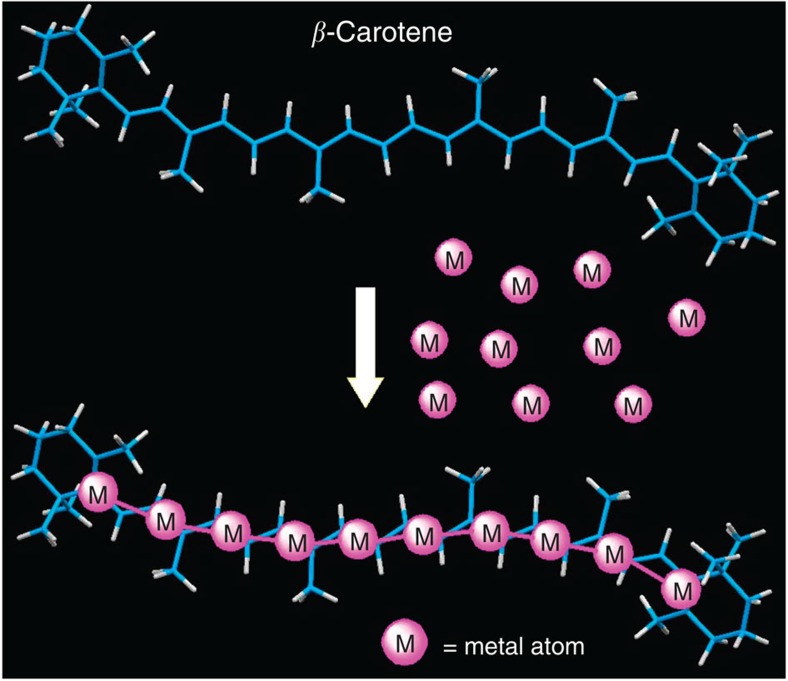
Carotenoids are naturally abundant pigments containing extended π-conjugated C=C double-bond arrays. The fascinating physical and chemical properties of carotenoids, such as light-harvesting, photo-protective, antioxidative and conductive properties have been explored not only for better understanding of their roles in biological processes but also for the use in artificial chemical systems1,2,3,4. An attractive, although yet undeveloped function of carotenoids is their metal-binding ability. While several early reports showed that carotenoids bind mononuclear metal moieties through conventional 1,3-butadiene-type tetrahapto–π-coordination5,6,7,8,9, the extended π-conjugated polyene moieties in their backbones may impart the ability to bind a large metal–metal (M-M)-bonded array through continuous multiple π-coordination bonds (Fig. 1). Such potential multidentate bridging π-coordination ability of carotenoids is highly attractive because of its usefulness in inorganic synthesis. Indeed, chemists have sought to use polynucleating multidentate ligands as the scaffolds or templates to control the metal assembly that provides methodology to synthesize molecularly well-defined metal clusters for catalysis and materials science10,11,12,13,14. However, the scaffold strategy has been applied mostly to the construction of metal clusters with small size owing to the difficulty to design practically useful large scaffold ligands that can assemble and then hold many metal atoms. In fact, only few synthetic scaffold ligands that can bind 10 or more metal atoms through multidentate bridging coordination have been developed. Recently, Peng and colleagues15 developed synthetic multidentate N-donor ligands that lead to isolation of Ni11 chain complexes. Shionoya and co-workers developed artificial metallo–DNA motifs that brought a method to construct a heterometal array of 10 metal ions, although direct M–M bonds are absent in the metal array16. While these previous studies used the σ-type scaffolds bearing multiple hetero-atom σ-binding sites, π-type extended π-conjugated unsaturated hydrocarbon scaffolds are also promising in light of their preferable rows of C=C π-binding sites at regular intervals comparable to M–M bond lengths17,18,19,20. Furthermore, relatively weak C=C π-coordination bond may cause dynamic metal binding at each C=C site, which would be a key for assembling many metal atoms in a convergent manner. Carotenes are the rational choice as the extended π-conjugated scaffold for many metal atoms, because carotenes are one of the most readily available extended unsaturated hydrocarbons containing more than ten π-conjugated C=C double bonds.
Figure 1. Schematic representation of multinuclear metal binding by β-carotene.
Many metal atoms are assembled on the π-conjugated plane of β-carotene.
Herein, we report remarkable multinuclear metal-binding ability of β-carotene through synthesis and characterization of bis-(β-carotene) decanuclear metal chain complexes. The metallo–carotene framework shows interesting multinuclear metalation–demetalation reactivity, allowing us to construct heterobimetallic decanuclear chain. The bis-(β-carotene) decanuclear metal chain complexes are stable rod-like sandwich molecules, exhibiting parallel π–π stacking self-assembly in the crystalline state.
Results
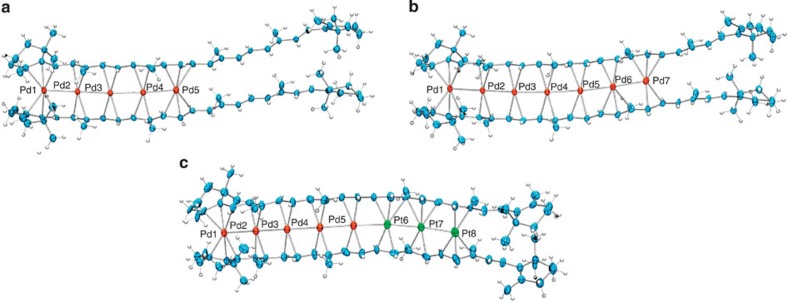
Synthesis and structure of bis-(β-carotene) decanuclear Pd complexes
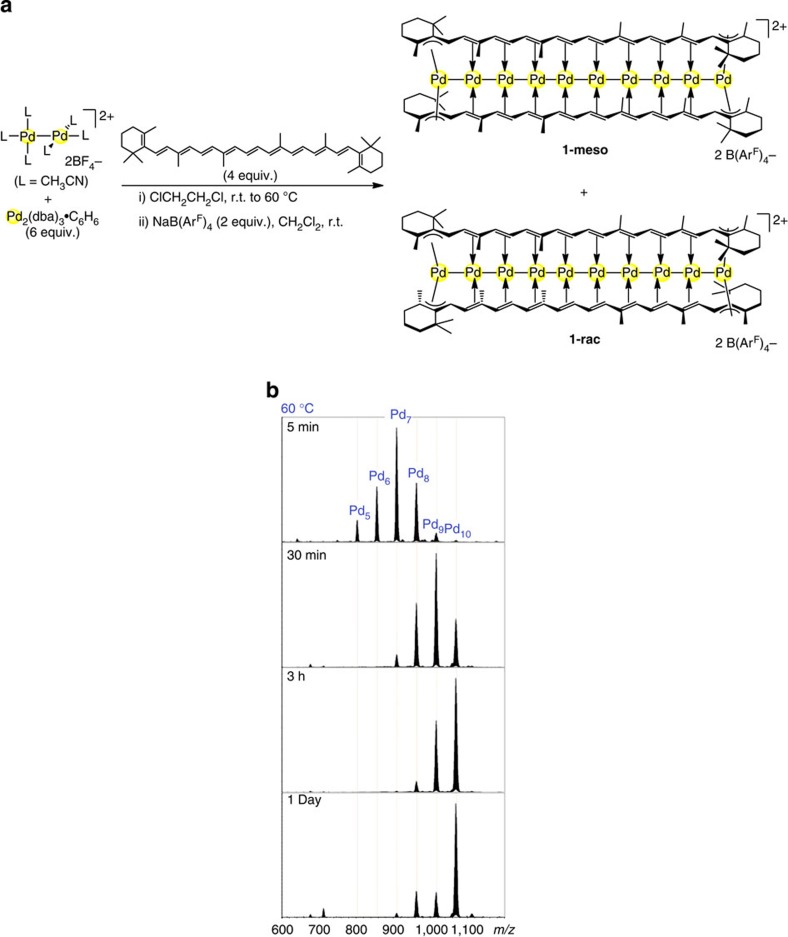
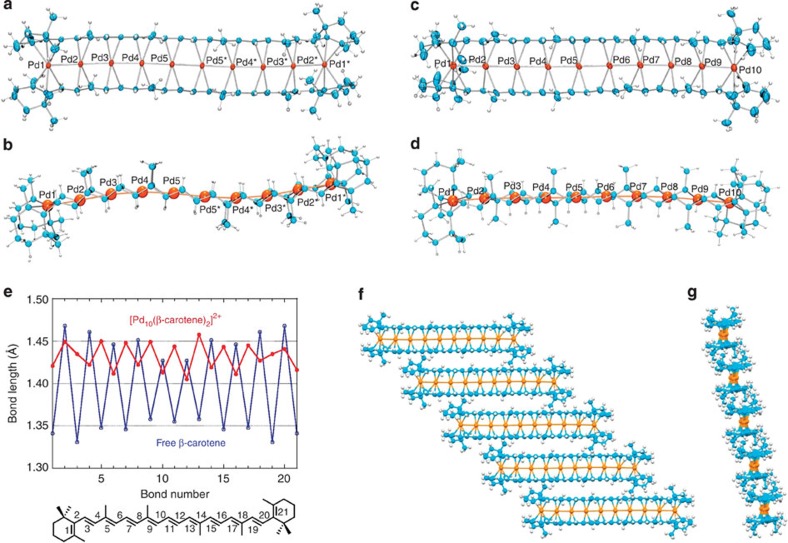
