ABSTRACT
Hepatitis E virus (HEV) is an important but extremely understudied human pathogen. Due largely to the lack of an efficient cell culture system for HEV, the molecular mechanisms of HEV replication and pathogenesis are poorly understood. Recently, a unique genotype 3 strain of HEV recovered from a chronically infected patient was adapted for growth in HepG2C3A human hepatoma cells. The adaptation of the Kernow C-1 P6 HEV to propagate in HepG2C3A cells selected for a rare virus recombinant that contains an insertion of a 171-nucleotide sequence encoding amino acids 21 to 76 of the human ribosomal protein S17 (RPS17) within the hypervariable region (HVR) of the HEV ORF1 protein. When the RPS17 insertion was placed into a strain of genotype 1 HEV which infects only humans, it expanded the host range of the virus, allowing it to infect cell lines from multiple animal species, including cow, dog, cat, chicken, and hamster. In this study, we utilized forward and reverse genetics to attempt to define which aspects of the RPS17 insertion allow for the ability of the Kernow C-1 P6 HEV to adapt in cell culture and allow for expanded host tropism. We demonstrate that the RPS17 sequence insertion in HEV bestows novel nuclear/nucleolar trafficking capabilities to the ORF1 protein of Kernow P6 HEV and that lysine residues within the RPS17 insertion, but not nuclear localization of the ORF1 protein, correlate with the enhanced replication of the HEV Kernow C-1 P6 strain. The results from this study have important implications for understanding the mechanism of cross-species infection and replication of HEV.
IMPORTANCE HEV is an important pathogen worldwide. The virus causes high mortality (up to 30%) in pregnant women and has been recognized to cause chronic hepatitis in immunocompromised populations. The life cycle of HEV has been understudied due to a lack of sufficient cell culture systems in which to propagate the virus. Recently, insertions and rearrangements of the hypervariable region (HVR) within the HEV genome, allowing for cell culture adaptation and expansion of the host range, have been reported. We utilized these cell culture-adapted HEV strains to assess how the HVR may be involved in virus replication and host range. We provide evidence that insertion of the RPS17 sequence in HEV likely confers nuclear trafficking capabilities to the nonstructural protein of the virus and that lysine residues within the RPS17 insertion are important for enhanced replication of the virus. These data will help to elucidate the mechanism of cross-species infection of HEV in the future.
INTRODUCTION
Hepatitis E virus (HEV) is the most common cause of acute viral hepatitis in many parts of the world (1, 2). Major outbreaks and epidemics are most common in developing countries, where a lack of proper sanitation conditions leads to waterborne spread of the disease. In industrialized countries, HEV infection is typically sporadic and endemic. Recently, chronic HEV infection has become an important clinical problem in immunosuppressed individuals, such as HIV/AIDS patients and those receiving solid organ transplants (3–5). HEV is a single-stranded positive-sense RNA virus of approximately 7.2 kb belonging to the family Hepeviridae. Within the genus Hepevirus, there exist at least four genotypes of HEV that infect humans. Genotypes 1 and 2 infect solely humans, whereas genotypes 3 and 4 are zoonotic, infecting humans and other animal species, such as swine (6, 7).
The genome of HEV carries three open reading frames (ORFs). ORF2 codes for the viral capsid protein. ORF3 encodes a small multifunctional phosphoprotein that interacts with host cellular proteins and may have a role in HEV virion release from the host cell (8–10). ORF1 encodes an approximately 186-kDa polyprotein with the following domain structure: NH2-methyltransferase (11), cysteine protease (12, 13), polyproline hypervariable region (HVR) (14, 15), ADP-ribose phosphorylase (X or macro domain), helicase (16, 17), and RNA-dependent RNA polymerase (RdRp) (18)–COOH. Analogy with other positive-strand RNA viruses suggests that these ORF1 domains are nonstructural proteins (15), although detailed information about posttranslational processing and virion assembly has been conflicted due to the absence of an efficient in vitro replication system for HEV (19).
The HVR of HEV was first reported as a putative protein hinge (15), although its function remains largely unknown. The HVR does not appear to be required for the replication of HEV (20). Deletions in the HVR did not abolish HEV infectivity in vivo or in vitro, although deletions of nearly full-length HVR attenuated the virus in animals, suggesting that the HVR may interact with viral and host factors to modulate HEV replication efficiency and/or pathogenesis (21). The HVR has been proposed to play a role in HEV persistence in immunocompromised individuals (22). Additionally, insertions and rearrangements within the ORF1 protein have given an enhanced ability to replicate in cell culture (23, 24).
Recently, unique strains of HEV containing insertions of 171 nucleotides in the HVR of the HEV genome, derived from the human ribosomal proteins S17 and S19 (RPS17 and RPS19), were identified from an English patient coinfected with human immunodeficiency virus and an American liver transplant patient chronically infected with HEV, respectively (24, 25). The RPS17 insertion mutant was further selected during serial passages of HEV-containing fecal materials in HepG2C3A cells and exhibited an expanded host range, infecting cells from pig, deer, chicken, cat, dog, mouse, and hamster in vitro (26). In the other case, the HVR of HEV had a 117-nucleotide in-frame insertion derived from the human RPS19 gene, inserting amino acids 93 to 132 (25), and the RSP19 insertion bestowed an enhanced cell culture replication efficiency of HEV, although not to the same degree as that with the RPS17 insertion. The mechanism by which RPS17 and RPS19 insertions into the HEV genome enhance virus replication and expand the in vitro host range remains unknown.
