ABSTRACT
Current influenza virus vaccines rely upon the accurate prediction of circulating virus strains months in advance of the actual influenza season in order to allow time for vaccine manufacture. Unfortunately, mismatches occur frequently, and even when perfect matches are achieved, suboptimal vaccine efficacy leaves several high-risk populations vulnerable to infection. However, the recent discovery of broadly neutralizing antibodies that target the hemagglutinin (HA) stalk domain has renewed hope that the development of “universal” influenza virus vaccines may be within reach. Here, we examine the functions of influenza A virus hemagglutinin stalk-binding antibodies in an endogenous setting, i.e., as polyclonal preparations isolated from human sera. Relative to monoclonal antibodies that bind to the HA head domain, the neutralization potency of monoclonal stalk-binding antibodies was vastly inferior in vitro but was enhanced by several orders of magnitude in the polyclonal context. Furthermore, we demonstrated a surprising enhancement in IgA-mediated HA stalk neutralization relative to that achieved by antibodies of IgG isotypes. Mechanistically, this could be explained in two ways. Identical variable regions consistently neutralized virus more potently when in an IgA backbone compared to an IgG backbone. In addition, HA-specific memory B cells isolated from human peripheral blood were more likely to be stalk specific when secreting antibodies of IgA isotypes compared to those secreting IgG. Taken together, our data provide strong evidence that HA stalk-binding antibodies perform optimally when in a polyclonal context and that the targeted elicitation of HA stalk-specific IgA should be an important consideration during “universal” influenza virus vaccine design.
IMPORTANCE Influenza viruses remain one of the most worrisome global public health threats due to their capacity to cause pandemics. While seasonal vaccines fail to protect against the emergence of pandemic strains, a new class of broadly neutralizing antibodies has been recently discovered and may be the key to developing a “universal” influenza virus vaccine. While much has been learned about the biology of these antibodies, most studies have focused only on monoclonal antibodies of IgG subtypes. However, the study of monoclonal antibodies often fails to capture the complexity of antibody functions that occur during natural polyclonal responses. Here, we provide the first detailed analyses of the biological activity of these antibodies in polyclonal contexts, comparing both IgG and IgA isotypes isolated from human donors. The striking differences observed in the functional properties of broadly neutralizing antibodies in polyclonal contexts will be essential for guiding design of “universal” influenza virus vaccines and therapeutics.
INTRODUCTION
Influenza A viruses (IAVs) remain one of the most pressing global public health concerns due to their widespread distribution, rapid evolution, and potential for reassortment (1). These traits contribute to the ability of IAVs to cause serious pandemics, four of which have occurred in the last 100 years. The potential severity of IAV pandemics is exacerbated by the striking paucity of effective antivirals, and inherent limitations in the speed of vaccine production and distribution when pandemics arise. Importantly, even the annual circulation of seasonal IAV strains carries substantial human and economic tolls.
It has been recognized that the most effective way to alleviate the human and economic impacts of IAV would be through the generation of a more broadly protective, or “universal” influenza virus vaccine. While several strategies have been proposed (2), one of the most promising to date involves the recent discovery of a subset of antibodies that are capable of neutralizing a wide range of IAVs through binding to the highly conserved stalk domain of the hemagglutinin (HA) protein (3). These antibodies seem to be boosted with the greatest magnitude upon sequential exposure to highly dissimilar HA subtypes, as happens during IAV pandemics (4–8). Studies in mice and ferrets have demonstrated that elicitation of these antibodies via vaccination with chimeric HA molecules can provide broad protection from challenge with a diverse array of IAV subtypes (9–11). However, one of the major concerns that has arisen in the development of vaccines designed to elicit broadly neutralizing antibodies (bnAbs) that target the HA stalk domain is that, in addition to their reduced prevalence relative to antibodies that bind to the HA head, they also appear to be less potently neutralizing in vitro (12–14). Yet, current understanding of the mechanisms governing the activity of bnAbs is largely reliant upon studies wherein monoclonal antibodies (primarily of IgG isotypes) have been analyzed in isolation (15). Although informative, this approach fails to recapitulate the complex interactions between differing antibody isotypes and clonotypes that occur naturally in humans as a consequence of the polyclonal response to antigens.
While cooperativity and synergism of monoclonal antibody mixtures have been reported in the past (16–19), emerging data have illustrated striking examples of situations wherein the activity of a monoclonal antibody can be drastically altered when assessed in the context of other antibodies (20, 21). Indeed, the benefits of using polyclonal antibody preparations to treat disease in therapeutic settings are just beginning to be realized (22). In addition, recent discovery of the remarkable effects of heavy-chain constant regions (CH) in allosterically modulating the affinity and specificity of identical variable (V) regions is fueling intensive new investigation into isotype-specific effects of antibodies (23–30).
Here, we address these issues by examining the differential properties of HA head and stalk-binding antibodies, as well as the effects of antibody isotypes in mediating neutralization of IAV in vitro using polyclonal preparations of antibodies isolated from human donors. We find that the deficiency in the neutralization potency of monoclonal HA stalk-binding antibodies relative to strain-specific head binding antibodies is greatly reduced in polyclonal contexts. Our results also demonstrate a surprising enhancement in both the quality and magnitude of HA stalk-binding antibodies of IgA subtype. Taken together, our findings highlight important differences in the activities of monoclonal versus polyclonal antibodies that bind to the HA glycoprotein of IAV and additionally reveal isotype-specific effects of these antibodies in both monoclonal and polyclonal contexts.
MATERIALS AND METHODS
Human serum samples.
Human serum samples were obtained from Innovative Research (Novi, Michigan) and were collected between March 2012 and April 2013. Samples were prescreened using HAI assay against wild-type Cal/09 virus. Donor information is shown in Table 1 and 2.
TABLE 1.