We examined the homoleptic carotene–metal systems in which all auxiliary ligands contained in the starting metal complexes are replaced by carotene to afford a sandwich-type multimetal-binding motif. Pd was used because of its feasibility to undergo convergent metal assembly with the aids of relatively weak metal–ligand and metal–metal bonds21,22. At first, we investigated the full metalation of the bis-carotene π-framework. The redox–condensation reaction of [Pd2(CH3CN)6][BF4]2 (ref. 23) and excess Pd2(dba)3·C6H6 (ref. 24) in the presence of β-carotene at 60 °C gave the decanuclear palladium complex [Pd10(μ10-β-carotene)2][B(ArF)4]2 (1, B(ArF)4=B(3,5-(CF3)2C6H3)4) in 33% yield as a mixture of two isomers (Fig. 2a, 1-meso:1-rac=7:3). It is noted that use of Pd2(dba)3·CHCl3 instead of Pd2(dba)3·C6H6 gave a complicated mixture from which 1 was not obtained. The major isomer (1-meso) is poorly soluble in CH3CN, whereas another isomer (1-rac) is soluble, enabling us to separate the two isomers. The molecular structures of thus obtained yellow complexes 1-meso and 1-rac were determined by X-ray crystallographic analysis (Fig. 3). Remarkably, two β-carotene molecules flank an array of 10 Pd atoms through unprecedentedly large μ10-bridging π-coordination. The two β-carotene ligands are stacking in an eclipsed form in 1-meso and in a staggered one in 1-rac. The nine Pd–Pd bond lengths (2.5827(7)–2.7172(6) Å in 1-meso; 2.6010(11)–2.7111(11) Å in 1-rac) are shorter than that of bulk Pd (2.76 Å), indicating that 10 Pd atoms in 1-meso or 1-rac are connected through Pd–Pd bonds (Supplementary Table 1). The calculated indices of Mayer bond order for each Pd–Pd are ranged from 0.23 to 0.14 for 1-meso; cf., for a typical Pd–Pd-bonded complex, Pd2Cl2(PH3)4, the index of Meyer bond order for Pd–Pd was calculated to be 0.60. The β-carotene ligands in 1-meso showed reduced and inversed C=C/C–C bond length alternation, that is, the long/short alternation (1.46/1.41 Å) was found for the inner nonaene substructure in the β-carotene ligands in 1-meso, being in between the C–C bond lengths in ethane (1.54 Å) and ethylene (1.34 Å; cf. 1.33/1.47 Å for the bond length alternation of free β-carotene; Fig. 3e). It is noted that the bis-β-carotene Pd10 chain dication of 1-meso formed infinite intermolecular π–π stacking columns (the shortest intermolecular C···C distance is 3.51 Å) in the crystalline state (Fig. 3f,g). Such intermolecular backbone π–π stacking represents the typical property of the planar π-conjugated system. The dication of 1-rac formed the π–π stacking dimer instead of the infinite column in the crystalline state. The 1H and 13C{1H} nuclear magnetic resonance (NMR) spectra of 1-meso and 1-rac in CD2Cl2 showed that all olefinic proton and carbon resonances of the β-carotene ligands appeared at the high-field region (olefinic moieties appeared at δ=3.5–2.6 ppm for 1H; δ=111–69 ppm for 13C), being consistent with the solid state structures determined by X-ray crystallography. The decanuclear sandwich complexes 1-meso and 1-rac are stable in solution even in the aerobic condition. Thus, it has been proven that bis-β-carotene π-framework can accommodate 10 Pd atoms array through remarkable multidentate bridging π-coordination. The decanuclear complexes 1-meso and 1-rac are the soluble and isolable organometallic clusters having a long metal chain. Existence of long inorganic palladium wires in solution was recently reported, where di- or tetranuclear palladium units are self-assembled through Pd–Pd interactions25,26. The extended (π-carbon framework)–(metal clusters) contact found in 1-meso and 1-rac may be related with the interface structure of the sp2-carbon material and metal clusters that is of current interests in materials science and catalysis27,28.
Figure 2. Synthesis of bis-(β-carotene) Pd10 chain complexes.
(a) Synthesis of [Pd10(μ10-β-carotene)2][B(ArF)4]2 (1), (b) ESI-MS monitoring of the formation of 1 at 60 °C showing the formation of metal-deficient intermediates [Pdn(β-carotene)2]2+ (n=5, 6, 7, 8 and 9).
Figure 3. Structures of bis-(β-carotene) Pd10 chain complexes.
(a) Thermal ellipsoid (50%) drawing of meso-[Pd10(μ10-β-carotene)2][B(ArF)4]2 (1-meso). (b) Ball-stick drawing of 1-meso. (c) Thermal ellipsoid (30%) drawing of rac-[Pd10(μ10-β-carotene)2][B(ArF)4]2 (1-rac). (d) Ball-stick drawing of 1-rac. (e) C–C bond lengths in 1-meso and free β-carotene (CCDC-253816), determined by X-ray structural analyses. (f) A view of a part of an intermolecular backbone π–π stacking column of 1-meso in the crystalline state. (g) A side view of a part of the π–π stacking column of 1-meso. For a–d,f,g, B(ArF)4 anions and non-coordinating solvent molecules were omitted for clarity.
Synthesis and structure of bis-(β-carotene) decanuclear PdPt complexes
To further explore the metal-binding ability of β-carotene, we next examined the binding of bimetallic chains by bis-β-carotene π-framework. It was difficult to obtain a single bimetallic chain product simply by using Pt2(dba)3 together with Pd2(dba)3 in the synthetic reaction. We then thought a stepwise synthesis, that is, if metal-deficient bis-β-carotene complexes [Pdn(β-carotene)2]2+ (n≤9) can be selectively constructed with Pd, subsequent incorporation of Pt may give bimetallic chains. The metal-deficient dications [Pdn(β-carotene)2]2+ (5≤n≤9) were indeed observed when the formation of 1 (Fig. 2a) was monitored by electrospray ionization mass spectroscopy (ESI-MS; Fig. 2b). Upon mixing the starting materials at ambient temperature, Pd6 and Pd7 complexes of β-carotene were detected as the major MS-detectable species after 3 h. Relatively small MS signals for Pd5, Pd8 and Pd9 complexes were also detected. Further incorporation of Pd into the bis-β-carotene framework proceeded gradually but was incomplete at ambient temperature after 2 days, resulting in that Pd7 and Pd8 complexes were the major MS-detectable species. Heating at 60 °C resulted in shift of the distribution of products to higher nuclearity species, eventually affording the Pd10 chain complexes as the major MS-detectable product. However, it has been difficult to isolate and characterize each of metal-deficient products from the reaction mixtures of the build-up reaction. We confirmed the existence of regioisomers for a short chain model, that is, [Pd2(1,10-diphenylpentaene)2][B(ArF)4]2, which was obtained by the reaction of [Pd2(1,4-diphenyl-1,3-butadiene)2][BF4]2 (ref. 29) with 1,10-diphenyl-1,3,5,7,9-decapentaene at room temperature (r.t.), contained four regioisomers (57:27:10:6) as shown in Supplementary Fig. 1.
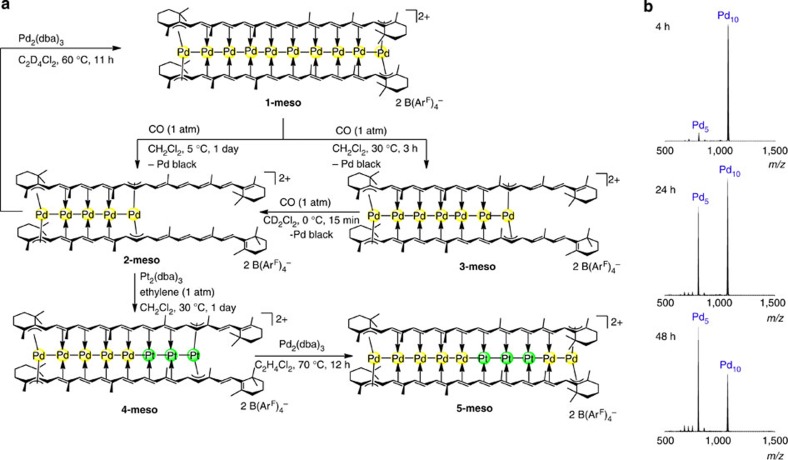
We then found that the metal-deficient complexes of β-carotene can be obtained as a single product by demetalation from the Pd10 complex 1-meso with CO. Thus, the reaction of 1 with CO (1 atmosphere (atm)) at 5 °C for 1 day afforded [Pd5(β-carotene)2][B(ArF)4]2 (2-meso) in 74% yield, together with a significant amount of Pd black (Fig. 4a). 13C{1H} NMR analysis as well as the single-crystal X-ray structure analysis of 2-meso showed that the Pd5 chain occupied the half-part of the bis-β-carotene framework (Fig. 5a). The ESI-MS monitoring experiments on the demetalation reaction with CO (1 atm) at 0 °C showed that the starting Pd10 complex 1-meso and the half-filled Pd5 complex 2-meso were present as the major MS-detectable species during the reaction (Fig. 4b). The prolonged reactions for 1 week resulted in gradual increase of the MS signal for the Pd4 complex. The Pd7 complex [Pd7(β-carotene)2][B(ArF)4]2 (3-meso) was also obtained by exposing 1-meso to CO (1 atm) at 30 °C for 3 h in 20% yield (Fig. 4a). During this reaction, the major ESI-MS-detectable species were the starting Pd10 complex and the Pd7 complex (Supplementary Fig. 2), while the prolonged reactions resulted in further demetalation. The 13C{1H} NMR analysis as well as the single-crystal X-ray structure analysis (Fig. 5b) showed that the Pd7 chain was located in the bis-β-carotene framework. The demetalation of 3-meso with CO (1 atm) at 0 °C occurred rapidly to afford 2-meso with complete consumption of 3-meso within 15 min. Thus, the pseudo-superposed β-carotene stacking structure was preserved during the demetalation under a CO atmosphere, giving a single regioisomer of metal-deficient sandwich. The results of the ESI-MS monitoring experiments suggested that the loss of a Pd0 atom from the Pd10 complex and from the Pd5 or Pd7 complex are relatively slow. The loss of Pd0 likely occurs from one end of the Pd chain, while it is not easy to explain the reason why the demetalation almost stopped at the Pd5 species or the Pd7 species in each reaction condition. There may be several factors that affect aggregation and dissociation of metals and organic ligands (for example, M–CO affinity30, M–carotene bond dissociation and M–M bond dissociation). In the case of associative ligand exchange, the relatively slow release of Pd0 from the filled Pd10 complex is probably due to the lower accessibility of the terminal Pd atoms, which are sterically hindered by the bulky terminal β-groups of the β-carotene ligands, to CO. Consistently, demetalation from the Pd7 complex, where one end of the Pd7 chain is less hindered by ligands, occurred much faster at 0 °C than that from the Pd10 complex.
Figure 4. Synthesis of bimetallic PdPt chain sandwich complexes of β-carotene.
(a) Demetalation and metalation of bis-β-carotene framework. Demetalation of 1-meso with CO afforded the metal-deficient complex [Pd5(μ5-β-carotene)2][B(ArF)4]2 (2-meso) or [Pd7(μ7-β-carotene)2][B(ArF)4]2 (3-meso). Subsequent metalation of 2-meso with Pt0 and then with Pd0 gave a mixed metal complex [Pd5Pt3(μ8-β-carotene)2][B(ArF)4]2 (4-meso) and [Pd5Pt3Pd2(μ10-β-carotene)2][B(ArF)4]2 (5-meso), respectively. (b) ESI-MS monitoring of the demetalation from 1-meso at 0 °C under CO (1 atm) atmosphere.
Figure 5. Structures of metal-deficient sandwich complexes of β-carotene.
(a) Thermal ellipsoid (30%) drawing of [Pd5(μ5-β-carotene)2][B(ArF)4]2 (2-meso). (b) Thermal ellipsoid (30%) drawing of [Pd7(μ7-β-carotene)2][B(ArF)4]2 (3-meso). (c) Thermal ellipsoid (30%) drawing of [Pd5Pt3(μ8-β-carotene)2][B(ArF)4]2 (4-meso). For a–c, B(ArF)4 anions and non-coordinating solvent molecules were omitted for clarity. The coordination modes of two β-carotene ligands in the crystalline 3-meso or 4-meso are slightly different. Furthermore, in the crystal structures of the metal-deficient complexes, 2-meso, 3-meso and 4-meso, the uncoordinated cyclohexenyl group are not superposed due to the rotation at C6–C7 bond. The NMR spectra of 2-meso, 3-meso or 4-meso showed only a single set of β-carotene signals.