Cal/09 HAI+ donor characteristics
| Donor | Age (yr) | Gender | Race | Collection date |
|---|---|---|---|---|
| 5-1 | 59 | F | Black | June 2012 |
| 2-2 | 54 | M | Caucasian | June 2012 |
| 4-2 | 19 | M | Black | June 2012 |
| 5-2 | 19 | M | Black | June 2012 |
| 6-2 | 49 | M | Caucasian | June 2012 |
| 7-2 | 51 | M | Black | March 2012 |
| 10-3 | 46 | M | Caucasian | April 2013 |
| 12-3 | 20 | F | Latino | April 2013 |
| 15-3 | 27 | F | Black | April 2013 |
| 16-3 | 35 | F | Latino | April 2013 |
TABLE 2.
Pre-2009 donor characteristicsa
| Donor | Race | Collection date |
|---|---|---|
| 1 | Black | November 2006 |
| 2 | Black | November 2006 |
| 3 | Black | November 2006 |
| 4 | Black | July 2004 |
| 5 | Black | August 2003 |
| 6 | Latino | November 2006 |
| 7 | Black | November 2006 |
| 8 | Black | November 2006 |
| 9 | Black | November 2006 |
| 10 | Black | November 2006 |
All donors were male. Donor ages are not known.
Expression and purification of recombinant influenza virus proteins.
HA proteins of cH6/1PR8 (H6 HA globular head domain from A/mallard/Sweden/81/02 and H1 HA stalk domain from A/PR/8/1934), cH9/1PR8 (H9 HA globular head domain from A/guinea fowl/Hong Kong/WF10/1999 and H1 HA stalk domain from A/PR/8/1934), Cal/09 and NC/99 were expressed in BTI-TN5B1-4 (High Five) cells as described previously (4, 9, 31). Briefly, coding sequences of each protein were cloned into a modified pFastBac vector (Invitrogen) with a C-terminal hexahistidine tag and a T4 trimerization domain. Recombinant baculovirus (rBV) expressing each HA was generated using the FastBac system (Invitrogen) according to the manufacturer's recommendations. High Five cells were infected with the rBVs at a multiplicity of infection (MOI) of 10. Cells were harvested 72 to 96 h after infection. Proteins were purified with Ni-nitrilotriacetic acid resin (Qiagen).
Cells and viruses.
MDCK cells (American Type Culture Collection) were grown in Dulbecco modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum (HyClone) and 100 U of penicillin and streptomycin (Gibco)/ml. The cH5/1 N3 virus (H5 HA globular head domain from A/Vietnam/1204/2004 and H1 HA stalk domain from A/PR/8/1934) and the recombinant Cal/09 N3 virus (H1 HA from A/California/04/09) were rescued as described previously (32). The N3 NA segment of these two viruses was derived from A/swine/Missouri/4296424/2006, and the remaining segments were derived from A/PR/8/1934.
HAI assays.
HA inhibition (HAI) assays were performed as described previously (4). Briefly, sera were inactivated with TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone)-trypsin at 56°C for 30 min. Viruses and sera were mixed and incubated at room temperature for 30 min. Chicken red blood cells (0.5%) were then added to each well, and the plates were incubated at 4°C for 30 min before reading. Plates were scored for the number of wells exhibiting HAI activity.
IgG and IgA purification.
Human serum was heat inactivated by incubation at 56°C for 30 min and was then diluted 1:4 in phosphate-buffered saline (PBS) for filtration through a 0.22-μm-pore-size filter unit. IgG was purified using a protein G/Sepharose resin (InvivoGen) on a gravity flow column (Qiagen). IgA was purified by a similar process over peptide M/Sepharose resin (InvivoGen). After sera were passed over the columns, the resins were washed with four column volumes of PBS. IgG/IgA was eluted using a 0.1 M glycine-HCl buffer at pH 2.7. The eluate was immediately neutralized in 2 M Tris-HCl buffer (pH 10). Purified IgG/IgA was then concentrated and dialyzed against PBS using Amicon Ultra centrifugal filter units (Millipore) with a 30-kDa molecular mass cutoff in a swinging-bucket rotor.
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed on 96-well plates as described previously (1). Briefly, plates were coated overnight with 2 μg of purified recombinant HA (Cal/09 and cH9/1PR8)/ml in bicarbonate-carbonate coating buffer (100 mM, pH 9.6). The plates were blocked with 5% nonfat milk for 1 h. Serum was diluted serially in 5% nonfat milk and then incubated on plates for 1 h at room temperature. Plates were washed with PBS–0.1% Tween 20 (PBS-T). Secondary goat anti-human IgG-horseradish peroxidase (HRP; Meridian Life Science, Inc.) or goat anti-human IgA-HRP (Millipore) was incubated on plates at 1:5,000 dilution in 5% nonfat milk for 1 h at room temperature, followed by extensive washing with PBS-T prior to addition of HRP substrate (Sigmafast OPD; Sigma-Aldrich). Reactions were stopped after ∼8 min by addition of 3 M HCl, and the optical densities were read at 490 nm on a Synergy 4 (Bio-Tek) plate reader. Curve fitting was performed with GraphPad Prism 6 software using a “log (agonist) versus normalized response minus variable slope (four-parameter) analysis” with a HillSlope set equal to 1.
Microneutralization assays.
For multicycle assays, antibodies and viruses (100 PFU/50 μl) were preincubated for 30 min at room temperature prior to inoculation of 96-well plates of confluent MDCK cells. The plates were incubated for 1 h at 37°C under 5% CO2 to allow for adsorption. After a washing step with PBS, the plates were reincubated with infection medium containing equivalent concentrations of diluted antibody supplemented with 1 μg of TPCK-trypsin (Sigma)/ml. After 20 h, the cells were fixed with 80% acetone and then blocked with 5% nonfat milk, followed by 3% hydrogen peroxide. The cells were incubated with a 1:2,000 dilution of biotin-conjugated mouse anti-NP (Millipore), followed by a 1:5,000 dilution of secondary HRP-conjugated streptavidin (Millipore). Peroxidase substrate (Sigmafast OPD) was added to the wells, and reactions were stopped with 3 M HCl. Curve fitting was performed with GraphPad Prism 6 software using a “log (inhibitor) versus normalized response minus variable slope (four-parameter) algorithm analysis” with a HillSlope set equal to 1. The 50% microneutralization (MNT50) titers were defined as the dilution of antibody that resulted in at least 50% inhibition of infectivity. For single-cycle assays, 1,000 PFU of each virus/50 μl was used as the inoculum, and incubations were performed in trypsin-free media. Cells were fixed at 12 h postinfection and stained as described above.