We next confirmed that metal-refilling reaction from isolated metal-deficient complexes proceeds smoothly by using the Pd5 complex 2-meso, that is, addition of Pd2(dba)3·C6H6 to 2-meso in C2D4Cl2 at 60 °C afforded the Pd10 complex 1-meso (41% yield). We then tested whether metal refilling of 2-meso with Pt0 is possible. Thus, the bimetallic Pd5Pt3 chain complex [Pd5Pt3(β-carotene)2][B(ArF)4]2 (4-meso) was formed by treatment of 2-meso with Pt2(dba)3·CHCl3 in the presence of ethylene (1 atm) at 30 °C for 1 day (59% yield; Fig. 4a). In the absence of ethylene, the metalation with Pt2(dba)3 did not proceed at the present condition. Addition of ethylene generated Pt–ethylene complexes in situ, which might be more reactive and soluble than Pt2(dba)3 (ref. 31). The Pd5−Pt3 mixed metal arrangement in 4-meso was confirmed by X-ray crystallographic analysis (Fig. 5c). The Pd5 chain (Pd–Pd=2.731(2)–2.629(2) Å) and the Pt3 chain (Pt–Pt=2.6612(9) and 2.6864(10) Å) are connected through Pd–Pt bond (2.657(2) Å). The Pd5Pt3 arrangement was also confirmed by 13C NMR analyses in CD2Cl2 where only one set of β-carotene signals was observed at 25 °C, and 13C signals for Pt-bound carbons appeared at relatively higher field compared with those for Pd-bound carbons. Further metalation of 4-meso with Pd2(dba)3·C6H6 at 70 °C gave decanuclear bimetallic chain complex [Pd5Pt3Pd2(β-carotene)2][B(ArF)4]2 (5-meso) (Fig. 4a). The alternative metal arrangement, Pd5–Pt3–Pd2 was confirmed by assignment of the upfield shifted Pt-bound carbons of the β-carotene ligands in 13C{1H} NMR analyses in CD2Cl2, that is, the substantial upfield shifts of the Pt-bound carbons of the β-carotene ligands in 5-meso relative to those in 1-meso (Δδ=8–13 ppm) were observed, while the chemical shifts of the Pd-bound carbons in 5-meso are similar to those in 1-meso (Δδ=0–2 ppm). Thus, β-carotene has the ability to bind bimetallic decanuclear chain, where stepwise demetalation–metalation sequence is useful in controlling the bimetal arrangement. Such reversible accommodation/liberation of multinuclear metal atoms has rarely been attained in metal cluster chemistry32, representing a facile dynamic metal-binding feature derived from weakly coordinating olefin π-coordination33,34 as well as M–M bonds in organometallic sandwich frameworks35.
Absorption spectra of bis-(β-carotene) Pd complexes
It is noted that [Pdn(β-carotene)2][B(ArF)4]2 showed a nuclearity-dependent absorption profile, that is, the red-shift of maximum absorption bands was observed according to decrease of the number of Pd atoms (362 nm for 1-meso, 373 nm for 3-meso and 468 nm for 2-meso) (Supplementary Fig. 3). The absorption spectra were well described by the time-dependent density functional theory calculations at the Coulomb-attenuated B3LYP level36, suggesting that (i) the absorption bands originate mainly from the ligand-to-metal charge transfer (Supplementary Fig. 4 and Supplementary Table 2) and (ii) the observed red-shift with decreasing number of Pd atoms reflects the increasing stabilization of lower-lying unoccupied molecular orbitals that have antibonding character for dσ(M)-dσ(M) and d(M)-p(L) orbital interactions (Supplementary Figs 5–7 and Supplementary Tables 3–5). Visible-light irradiation resulted in the formation of 1 without heating, that is, the reaction similar to Fig. 2a with visible light irradiation (Xenon lamp, >385 nm) yielded 1 at 20 °C in 24% yield (1-meso:1-rac=7:3) (Supplementary Fig. 8 and Supplementary Methods).
Discussion
In this report, it has been proven that β-carotene, a naturally abundant and readily available unsaturated hydrocarbon pigment, has the ability to bind decanuclear homo- and heterometal chains through unprecedentedly large μ10-bridging π-coordination. The present results showed that natural extended π-conjugated unsaturated hydrocarbons can be utilized as the multidentate π-scaffolds for the construction of giant metal clusters. Future studies will focus on the physical and chemical properties of the rod-like bis-carotene decametal chain sandwich complexes, such as self-assembling behaviour, multielectron redox behaviour and charge mobility.
Methods
Synthesis and characterization of compounds
All manipulations were conducted under a nitrogen atmosphere using standard Schlenk or drybox techniques. The β-carotene–metal complexes were characterized by elemental analyses, ESI-MS analyses and NMR. The assignment of each resonance in NMR analysis was made with aid of heteronuclear single-quantum correlation (HSQC) or heteronuclear multiple-quantum correlation (HMQC) and heteronuclear multiple-bond correlation (HMBC) techniques. Furthermore, the five complexes (1-meso, 1-rac, 2-meso, 3-meso and 4-meso) were structurally determined by X-ray crystallographic analyses (Supplementary Data 1, 2, 3, 4, 5).
Synthesis of [Pd10(β-carotene)2][B(ArF)4]2 (1-meso) and (1-rac)
To a suspension of β-carotene (679 mg, 1.27 mmol) in ClCH2CH2Cl (200 ml) were added Pd2(dba)3·(C6H6) (1.89 g, 1.90 mmol) and [Pd2(CH3CN)6][BF4]2 (200 mg, 0.316 mmol) at r.t. The reaction mixture was stirred under nitrogen atmosphere at 60 °C for 1 day. The reaction mixture was filtered and the filtrate was dried in vacuo. The obtained brown powder and NaB(ArF)4 (560 mg, 0.632 mmol) was added to CH2Cl2, and the mixture was stirred for 5 min at r.t. Et2O was added to the solution and the mixture was filtered. The filtrate was dried in vacuo to yield a red powder. After washing with CH3CN, [Pd10(β-carotene)2][B(ArF)4]2 (1-meso) was isolated as an yellow powder (290 mg, 24%). [Pd10(β-carotene)2][B(ArF)4]2 (1-rac) was obtained by recrystallization from the CH3CN solution (107 mg, 9%). For 1-meso: 1H NMR (400 MHz, CD2Cl2, 25 °C): δ–0.27 (s, 12H), –0.15 (s, 12H), 0.33 (s, 12H), 1.54 (s, 12H), 1.64 (m, 4H), 1.78 (m, 4H), 1.99 (m, 4H), 2.08 (m, 4H), 2.10 (s, 12H), 2.65 (d, J=12 Hz, 4H), 2.66 (d, J=12 Hz, 4H), 2.86 (dd, J=3 Hz, J=9 Hz, 4H), 2.95 (d, J=12 Hz, 4H), 3.04–3.11 (m, 8H), 3.28–3.45 (m, 8H), 3.47 (t, J=12 Hz, 4H), 7.50 (s, 8H, p-B(ArF)4), 7.65 (s, 16H, o-B(ArF)4). 13C NMR (100 MHz, CD2Cl2, 25 °C): δ 14.3, 15.0, 20.5, 23.7, 28.1, 31.8, 34.9, 36.8, 43.5, 74.1, 74.7, 80.7, 82.1, 83.0, 83.3, 84.5, 98.0, 103.3, 109.7, 110.1, 117.8 (p-B(ArF)4), 125.4 (CF3-B(ArF)4), 129.2 (m-B(ArF)4), 135.1 (o-B(ArF)4), 162.1 (ipso-B(ArF)4). MS (ESI) m/z calcd. for [C80H112Pd10]2+: 1,068.9601, found: 1,068.9647. Anal. calcd. For C144H136B2F48Pd10: C, 44.76; H, 3.55, found: C, 44.77; H, 3.73. A single crystal suitable for X-ray crystallographic analysis was grown from a CH2Cl2-Toluene solution (Supplementary Methods). For 1-rac: 1H NMR (400 MHz, CD2Cl2, 25 °C): δ–0.72 (s, 12H), 0.20 (s, 12H), 0.38 (s, 12H), 1.40 (m, 4H), 1.70 (m, 4H), 1.73 (s, 12H), 1.80 (m, 4H), 1.83 (s, 12H), 2.08 (m, 4H), 2.77 (d, J=12 Hz, 4H), 2.80 (d, J=12 Hz, 4H), 2.85 (d, J=12 Hz, 4H), 3.03 (d, J=12 Hz, 4H), 3.07 (dd, J=3 Hz, J=9 Hz, 4H), 3.33 (m, 4H), 3.34 (t, J=12 Hz, 4H), 3.46 (dd, J=3 Hz, J=9 Hz, 4H), 3.52 (m, 4H), 7.30 (s, 8H, p-B(ArF)4), 7.65 (s, 16H, o-B(ArF)4). 13C NMR (100 MHz, CD2Cl2, 25 °C): δ 13.7, 13.8, 20.0, 24.4, 29.4, 29.7, 34.4, 36.5, 43.1, 69.3, 76.5, 79.5, 84.0, 87.5, 88.1, 89.3, 95.0, 96.1, 99.4, 108.1, 117.8 (p-B(ArF)4), 125.4 (CF3-B(ArF)4), 129.2 (m-B(ArF)4), 135.1 (o-B(ArF)4), 162.1 (ipso-B(ArF)4). MS (ESI) m/z calcd. for [C80H112Pd10]2+: 1,068.9601, found: 1,068.9432. Anal. calcd. For C144H136B2F48Pd10·C6H14: C, 45.60; H, 3.83, found: C, 45.48; H, 3.99. A single crystal suitable for X-ray crystallographic analysis was grown from a diethylether−hexane solution (Supplementary Methods).
Synthesis of [Pd5(β-carotene)2][B(ArF)4]2 (2-meso)
CO gas (1 atm) was bubbled in a CH2Cl2 solution (220 ml) of [Pd10(β-carotene)2][B(ArF)4]2 (1-meso) (340 mg, 88.0 μmol) at 5 °C for 24 h. The reaction mixture was filtered and the filtrate was dried in vacuo to give a dark brown powder. After extraction with CH3CN, the volatiles were removed in vacuo to give [Pd5(β-carotene)2][B(ArF)4]2 (2-meso) as a dark brown powder (218 mg, 74%). 1H NMR (400 MHz, CD2Cl2, 25 °C): δ–0.29 (s, 6H), 0.05 (s, 6H), 0.90 (s, 6H), 1.05 (s, 6H), 1.06 (s, 6H), 1.47 (m, 4H), 1.60 (s, 6H), 1.60–1.68 (m, 8H), 1.74 (s, 6H), 1.92–2.08 (m, 6H), 1.94 (s, 6H), 1.98 (s, 6H), 2.02 (d, J=12 Hz, 2H), 2.06 (s, 6H), 2.12 (m, 2H), 2.39 (d, J=12 Hz, 2H), 2.52 (d, J=12 Hz, 2H), 2.92 (t, J=12 Hz, 2H), 2.96 (t, J=12 Hz, 2H), 3.3 (m, 4H), 3.67 (t, J=12 Hz, 2H), 5.13 (t, J=12 Hz, 2H), 5.85 (t, J=12 Hz, 2H), 6.10 (d, J=12 Hz, 2H), 6.15 (d, J=16 Hz, 2H), 6.22 (d, J=12 Hz, 2H), 6.26 (d, J=16 Hz, 2H), 6.29 (d, J=16 Hz, 2H), 6.91 (dd, J=12 Hz, J=16 Hz, 2H), 7.49 (s, 8H, p-B(ArF)4), 7.64 (s, 16H, o-B(ArF)4). 13C NMR (100 MHz, CD2Cl2, 25 °C): δ 13.0, 13.3, 14.0, 14.2, 19.6, 19.9, 22.1, 24.0, 28.0, 29.2, 29.4, 31.6, 33.7, 34.6, 35.0, 36.8, 40.1, 42.7, 76.8, 78.9, 79.7, 89.2, 89.4, 89.6, 91.8, 92.5, 103.5, 111.8, 112.3, 117.8 (p-B(ArF)4), 123.4, 125.5 (CF3-B(ArF)4), 126.7, 129.2 (m-B(ArF)4), 129.3, 129.9, 130.4, 130.6, 135.1 (o-B(ArF)4), 135.7, 137.6, 138.0, 140.3, 143.9, 162.1 (ipso-B(ArF)4). MS (ESI) m/z calcd. for [C80H112Pd5]2+: 802.6996, found: 802.7014. Anal. calcd. For C144H136B2F48Pd5·C6H6: C, 52.83; H, 4.20, found: C, 52.65; H, 4.35. A single crystal suitable for X-ray crystallographic analysis was grown from a diethylether–benzene solution (Supplementary Methods).