Plaque reduction (PR) neutralization assay.
cH5/1PR8 N3 virus or Cal/09 N3 virus (100 PFU) was incubated in minimal essential medium (MEM) with serially diluted purified IgG or IgA (initial concentration of 0.5 mg/ml, followed by 2-fold serial dilutions) for 30 min at room temperature. The virus-antibody mixture was then transferred onto 12-well plates of confluent MDCK cells, followed by incubation at 37°C for 1 h. The virus-antibody mixture was removed, and the cells were overlaid with an agar-MEM mixture containing equivalent concentrations of diluted antibody, as found in the initial inoculum, as well as TPCK-trypsin (1 μg/ml) and DEAE-dextran (0.1%). After incubation at 37°C for 48 h, the cells were fixed with 3.7% paraformaldehyde and blocked with 3% bovine serum albumin. The cells were then incubated with a 1:2,000 dilution of biotin-conjugated mouse anti-NP (Millipore), followed by a 1:5,000 dilution of secondary HRP-conjugated streptavidin (Millipore). TrueBlue HRP substrate (KPL) was added to wells to visualize plaques.
B cell enzyme-linked immunosorbent spot assays (ELISpots).
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats by centrifugation through Lymphoprep (Stemcell Technologies) according to the manufacturer's recommendations. To stimulate antibody production by memory B cells (MBCs), PBMCs were cultured for 6 days in B cell stimulation media consisting of RPMI (Gibco) supplemented with 10% fetal bovine serum (FBS; HyClone), penicillin-streptomycin (Gibco), fixed Staphylococcus aureus Cowan strain (1:10,000), pokeweed mitogen (1:1,000; Sigma), 50 μM β-mercaptoethanol, and 1 μg of R848 (Invivogen)/ml. Multiscreen 96-well filtration plates were coated with 2 μg/ml of either goat anti-human IgG (Sigma), goat anti-human IgA (Millipore), recombinant NC/99 HA, recombinant Cal/09 HA, or recombinant cH6/1PR8 HA overnight in PBS at 4°C. The next day, plates were blocked with RPMI supplemented with 10% FBS for 2 h at 37°C. After extensive washing in RPMI, stimulated PBMCs were plated and serially diluted prior to overnight incubation at 37°C. The spots were read by using an immunospot analyzer (CTL, Shaker Heights, OH).
RESULTS
Monoclonal HA stalk-binding antibodies exhibit reduced in vitro neutralization potency compared to monoclonal antibodies that bind to the HA globular head.
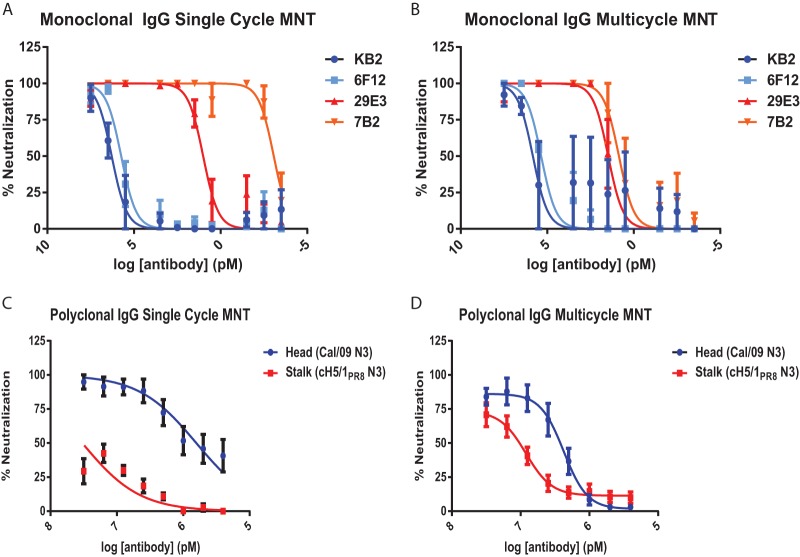
In order to compare the potency with which antibodies that bind to the head or stalk domain of the HA molecule neutralize virus, MNT assays were performed using four murine monoclonal antibodies previously described by our group (14, 32–34). 6F12 and KB2 bind to the HA stalk domain of H1 viruses, whereas 7B2 and 29E3 have specificity for the head domain of A/California/04/2009 (Cal/09) HA. Antibodies were preincubated with a recombinant Cal/09 N3 virus prior to infection of Madin-Darby canine kidney (MDCK) cells and maintained in the media during multicycle virus replication. The concentration at which each antibody achieved 50% microneutralization (i.e., MNT50) relative to control wells containing no antibody was then calculated. Indeed, striking differences were observed in the potency of neutralization when comparing the head-binding and stalk-binding antibodies. HA head-binding antibodies neutralized Cal/09 N3 virus with an MNT50 of 0.02 to 0.68 pM, while the stalk-binding antibodies had MNT50 values of 10,719 to 131,414 pM (Fig. 1A). To control for specificity, the MNT assay was repeated with a recombinant cH5/1PR8 N3 virus (Fig. 1B). The MNT50 values of 6F12 and KB2 against cH5/1PR8 N3 virus were similar to those observed against Cal/09 N3 virus, indicating that the antibodies are not highly sensitive to small differences within H1 stalks. As expected, neither 7B2 nor 29E3 neutralized the cH5/1PR8 N3 virus effectively, since they are specific for the head domain of Cal/09 HA. To further confirm the magnitude of the potency differences observed by MNT assay, plaque reduction (PR) assays were performed with Cal/09 N3 virus. As expected, the striking differences in neutralization potency between HA head and stalk-binding antibodies were maintained (data not shown).
FIG 1.