Synthesis of [Pd7(β-carotene)2][B(ArF)4]2 (3-meso)
CO gas (1 atm) was bubbled in a CH2Cl2 solution (100 ml) of [Pd10(β-carotene)2][B(ArF)4]2 (1-meso) (107 mg, 27.8 μmol) at 30 °C for 3 h in the dark. The reaction mixture was filtered and the filtrate was dried in vacuo to give a dark red powder. The resultant powder was washed with CH3CN and dried in vacuo. Et2O was added and the mixture was filtered. The filtrate was dried in vacuo to give [Pd7(β-carotene)2][B(ArF)4]2 (3-meso) as a red powder (20.0 mg, 20%). 1H NMR (400 MHz, CD2Cl2, 25 °C): δ–0.27 (s, 6H), –0.04 (s, 6H), 0.07 (s, 6H), 0.67 (s, 6H), 1.12 (s, 6H), 1.13 (s, 6H), 1.51 (m, 4H), 1.59 (s, 6H), 1.61–1.70 (m, 6H), 1.79 (s, 6H), 1.97 (m, 2H), 2.06 (s, 6H), 2.06–2.15 (m, 8H), 2.23 (d, J=12 Hz, 2H), 2.33 (d, J=12 Hz, 2H), 2.34 (s, 6H), 2.40 (t, J=12 Hz, 2H), 2.79 (d, J=12 Hz, 2H), 2.85 (d, J=12 Hz, 2H), 3.03 (d, J=12 Hz, 2H), 3.27 (d, J=12 Hz, 2H), 3.34 (m, 4H), 3.56 (t, J=12 Hz, 2H), 3.65 (d, J=12 Hz, 2H), 4.50 (d, J=12 Hz, 2H), 6.08 (d, J=12 Hz, 2H), 6.28 (d, J=16 Hz, 2H), 6.44 (d, J=16 Hz, 2H), 6.46 (t, J=12 Hz, 2H), 7.48 (s, 8H, p-B(ArF)4), 7.63 (s, 16H, o-B(ArF)4). 13C NMR (100 MHz, CD2Cl2, 25 °C): δ 13.6, 14.4, 14.6, 14.7, 19.6, 20.2, 22.2, 23.9, 28.0, 29.3, 29.5, 31.7, 34.0, 34.5, 34.9, 36.7, 40.2, 43.0, 75.5, 76.8, 79.0, 80.3, 80.6, 86.7, 87.9, 88.8, 89.2, 95.5, 96.1, 96.4, 100.9, 103.5, 111.2, 117.1, 117.8 (p-B(ArF)4), 125.5 (CF3-B(ArF)4), 127.2, 129.2 (m-B(ArF)4), 129.4, 131.5, 135.1 (o-B(ArF)4), 136.6, 137.6, 140.4, 162.1 (ipso-B(ArF)4). MS (ESI) m/z calcd. for [C80H112Pd7]2+: 909.1038, found: 909.1082. Anal. calcd. For C144H136B2F48Pd7·(C6H6)2: C, 50.62; H, 4.03, found: C, 50.73; H, 4.06. A single crystal suitable for X-ray crystallographic analysis was grown from a diethylether–benzene solution (Supplementary Methods).
Synthesis of [Pd5Pt3(β-carotene)2][B(ArF)4]2 (4-meso)
Ethylene gas (1 atm) was bubbled in a CH2Cl2 solution (30 ml) of [Pd5(β-carotene)2][B(ArF)4]2 (2-meso) (54.0 mg, 16.2 μmol) and Pt2(dba)3·(CHCl3) (100 mg, 82.5 μmol) at r.t. for 5 min. After the solution was stirred at 30 °C for 1 day, the colour turned dark brown. The reaction mixture was dried in vacuo to give a dark brown powder. After extraction with Et2O and washing with C6H6, [Pd5Pt3(β-carotene)2][B(ArF)4]2 (4-meso) was isolated as a yellow solid (37.6 mg, 59%). 1H NMR (400 MHz, CD2Cl2, 25 °C): δ–0.28 (s, 6H), –0.11 (s, 6H), 0.50 (s, 12H), 1.17 (s, 6H), 1.21 (s, 6H), 1.56 (s, 6H), 1.56–1.59 (m, 4H), 1.60–1.72 (m, 8H), 1.84 (s, 6H), 1.98 (m, 2H), 2.01 (s, 6H), 2.07 (s, 6H), 2.09 (m, 2H), 2.13–2.18 (m, 4H), 2.34 (d, J=12 Hz, 2H), 2.36 (d, J=12 Hz, 2H), 2.43 (t, J=12 Hz, 2H), 2.71 (d, J=12 Hz, 2H), 2.76 (d, J=14 Hz, 2H), 2.81 (d, J=12 Hz, 2H), 3.00 (t, J=11 Hz, 2H), 3.04 (d, J=12 Hz, 2H), 3.11 (d, J=12 Hz, 2H), 3.3 (m, 4H), 3.40 (m, 2H), 3.48 (t, J=12 Hz, 2H), 3.79 (d, J=11 Hz, 2H), 6.47 (d, J=16 Hz, 2H), 6.68 (d, J=16 Hz, 2H), 7.49 (s, 8H, p-B(ArF)4), 7.65 (s, 16H, o-B(ArF)4). 13C NMR (100 MHz, CD2Cl2, 25 °C): δ 14.3, 14.6, 15.5, 17.5, 19.7, 20.3, 22.2, 23.8, 28.0, 29.2, 29.7, 31.7, 33.9, 34.7, 34.9, 36.8, 40.1, 43.2, 64.4, 67.3, 74.9, 75.1, 75.9, 78.1, 78.7, 79.9, 80.6, 85.2, 85.7, 87.6, 96.9, 98.4, 98.6, 103.5, 110.6, 114.4, 117.8 (p-B(ArF)4), 125.5 (CF3-B(ArF)4), 129.1, 129.2 (m-B(ArF)4), 132.3, 135.1 (o-B(ArF)4), 136.0, 136.5, 162.1 (ipso-B(ArF)4). MS (ESI) m/z calcd. for [C80H112Pd5Pt3]2+: 1,095.1460, found: 1095.1291. Anal. calcd. For C144H136B2F48Pd5Pt3·(C6H6)2: C, 45.99; H, 3.66, found: C, 46.10; H, 3.86. A single crystal suitable for X-ray crystallographic analysis was grown from a dichloromethane–benzene solution (Supplementary Methods).
Synthesis of [Pd5Pt3Pd2(β-carotene)2][B(ArF)4]2 (5-meso)
To a solution of [Pd5Pt3(β-carotene)2][B(ArF)4]2 (4-meso) (58.0 mg, 14.8 μmol) in ClCH2CH2Cl (50 ml) was added Pd2(dba)3·(C6H6) (200 mg, 201 μmol), and the reaction mixture was stirred under nitrogen atmosphere at 70 °C for 12 h. The mixture was filtered and the filtrate was dried in vacuo. After reprecipitation with CH2Cl2/hexane, the yellow powder was obtained. After drying in vacuo, the product was analysed by NMR, showing that [Pd5Pt3Pd2(β-carotene)2][B(ArF)4]2 (5-meso) was formed as a major product with an unidentified minor product (major:minor=8:2; a mixture of two products: 43 mg). For 5-meso: 1H NMR (600 MHz, CD2Cl2, 25 °C): δ–0.27 (s, 6H), –0.22 (s, 6H), –0.17 (s, 6H), 0.11 (s, 6H), 0.31 (s, 6H), 1.01 (s, 6H), 1.48 (s, 6H), 1.54 (s, 6H), 1.63 (m, 4H), 1.78 (m, 4H), 1.98 (m, 4H), 2.05 (s, 6H), 2.08 (m, 4H), 2.09 (s, 6H), 2.52 (d, J=12 Hz, 2H), 2.56 (d, J=12 Hz, 2H), 2.60 (t, J=12 Hz, 2H), 2.62 (d, J=12 Hz, 2H), 2.66 (d, J=12 Hz, 2H), 2.74 (d, J=12 Hz, 2H), 2.90 (d, J=12 Hz, 4H), 2.91 (d, J=12 Hz, 2H), 2.95 (d, J=12 Hz, 2H), 3.07 (d, J=12 Hz, 2H), 3.19 (t, J=12 Hz, 2H), 3.30 (m, 8H), 3.40 (t, J=12 Hz, 2H), 3.42 (t, J=12 Hz, 2H), 7.51 (s, 8H, p-BArF4), 7.66 (s, 16H, o-BArF4). 13C NMR (150 MHz, CD2Cl2, 25 °C): δ 14.1, 14.3, 14.8, 15.8, 20.4, 20.5, 23.7, 28.1, 28.2, 31.8, 31.9, 34.9, 36.8, 36.9, 43.4, 43.5, 70.0, 71.1, 71.2, 71.3, 71.8, 73.6, 74.1, 74.6, 74.8, 80.5, 81.8, 83.1, 83.4, 84.6, 89.9, 98.2, 103.5, 105.3, 109.8, 110.4, 111.4, 117.9 (p-BArF4), 125.0 (CF3-BArF4), 129.2 (m-BArF4), 135.2 (o-BArF4), 162.1 (ipso-BArF4). MS (ESI) m/z calcd. for [C80H112Pd7Pt3]2+: 1,201.5503, found: 1,201.5553. Anal. calcd. For C144H136B2F48Pd7Pt3·(C6H6): C, 42.81; H, 3.40, found: C, 42.87; H, 3.46. The sample suitable for elemental analysis was obtained from a dichloromethane–benzene solution. The minor product might be an isomer of 5-meso having a different metal arrangement (Supplementary Methods).
Synthesis of [Pd2(1,10-diphenylpentaene)2][B(ArF)4]2
To a suspension of 1,10-diphenyl-1,3,5,7,9-decapentaene (80.0 mg, 0.28 mmol) in CH2Cl2 (30 ml) was added [Pd2(1,4-diphenyl-1,3-butadiene)][B(ArF)4]2 (299.6 mg, 0.13 mmol). The mixture was stirred for 1 h at r.t. The reaction mixture was filtered and poured into hexane to give deep-green precipitation. [Pd2(1,10-diphenylpentaene)2][B(ArF)4]2was isolated as a mixture of four isomers by recrystallization (262.1 mg, 82%, isomer ratio 57:27:10:6). 1H NMR (600 MHz, CD2Cl2, 25 °C) of vinyl protons in A (57% of products): δ 3.30 (dd, J=12 Hz, J=12 Hz, 1H), 3.38 (dd, J=12 Hz, J=12 Hz, 1H), 3.54 (dd, J=12 Hz, J=12 Hz, 1H), 3.75 (dd, J=11 Hz, J=12 Hz, 1H), 4.29 (dd, J=11 Hz, J=13 Hz, 1H), 4.33 (dd, J=11 Hz, J=12 Hz, 1H), 4.48 (dd, J=12 Hz, J=13 Hz, 1H), 5.07 (dd, J=12 Hz, J=14 Hz, 1H), 5.52 (dd, J=12 Hz, J=13 Hz, 1H), 5.77 (dd, J=11 Hz, J=13 Hz, 1H), 5.90 (dd, J=11 Hz, J=15 Hz, 1H), 6.05 (dd, J=12 Hz, J=14 Hz, 1H), 6.08 (dd, J=11 Hz, J=15 Hz, 1H), 6.14 (d, J=14 Hz, 1H), 6.21 (dd, J=11 Hz, J=15 Hz, 1H), 6.40 (dd, J=11 Hz, J=11 Hz, 1H), 6.47 (dd, J=11 Hz, J=14 Hz, 1H), 6.50 (d, J=15 Hz, 1H), 6.69 (d, J=15 Hz, 1H), 6.71 (dd, J=15 Hz, 1H). B or C (27% of products): δ 3.34 (dd, J=11 Hz, J=11 Hz, 2H), 3.69 (dd, J=11 Hz, J=11 Hz, 2H), 4.32 (dd, J=11 Hz, J=13 Hz, 2H), 4.98 (dd, J=11 Hz, J=14 Hz, 2H), 5.46 (dd, J=12 Hz, J=14 Hz, 2H), 5.76 (dd, J=12 Hz, 13 Hz, 2H), 6.21 (dd, J=11 Hz, J=14 Hz, 2H), 6.42 (d, J=14 Hz, 2H), 6.61 (dd, J=11 Hz, J=15 Hz, 2H), 6.80 (dd, J=15 Hz, 2H). B or C (10% of products): δ 3.30 (dd, J=11 Hz, J=11 Hz, 2H), 3.78 (dd, J=11 Hz, J=11 Hz, 2H), 4.23 (dd, J=11 Hz, J=13 Hz, 2H), 5.03 (dd, J=11 Hz, J=14 Hz, 2H), ca. 5.46 (2H), 6.13 (d, J=14 Hz, 2H), 6.31 (dd, J=11 Hz, J=15 Hz, 2H), ca. 6.51 (2H), 6.56 (dd, J=11 Hz, J=14 Hz, 2H), 7.01 (dd, J=15 Hz, 2H). D (6% of products): δ 3.48 (m, 4H), 4.49 (m, 4H), 6.01 (dd, J=11 Hz, J=13 Hz, 4H), 6.18 (dd, J=11 Hz, J=15 Hz, 4H), 6.69 (d, J=15.6 Hz, 4H). Anal. calcd. For C108H64B2F48Pd2: C, 51.72; H, 2.57, found: C, 51.59; H, 2.58 (Supplementary Methods).
Author contributions
The idea and plans of this research were made by T.Mu. Experiments and data analysis were performed by S.H., Y.T., M.Y., K.Y., K.M., K.T., T.Ma., S.K. and T.Mu. The theoretical calculations were performed by Y.K. and T.Y. The manuscript was co-written by T.Mu., Y.K. and T.Y. All authors discussed the results.
Additional information
Accession codes: The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 1001723-1001727. These data can be obtained free of charge from The CCDC via www.ccdc.cam.ac.uk/data_request/cif.
How to cite this article: Horiuchi, S. et al. Multinuclear metal-binding ability of a carotene. Nat. Commun. 6:6742 doi: 10.1038/ncomms7742 (2015).
Supplementary Material
Supplementary Figures 1-7, Supplementary Tables 1-2, Supplementary Methods and Supplementary References
Crystallographic Information File for 1-meso.
Crystallographic Information File for 1-rac.
Crystallographic Information File for 2-meso.
Crystallographic Information File for 3-meso.
Crystallographic Information File for 4-meso.
Acknowledgments
This work was supported by JST through PRESTO programme, JSPS and MEXT through Grants-in-aid for Scientific Research and Institute for Molecular Science. We thank Professor H. Kurosawa and Professor S. Ogoshi for discussion.