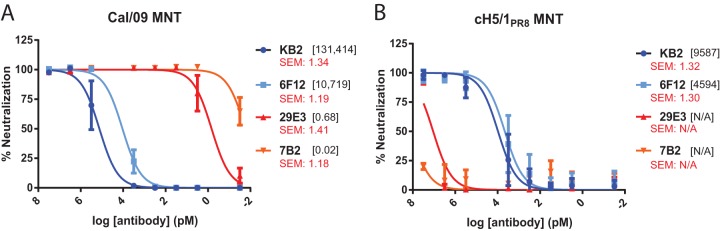
HA stalk-binding monoclonal antibodies neutralize virus in vitro with much less potency than monoclonal antibodies that recognize the HA head domain. A total of 100 PFU of Cal/09 N3 virus (A) or cH5/1PR8 N3 virus (B) were preincubated with the indicated concentration of 6F12, KB2, 29E3, or 7B2 prior to infection of MDCK cells. At 20 h postinfection, the cells were fixed, and infectivity was assessed by anti-NP staining. The data representative the means and standard errors of three independent experiments. The x-axis scale numbers indicate MNT50 antibody concentrations in pM. Standard error measurements of MNT50 values are reported in red.
Potency of HA stalk-mediated neutralization is enhanced in polyclonal contexts.
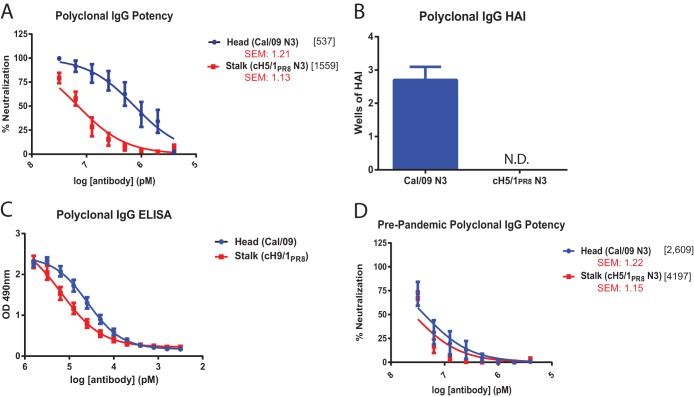
To determine whether relative differences in the in vitro neutralization potency of HA head and stalk-mediated monoclonal antibodies would be recapitulated in the more natural setting of a polyclonal response, IgG was purified from the sera of individuals who were seropositive for Cal/09 (Table 1). Previous work has demonstrated that exposure to Cal/09 virus is capable of robustly boosting the titers of HA stalk-reactive antibodies in individuals who have previously been exposed to other group 1 HA IAVs (4–6, 35, 36). MNT assays were thus performed using the Cal/09 N3 virus to assess HA head-mediated neutralization, and the cH5/1PR8 N3 virus to assess HA stalk-mediated neutralization. Unlike the multilog potency differences observed using monoclonal antibodies, the human polyclonal IgG preparations neutralized Cal/09 N3 virus and cH5/1PR8 N3 virus with differences of only 3-fold (Fig. 2A). Again, similar results were obtained by PR assay (data not shown). To confirm that the contraction in potency observed in polyclonal sera was not due to the presence of HAI-positive antibodies binding to the cH5/1PR8 HA head domain, HAI assays were performed using the Cal/09 N3 virus and the cH5/1PR8 N3 virus. Viruses were mixed with equivalent dilutions of purified IgG, as were used in the MNT assays. These antibodies produced, on average, 2.7 wells of HAI activity against the Cal/09 N3 virus, while no HAI activity was observed against cH5/1PR8 N3 (Fig. 2B). Differences in reactivity of the IgG preparations to recombinant Cal/09 HA or cH9/1PR8 HA (used to measure HA stalk binding) as measured by ELISA were even more modest, suggesting that binding alone does not account for the observed differences in neutralization potency and that differences in mechanisms of neutralization are likely to have an influence (Fig. 2C). Consistent with previous findings (4, 35), polyclonal IgG purified from the sera of Cal/09 HAI-negative individuals neutralized Cal/09 N3 and cH5/1PR8 N3 virus poorly and with similar potency (Fig. 2D).
FIG 2.
The potency of HA stalk antibody-mediated neutralization is enhanced by the polyclonal response. (A to C) Polyclonal IgG was purified from the sera of ten Cal/09 HAI-positive individuals (Table 1). (B) HAI assays were performed according to standard procedures starting with the maximum dilution of antibodies found in MNT assays (7.5 pM). The number of wells containing sufficient amounts of antibody to retain HAI activity was then assessed. N.D., not detected. (C) Whole HA- and HA stalk-directed reactivity of polyclonal IgG isolated from Cal/09 HAI-positive individuals were assessed by ELISA on plates coated with recombinant Cal/09 HA or cH9/1PR8 HA, respectively. (A and D) A total of 100 PFU of the indicated viruses was incubated with corresponding dilutions of antibodies prior to infection of MDCK cells. At 20 h postinfection, the cells were fixed, and the infectivity was assessed by anti-NP staining. (D) Polyclonal IgG was also purified from the sera of 10 Cal/09 HAI-negative individuals (Table 2) as a control for nonspecific Cal/09 neutralizing antibody titers. The x-axis scale numbers indicate MNT50 antibody concentrations in pM. Standard error measurements of MNT50 values are reported in red.
HA stalk-mediated neutralization is most potent during multicycle replication.
Previous studies have reported unique mechanisms through which HA head and stalk domain-binding antibodies mediate virus neutralization. Although HA head-binding antibodies primarily block viral attachment and release, stalk-binding antibodies function intracellularly to block fusion of the viral and endosomal membranes, and extracellularly to inhibit proteolytic cleavage of HA (37). To determine whether the mechanism of neutralization exerted by HA head- and stalk-binding antibodies differentially affected neutralization potency at specific stages in the viral life cycle, we performed MNT assays in the context of single-cycle and multicycle infections. For single cycle assays, virus was preincubated with antibody prior to adsorption and cells were infected at high MOIs (Fig. 3A and C). Multicycle assays were performed by adding antibody to media after adsorption of virus at low MOI (Fig. 3B and D). HA stalk-binding monoclonal antibodies KB2 and 6F12 neutralized virus at nearly equivalent potency during both single-cycle and multicycle infections (Fig. 3A and B). In contrast, differences were observed in the neutralizing properties of the head binding monoclonal antibodies 29E3 and 7B2. Although 29E3 was equally potent in neutralizing virus during single-cycle and multicycle growth, 7B2 exhibited markedly less potent neutralization during multicycle replication than during single-cycle replication (Fig. 3A and B). This is likely related to subtle differences in the head domain epitopes recognized by each antibody. For example, the binding site of 7B2 may allow the antibody to efficiently block binding of viral particles to the cell surface during single-cycle replication but may be less efficient in preventing egress/budding during multicycle replication. Similarly, although substantial potency differences were observed in the ability of polyclonal IgGs to neutralize virus via primarily the head (Cal/09 N3) or stalk (cH5/1PR8 N3) domains during single cycle replication (Fig. 3C), this potency difference was substantially contracted in the context of multicycle replication (Fig. 3D). This is likely a reflection of the unique mechanisms of neutralization mediated by HA head and stalk binding antibodies, which have been reported previously (37, 38).
FIG 3.
Polyclonal HA stalk-mediated neutralization is most potent during multicycle replication. MNT assays were performed against Cal/09 N3 virus with monoclonal HA stalk-binding antibodies (6F12 and KB2) and monoclonal HA head-binding antibodies (A and B) or using polyclonal IgG and Cal/09 N3 virus (to assess primarily HA head-mediated neutralization) and cH5/1PR8 N3 virus (to assess HA stalk-mediated neutralization) (C and D). (A and C) For single-cycle assays, antibody dilutions were preincubated with 1,000 PFU of virus before inoculation of MDCK cells. After adsorption, the cells were incubated in antibody-free and trypsin-free media for 12 h prior to fixation and staining. (B and D) Multicycle assays were performed by inoculating MDCK cells with 100 PFU of virus for 1 h at 37°C. After adsorption, the cells were washed and reincubated in medium containing trypsin and antibody for 20 h prior to fixation and staining. In panels A and B, the data represent the means and standard errors of three independent experiments. For panels C and D, n = 10 (see Table 1).
Preferential stalk-specificity exhibited by human IgA.
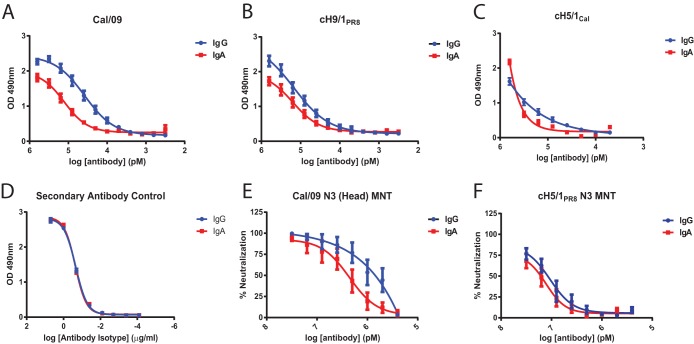
Despite the known importance of IgA in maintenance of mucosal immunity, little is known about the biology of HA stalk-reactive antibodies of isotypes other than IgG. Therefore, polyclonal serum-derived IgG and IgA were purified from 10 individuals as matched samples. As expected, the serum-derived IgA was mainly monomeric (39). Reactivity of the donor-matched polyclonal IgG and IgA preparations were first compared by ELISA against recombinant Cal/09 HA (Fig. 4A) and against recombinant cH9/1PR8 and cH5/1Cal HA to measure stalk-specific reactivity (Fig. 4B and C). Although the EC50 of IgA binding to Cal/09 HA was ∼4-fold lower than that of IgG (Fig. 4A), the difference in binding to the HA stalk was minimal (Fig. 4B and C). These differences were not due to differences in detection by secondary antibodies, since the antibodies produced equivalent signals when incubated with equal concentrations of IgG or IgA (Fig. 4D). To evaluate whether these differences in binding corresponded to functional differences in neutralization, MNT and PR assays were performed using Cal/09 N3 and cH5/1PR8 N3 viruses (Fig. 4E and F and data not shown). In agreement with the ELISA data, IgG preparations neutralized Cal/09 N3 virus 2- to 4-fold more potently than IgA preparations (Fig. 4E), whereas neutralization of cH5/1PR8 N3 was essentially equivalent for both isotypes (Fig. 4F).
FIG 4.
Preferential targeting of the HA stalk domain by human IgA. Matched preparations of polyclonal human IgG and IgA were isolated from the sera of 10 donors (Table 1). (A to C) ELISAs were performed to compare the binding of polyclonal IgG and IgA to whole recombinant Cal/09 HA (A), recombinant cH9/1PR8 HA (B), or recombinant cH5/1Cal HA (C) to evaluate stalk binding. (D) Control for secondary antibody reactivity showing that when equivalent concentrations of IgG and IgA are present, no differences are observed in the optical density (OD) values. (E and F) MNT assays were performed to assess neutralization potency using Cal/09 N3 virus (E) and cH5/1PR8 N3 virus (F) to measure stalk-mediated neutralization, as described in Fig. 2A. IgG data from Fig. 2A have been reproduced here to facilitate comparison. The data points represent the mean and standard error of three replicates. The x-axis scale numbers indicate MNT50 antibody concentrations in pM. Standard error measurements of MNT50 values are reported in red.
Isotype-intrinsic differences in the potency of HA stalk binding and neutralization.