References
- Polivka T. & Sundstrom V. Ultrafast dynamics of carotenoid excited states−from solution to natural and artificial systems. Chem. Rev. 104, 2021–2072 (2004) . [DOI] [PubMed] [Google Scholar]
- Polivka T. & Frank H. A. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc. Chem. Res. 43, 1125–1134 (2010) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaichi S., Mimuro M. & Tomita Y. Carotenoids -Biological Functions and Diversity Shokabo (2006) . [Google Scholar]
- Visoly-Fisher I. et al. Conductance of a biomolecular wire. Proc. Natl. Acad. Sci. USA 103, 8686–8690 (2006) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M., Tsutsui M. & Vohwinkel F. Iron carbonyl complexes of β-carotene and lycopene. Z. Naturforsch. B 22, 376–379 (1967) . [DOI] [PubMed] [Google Scholar]
- Nakamura A. & Tsutsui M. π-Complexes with biologically significant materials. III. Vitamin A acetate iron tricarbonyl. J. Med. Chem. 7, 335–337 (1964) . [DOI] [PubMed] [Google Scholar]
- Birch A. J., Fitton H., Mason R., Robertson G. B. & Stangroom J. E. Vitamin-A aldehyde iron tricarbonyl. Chem. Commun. 613–614 (1966) . [Google Scholar]
- Mason R. & Robertson G. B. Crystal and molecular structure of (vitamin-A aldehyde)tricarbonyliron. J. Chem. Soc. A 1229–1234 (1970) . [Google Scholar]
- Mashima K. & Nakamura A. Adaptable coordination modes of conjugated 1,3-diene: uniqueness of s-trans coordination. J. Organomet. Chem. 663, 5–12 (2002) . [Google Scholar]
- Bera J. K. & Dunbar K. R. Chain compounds based on transition metal backbones: new life for an old topic. Angew. Chem. Int. Ed. 41, 4453–4457 (2002) . [DOI] [PubMed] [Google Scholar]
- Berry J. F. Metal-metal bonds in chains of three or more metal atoms: from homometallic to heterometallic chains. Struct. Bond 136, 1–28 (2010) . [Google Scholar]
- Zhao Q., Harris T. D. & Betley T. A. [(HL)2Fe6(NCMe)m]n+ (m=0, 2, 4, 6; n=-1, 0, 1, 2, 3, 4, 6): An electron-transfer series featuring octahedral Fe6 clusters supported by a hexaamide ligand platform. J. Am. Chem. Soc. 133, 8293–8306 (2011) . [DOI] [PubMed] [Google Scholar]
- Kanady J. S., Tsui E. Y., Day M. W. & Agapie T. A synthetic model of the Mn3Ca subsite of the oxygen-evolving complex in photosystem II. Science 333, 733–736 (2011) . [DOI] [PubMed] [Google Scholar]
- Sunada Y., Haige R., Otsuka K., Kyushin S. & Nagashima H. A ladder polysilane as a template for folding palladium nanosheets. Nat. Commun. 4, 2014 (2013) . [DOI] [PubMed] [Google Scholar]
- Ismayilov R. H. et al. Two Linear undecanickel mixed-valence complexes: Increasing the size and the scope of the electronic properties of nickel metal strings. Angew. Chem. Int. Ed. 50, 2045–2048 (2011) . [DOI] [PubMed] [Google Scholar]
- Tanaka K. et al. Programmable self-assembly of metal ions inside artificial DNA duplexes. Nat. Nanotechnol. 1, 190–194 (2006) . [DOI] [PubMed] [Google Scholar]
- Murahashi T., Mochizuki E., Kai Y. & Kurosawa H. Organometallic sandwich chains made of conjugated polyenes and metal-metal chains. J. Am. Chem. Soc. 121, 10660–10661 (1999) . [Google Scholar]
- Tatsumi Y., Shirato K., Murahashi T., Ogoshi S. & Kurosawa H. Sandwich complexes containing bent palladium chains. Angew. Chem. Int. Ed. 45, 5799–5803 (2006) . [DOI] [PubMed] [Google Scholar]
- Murahashi T. et al. Discrete sandwich compounds of monolayer palladium sheets. Science 313, 1104–1107 (2006) . [DOI] [PubMed] [Google Scholar]
- Murahashi T., Inoue R., Usui K. & Ogoshi S. Square tetrapalladium sheet sandwich complexes: Cyclononatetraenyl as a versatile face-capping ligand. J. Am. Chem. Soc. 131, 9888–9889 (2009) . [DOI] [PubMed] [Google Scholar]
- Mednikov E. G. & Dahl L. F. Palladium: It forms unique nano-sized carbonyl clusters. J. Chem. Edu. 86, 1135 (2009) . [Google Scholar]
- Murahashi T. & Kurosawa H. Organopalladium complexes containing palladium-palladium bonds. Coord. Chem. Rev. 231, 207–228 (2002) . [Google Scholar]
- Murahashi T., Nagai T., Okuno T., Matsutani T. & Kurosawa H. Synthesis and ligand substitution reactions of a homoleptic acetonitrile dipalladium(I) complex. Chem. Commun. 1689–1690 (2000) . [Google Scholar]
- Ukai T., Kawazura H., Ishii Y., Bonnet J. J. & Ibers J. A. Chemistry of dibenzylideneacetone-palladium(0) complexes: I. Nobel tris(dibenzylideneacetone)dipalladium(solvent) complexes and their reactions with quinones. J. Organomet. Chem. 65, 253–266 (1974) . [Google Scholar]
- Campbell M. G. et al. Synthesis and structure of solution-stable one-dimensional palladium wires. Nat. Chem. 3, 949–953 (2011) . [DOI] [PubMed] [Google Scholar]
- Nakamae K. et al. Self-alignment of low-valent octanuclear palladium atoms. Angew. Chem. Int. Ed. 54, 1016–1021 (2015) . [DOI] [PubMed] [Google Scholar]
- Sarkar S. et al. Metals on graphene and carbon nanotube surfaces: From mobile atoms to atomtronics to bulk metals to clusters and catalysts. Chem. Mater. 26, 184–195 (2014) . [Google Scholar]
- Chambers A., Nemes T., Rodriguez N. M. & Baker R. T. K. Catalytic behavior of graphite nanofiber supported nickel particles. 1. Comparison with other support media. J. Phys. Chem. B 102, 2251–2258 (1998) . [Google Scholar]
- Tatsumi Y., Nagai T., Nakashima H., Murahashi T. & Kurosawa H. Stepwise growth of polypalladium chains in 1,4-diphenyl-1,3-butadiene sandwich complexes. Chem. Commun. 1430–1431 (2004) . [DOI] [PubMed] [Google Scholar]
- Diefenbach A., Bickelhaupt F. M. & Frenking G. The nature of the transition metal-carbonyl bond and the question about the valence orbitals of transition metals. A bond-energy decomposition analysis of TM(CO)6q (TMq=Hf2−, Ta−, W, Re+, Os2+, Ir3+). J. Am. Chem. Soc. 122, 6449–6458 (2000) . [Google Scholar]
- Murahashi T., Usui K., Tachibana Y., Kimura S. & Ogoshi S. Selective construction of Pd2Pt and PdPt2 triangles in a sandwich framework: carbocyclic ligands as scaffolds for a mixed-metal system. Chem. Eur. J. 18, 8886–8890 (2012) . [DOI] [PubMed] [Google Scholar]
- Takemoto S. & Matsuzaka H. Recent advances in the chemistry of ruthenium carbido complexes. Coord. Chem. Rev. 256, 574–588 (2012) . [Google Scholar]
- Uddin J., Dapprich S., Frenking G. & Yates B. F. Nature of the metal-alkene bond in platinum complexes of strained olefins. Organometallics 18, 457–465 (1999) . [Google Scholar]
- Stromberg S., Svensson M. & Zetterberg K. Binding of ethylene to anionic, neutral, and cationic nickel(II), palladium(II), and platinum(II) cis/trans chloride ammonia complexes. A theoretical study. Organometallics 16, 3165–3168 (1997) . [Google Scholar]
- Murahashi T. et al. Redox-induced reversible metal assembly through translocation and reversible ligand coupling in tetranuclear metal sandwich frameworks. Nat. Chem. 4, 52–58 (2012) . [DOI] [PubMed] [Google Scholar]
- Yanai T., Tew D. P. & Handy N. C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393, 51–57 (2004) . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-7, Supplementary Tables 1-2, Supplementary Methods and Supplementary References
Crystallographic Information File for 1-meso.
Crystallographic Information File for 1-rac.
Crystallographic Information File for 2-meso.
Crystallographic Information File for 3-meso.
Crystallographic Information File for 4-meso.