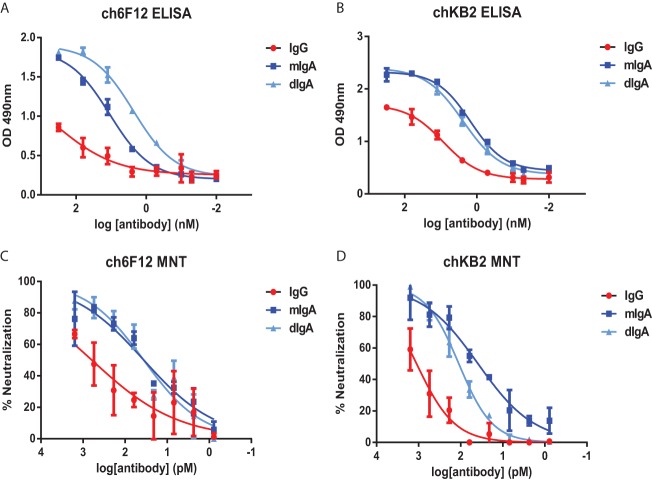
To explore the possibility that antibody isotype might influence the binding and neutralizing properties of HA stalk-binding antibodies, chimeric IgG and IgA antibodies were generated by cloning the variable regions of the group 1 HA stalk-binding monoclonal antibodies, 6F12 and KB2, into human IgG and IgA backbones (chKB2 and ch6F12). Both monomeric and dimeric IgA molecules were expressed for each variable (V) region. Comparable binding of mIgA and dIgA to recombinant PR8 HA was observed when the antibodies were added at equal molarity. However, in the case of both ch6F12 and chKB2, the IgG antibodies bound substantially weaker (Fig. 5A and B). MNT assays were then performed to determine whether isotype would influence the in vitro neutralization potency of antibodies with identical V regions (Fig. 5C and D). For both ch6F12 and chKB2, the most potent neutralization was exerted by the IgA molecules. In agreement with the ELISA data, IgG molecules consistently exhibited the weakest neutralization potency (Fig. 5C and D). Thus, structural features of the IgA backbone appear to increase the binding and neutralization potency of HA stalk-specific V regions.
FIG 5.
IgA exhibits an isotype-intrinsic enhancement in HA-stalk binding and neutralization activity. (A to D) The variable regions of mouse monoclonal HA stalk-binding antibodies, KB2 and 6F12, were cloned into human IgG or IgA backbones to generate chimeric antibodies. Dimeric IgA was generated by coexpression of human J chain. (A and B) ELISA plates were coated with recombinant PR8 protein in order to compare the binding properties of ch6F12 (A) and chKB2 (B) antibodies. (C and D) MNT assays were performed against cH5/1PR8 N3 virus using the indicated dilutions of ch6F12 (C) or chKB2 (D). The data represent the means and standard errors of three independent experiments.
The IgA-secreting memory B cell compartment is biased toward HA stalk specificity.
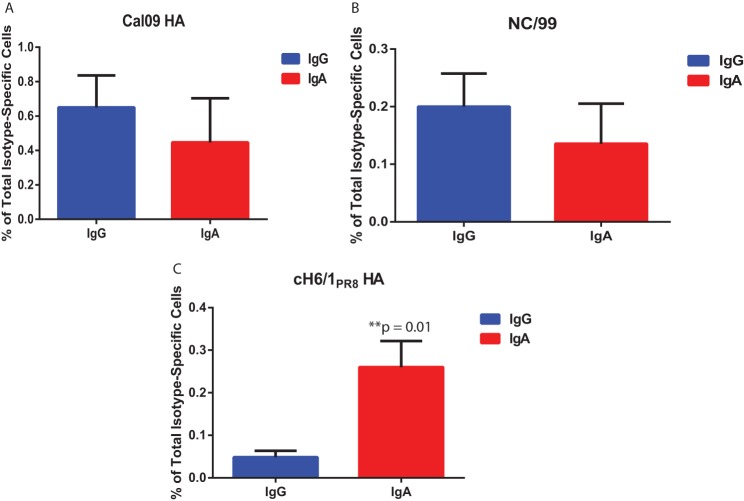
Finally, B cell ELISpots were performed using PBMCs isolated from human donors and stimulated in vitro to determine whether differences existed in the frequency of stalk-specific MBCs belonging to IgG and IgA pools. Although no significant differences were observed in the frequency of total IgG-secreting cells versus total IgA-secreting cells with specificity for Cal/09 HA (Fig. 6A) or A/New Caledonia/20/99 (NC/99) HA (Fig. 6B), a substantially greater proportion of total IgA-secreting cells were stalk specific relative to the pool of IgG-secreting cells (Fig. 6C). These findings suggest a previously unappreciated epitope-specific bias for antibodies of unique isotypes. Whether the basis for this skewing is genetic in nature or a result of exposure route is the source of ongoing investigation.
FIG 6.
IgA-secreting MBCs exhibit a bias toward HA stalk specificity. (A to C) PBMCs were isolated from the blood of seven healthy donors, and MBCs were stimulated in vitro for 6 days to elicit antibody production. ELISpots were performed to evaluate the total number of IgG- and IgA-secreting cells from each donor, as well as the number of IgG- or IgA-secreting cells specific for recombinant Cal/09 HA (A), recombinant NC/99 HA (B), or recombinant cH6/1PR8 HA (C). P values were determined by using a paired Student t test.
DISCUSSION
The discovery of bnAbs that bind to the HA stalk domain has opened new frontiers in both “universal” influenza virus vaccine design, and in biological anti-influenza virus therapeutics. Although much has been learned from studies of monoclonal stalk-binding IgGs derived from both mice and humans, the activities of HA stalk binding antibodies in polyclonal contexts and in non-IgG isotypes has gone largely unstudied. However, these issues are nontrivial. Indeed, the activities of monoclonal antibodies can change drastically in polyclonal mixtures (20, 21), and vaccine regimens invariably elicit polyclonal responses. In addition, IgA isotype antibodies play an important role in the maintenance of mucosal immunity against respiratory pathogens and may be of particular relevance in terms of protection afforded to individuals immunized with live-attenuated influenza virus vaccines (40–42).
Despite the broad neutralizing capacity of HA stalk-binding antibodies relative to those that bind to the head domain, concerns have been raised about their suboptimal neutralization potency (12, 13). Indeed, when compared directly in vitro, the neutralization potencies of HA stalk-binding monoclonal antibodies were several orders of magnitude weaker than HAI-positive monoclonal antibodies that bind to the HA head domain. Previous work has indicated that HA stalk-binding antibodies appear to be capable of accessing their epitope on virions (43), and thus their inferior potency is likely due to their inability to inhibit HA binding to cell surface receptors: the robust means of neutralization achieved by HA head-binding antibodies (37). A recent study has also suggested that stalk-binding monoclonal antibodies have limited activity against cell-bound viruses, which would further limit their neutralization potency (44). Despite this inferior capacity to neutralize virus, passive transfer experiments in mice have demonstrated a strong propensity for these antibodies to activate antibody-dependent cellular cytotoxicity (ADCC), which correlates with enhanced protection in vivo (13). Importantly, we found that the in vitro potency of HA stalk-mediated neutralization was also enhanced by several orders of magnitude relative to conventional HAI-mediated neutralization when the antibodies were evaluated in a polyclonal setting. Taken together, these findings are particularly encouraging when considering the feasibility of “universal” influenza virus vaccine approaches seeking to exploit HA stalk binding antibodies. Indeed, the inferior in vitro neutralization potency of HA stalk-binding monoclonal antibodies can likely be overcome or substantially reduced in the context of polyclonal responses and ADCC, which occur in vivo.
Given that influenza viruses initiate infection at the respiratory mucosa, understanding the biology of HA-stalk binding antibodies of IgA isotype is of critical importance. We demonstrate here that serum-derived polyclonal monomeric IgA possesses an equivalent capacity to neutralize virus in vitro compared to IgG from matched donors. Using a reductionist approach, we observed that when expressing identical variable regions, IgA molecules bound and neutralized virus via the HA stalk domain with elevated potency compared to corresponding IgG antibodies. This enhancement in the potency of IgA-mediated neutralization was also recently reported for HAI-positive antibodies that bind to the head domain (30) and may therefore reflect a more general structural feature of IgA constant regions that contribute to enhanced binding and neutralization. Stalk-binding V-regions expressed in the context of IgA constant regions may experience subtle conformational differences that promote their binding to the relatively discrete and well-characterized neutralizing epitope found in the HA stalk/stem.
In addition to the antibody-intrinsic enhancement of IgA stalk-mediated neutralization, analysis of MBCs present in human PBMCs highlighted an enrichment in stalk-specific antibody secreting cells of the IgA subtype. Although the source of this repertoire biasing remains under investigation, it could reflect either a genetic predisposition for particular V regions during class-switch recombination or an environmental factor, such as exposure route, which results in more potent elicitation of HA stalk-specific antibodies of IgA subtype during natural infection at respiratory surfaces.
Taken together, our findings provide important new insights regarding the biological activity of HA stalk-specific antibodies in the natural context of polyclonal human sera. The enhanced activity of these antibodies in polyclonal contexts and in the context of IgA should be carefully considered in the design of HA stalk antibody therapeutics and for design of “universal” influenza virus vaccines.
ACKNOWLEDGMENTS
This study was partially supported by a Centers for Excellence for Influenza Research and Surveillance grant (HHSN266200700010C) and by National Institutes of Health grants U19 AI109946, P01 AI097092 (P.P.), and HHSN266200500021C (T.M.M.). M.S.M. was supported in part by a CIHR postdoctoral fellowship.
REFERENCES
- 1.Miller MS, Palese P. 2014. Peering into the crystal ball: influenza pandemics and vaccine efficacy. Cell 157:294–299. doi: 10.1016/j.cell.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Pica N, Palese P. 2013. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med 64:189–202. doi: 10.1146/annurev-med-120611-145115. [DOI] [PubMed] [Google Scholar]
- 3.Ekiert DC, Wilson IA. 2012. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr Opin Virol 2:134–141. doi: 10.1016/j.coviro.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MS, Tsibane T, Krammer F, Hai R, Rahmat S, Basler CF, Palese P. 2013. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J Infect Dis 207:98–105. doi: 10.1093/infdis/jis652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P. 2013. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med 5:198ra107. doi: 10.1126/scitranslmed.3006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G-M, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng N-Y, Lee J-H, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. 2012. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wrammert J, Koutsonanos D, Li G-M, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng N-Y, Lee J-H, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L, Silva V, Diederich S, Jones RB, Gubbay J, Pasick J, Petric M, Jean F, Allen VG, Brown EG, Rini JM, Schrader JW. 2012. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front Immunol 3:87. doi: 10.3389/fimmu.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, García-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado V, Ryder AB, Miller MS, Rose JK, Palese P, García-Sastre A, Albrecht RA. 2014. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 88:3432–3442. doi: 10.1128/JVI.03004-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellebedy AH, Ahmed R. 2012. Re-engaging cross-reactive memory B cells: the influenza puzzle. Front Immunol 3:53. doi: 10.3389/fimmu.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dilillo DJ, Tan GS, Palese P, Ravetch JV. 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med 20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. 2012. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol 86:6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laursen NS, Wilson IA. 2013. Broadly neutralizing antibodies against influenza viruses. Antivir Res 98:476–483. doi: 10.1016/j.antiviral.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng LW, Stanker LH, Henderson TD, Lou J, Marks JD. 2009. Antibody protection against botulinum neurotoxin intoxication in mice. Infect Immun 77:4305–4313. doi: 10.1128/IAI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demarest SJ, Hariharan M, Elia M, Salbato J, Jin P, Bird C, Short JM, Kimmel BE, Dudley M, Woodnutt G, Hansen G. 2010. Neutralization of Clostridium difficile toxin A using antibody combinations. MAbs 2:190–198. doi: 10.4161/mabs.2.2.11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varshney AK, Wang X, Cook E, Dutta K, Scharff MD, Goger MJ, Fries BC. 2011. Generation, characterization, and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B-induced lethal shock. J Biol Chem 286:9737–9747. doi: 10.1074/jbc.M110.212407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngundi MM, Meade BD, Little SF, Quinn CP, Corbett CR, Brady RA, Burns DL. 2012. Analysis of defined combinations of monoclonal antibodies in anthrax toxin neutralization assays and their synergistic action. Clin Vaccine Immunol 19:731–739. doi: 10.1128/CVI.05714-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow S-K, Smith C, MacCarthy T, Pohl MA, Bergman A, Casadevall A. 2013. Disease-enhancing antibodies improve the efficacy of bacterial toxin-neutralizing antibodies. Cell Host Microbe 13:417–428. doi: 10.1016/j.chom.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pohl MA, Rivera J, Nakouzi A, Chow S-K, Casadevall A. 2013. Combinations of monoclonal antibodies to anthrax toxin manifest new properties in neutralization assays. Infect Immun 81:1880–1888. doi: 10.1128/IAI.01328-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X-Z, Coljee VW, Maynard JA. 2013. Back to the future: recombinant polyclonal antibody therapeutics. Curr Opin Chem Eng 2:405–415. doi: 10.1016/j.coche.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritsch O, Hudry-Clergeon G, Buckle M, Petillot Y, Bouvet JP, Gagnon J, Dighiero G. 1996. Can immunoglobulin C(H)1 constant region domain modulate antigen binding affinity of antibodies? J Clin Invest 98:2235–2243. doi: 10.1172/JCI119033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correa A, Trajtenberg F, Obal G, Pritsch O, Dighiero G, Oppezzo P, Buschiazzo A. 2013. Structure of a human IgA1 Fab fragment at 1.55 Å resolution: potential effect of the constant domains on antigen-affinity modulation. Acta Crystallogr D Biol Crystallogr 69:388–397. doi: 10.1107/S0907444912048664. [DOI] [PubMed] [Google Scholar]
- 25.Janda A, Eryilmaz E, Nakouzi A, Cowburn D, Casadevall A. 2012. Variable region identical immunoglobulins differing in isotype express different paratopes. J Biol Chem 287:35409–35417. doi: 10.1074/jbc.M112.404483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eryilmaz E, Janda A, Kim J, Cordero RJB, Cowburn D, Casadevall A. 2013. Global structures of IgG isotypes expressing identical variable regions. Mol Immunol 56:588–598. doi: 10.1016/j.molimm.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casadevall A, Janda A. 2012. Immunoglobulin isotype influences affinity and specificity. Proc Natl Acad Sci U S A 109:12272–12273. doi: 10.1073/pnas.1209750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia Y, Janda A, Eryilmaz E, Casadevall A, Putterman C. 2013. The constant region affects antigen binding of antibodies to DNA by altering secondary structure. Mol Immunol 56:28–37. doi: 10.1016/j.molimm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tudor D, Yu H, Maupetit J, Drillet A-S, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel M. 2012. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci U S A 109:12680–12685. doi: 10.1073/pnas.1200024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muramatsu M, Yoshida R, Yokoyama A, Miyamoto H, Kajihara M, Maruyama J, Nao N, Manzoor R, Takada A. 2014. Comparison of antiviral activity between IgA and IgG specific to influenza virus hemagglutinin: increased potential of IgA for heterosubtypic immunity. PLoS One 9:e85582. doi: 10.1371/journal.pone.0085582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heaton NS, Leyva-Grado VH, Tan GS, Eggink D, Hai R, Palese P. 2013. In vivo bioluminescent imaging of influenza A virus infection and characterization of novel cross-protective monoclonal antibodies. J Virol 87:8272–8281. doi: 10.1128/JVI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manicassamy B, Medina RA, Hai R, Tsibane T, Stertz S, Nistal-Villán E, Palese P, Basler CF, García-Sastre A. 2010. Protection of mice against lethal challenge with 2009 H1N1 influenza A virus by 1918-like and classical swine H1N1 based vaccines. PLoS Pathog 6:e1000745. doi: 10.1371/journal.ppat.1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, García-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng N-Y, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandenburg B, Koudstaal W, Goudsmit J, Klaren V, Tang C, Bujny MV, Korse HJWM, Kwaks T, Otterstrom JJ, Juraszek J, van Oijen AM, Vogels R, Friesen RHE. 2013. Mechanisms of hemagglutinin targeted influenza virus neutralization. PLoS One 8:e80034. doi: 10.1371/journal.pone.0080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan GS, Lee PS, Hoffman RMB, Mazel-Sanchez B, Krammer F, Leon PE, Ward AB, Wilson IA, Palese P. 2014. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza A virus hemagglutinin. J Virol 88:13580–13592. doi: 10.1128/JVI.02289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woof JM, Kerr MA. 2004. IgA function: variations on a theme. Immunology 113:175–177. doi: 10.1111/j.1365-2567.2004.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seibert CW, Rahmat S, Krause JC, Eggink D, Albrecht RA, Goff PH, Krammer F, Duty JA, Bouvier NM, García-Sastre A, Palese P. 2013. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J Virol 87:7793–7804. doi: 10.1128/JVI.00979-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sealy R, Webby RJ, Crumpton JC, Hurwitz JL. 2013. Differential localization and function of antibody-forming cells responsive to inactivated or live-attenuated influenza virus vaccines. Int Immunol 25:183–195. doi: 10.1093/intimm/dxs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambrose CS, Wu X, Jones T, Mallory RM. 2012. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine 30:6794–6801. doi: 10.1016/j.vaccine.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Harris AK, Meyerson JR, Matsuoka Y, Kuybeda O, Moran A, Bliss D, Das SR, Yewdell JW, Sapiro G, Subbarao K, Subramaniam S. 2013. Structure and accessibility of HA trimers on intact 2009 H1N1 pandemic influenza virus to stem region-specific neutralizing antibodies. Proc Natl Acad Sci U S A 110:4592–4597. doi: 10.1073/pnas.1214913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wyrzucki A, Bianchi M, Kohler I, Steck M, Hangartner L. 2015. Heterosubtypic antibodies to influenza A virus have limited activity against cell-bound virus but are not impaired by strain-specific serum antibodies. J Virol 89:3136–3144. doi: 10.1128/JVI.03069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]