The research which led to the discovery of the cell culture-adapted strain of HEV also produced an infectious cDNA clone, passage 1 Kernow-C1 HEV (GenBank accession no. JQ679014), derived from the first passage of the Kernow-C1 virus in HepG2C3A cells directly inoculated with virus from the chronically infected patient. The passage 1 Kernow-C1 HEV lacked the RPS17 insertion and had 25 additional amino acid differences from the passage 6 Kernow-C1 P6 strain (GenBank accession no. JQ679013). The Kernow-C1 P1 strain expressed the HEV ORF2 protein in less than 2% of Huh7 S10-3 liver cells transfected with viral RNA, whereas the Kernow-C1 P6 HEV strain expressed the HEV ORF2 protein in 10 to 45% of transfected Huh7 S10-3 liver cells (24). Having two infectious cDNA clones derived from the same patient fecal sample, with one lacking elements required for enhanced replication, gave us a unique opportunity to assess the molecular determinants allowing the Kernow-C1 P6 strain to replicate better than the Kernow-C1 P1 strain.
RPS17 is a basic protein with a predicted molecular mass of 15.5 kDa that is composed of 135 amino acids. It is transcribed from five exons located on human chromosome 15 (27, 28). RPS17 is part of the 40S ribosomal subunit. It lies in close proximity to RPS13, RPS16, and RPS19, which are involved in elongation initiation factor 2 (eIF-2) binding (29). RPS17 along with RPS19 and RPS24 has been linked to diseases in humans, including Diamond-Blackfan anemia (30, 31), an erythroid aplasia resulting in a deficiency of red blood cell precursors in the bone marrow. In Escherichia coli, RPS17 is one of the six proteins which bind independently to 16S rRNA (30).
A common feature shared by both RPS17 and RPS19 is an active nuclear/nucleolar localization signal. RPS19 contains a nucleolar localization signal within amino acids glycine 120 to asparagine 142 (32), whereas the RPS17 protein contains a functional nuclear localization signal (NLS) within amino acids 32 to 50 and a nucleolar trafficking domain within amino acids 60 to 70 (our unpublished data). As the HEV HVR insertion of the RPS19 sequence contains amino acids 93 to 132 and the HVR insertion of the RPS17 sequence contains amino acids 21 to 76, both insertions in the HEV HVR should bestow nuclear/nucleolar trafficking properties to the HEV ORF1 polyprotein. Therefore, in this study, we asked whether the RPS17 insertion into the HVR of HEV bestows nuclear trafficking capabilities to the viral ORF1 protein and whether this acquired nuclear localization function is responsible for the observed enhancement of virus replication and expanded in vitro host range of the Kernow C-1 P6 HEV strain.
MATERIALS AND METHODS
Expression vectors, HEV infectious cDNA clones, and cells.
Passage 1 (lacking the RPS17 insertion) and passage 6 (containing the RPS17 insertion) strains of genotype 3 Kernow-C1 HEV infectious clones in the pBlueScript SK(+) vector were described previously (24). The swine HEV infectious cDNA clone was pSHEV-3, which was described previously (34). Enhanced yellow fluorescent protein (EYFP-N1) was obtained from Clontech. The triple eYFP-N1 vector was created by amplifying eYFP from the eYFP-N1 vector and cloning two additional copies in frame with eYFP-N1. The Huh-7 subclone 10-3 cell line (35), human HepG2C3A (CRL-10741) cell line, and hamster BHK-21 (CCL-10) cell line were used for imaging and virus infectivity assays.
Construction of recombinant vectors expressing HEV ORF1 fluorescently tagged fusion proteins.
HEV ORF1 was amplified by PCR from the P1 and P6 Kernow C-1 HEV strain cDNAs by using the primers spk402 (5′-CATCATGCTAGCATGGAGGCCCACCAGTTC-3′) and spk403 (5′-CATCATAAGCTTACATTCTACCCGCTGTATGATGG-3′) and subsequently cloned in frame with EYFP, using NheI and HindIII restriction sites. Additionally, the HVRs of passage 1 virus, passage 6 virus, and passage 6 virus containing point mutations within RPS17 or passage 6 virus containing substitutions for the RPS17 insertion were amplified by PCR, using primers spk437 (5′-CATCATCTCGAGATGCCAGAGCAGTATGTCCTGTC-3′) and spk438 (5′-CATCATAAGCTTCAGCCAATCACAGTCTGATTCAAA-3′), and subsequently cloned into the triple YFP vector, using the restriction enzymes XhoI and HindIII. The spk437 primer introduces a methionine start codon into the HVR of the proteins.
Imaging of subcellular localization of HEV ORF1 and HVR YFP fusion proteins.
Huh7 S10-3 cells were seeded in 6-well plates with coverslips. Cells were transfected with plasmid DNA encoding the full-length ORF1 protein or amino acids 587 to 875 (P6 numbering) in frame with YFP, using either XTreme Gene 9 (Roche) or Lipofectamine LTX (Invitrogen), fixed in 4% paraformaldehyde at 17 to 20 h posttransfection, incubated with 4′,6-diamidino-2-phenylindole (DAPI), and mounted on slides by use of Aquapolymount (Polysciences Inc.). Cells were imaged with either a Nikon TE2000 inverted microscope using a Photometrics HQ2 camera or a Zeiss 510 LSM confocal microscope.
Introduction of unique NheI and StuI sites flanking the RPS17 insertion in the Kernow-C1 P6 HEV infectious cDNA clone.
To facilitate mutation of the HVR region of the P6 Kernow HEV containing the RPS17 insertion, we utilized overlap extension mutagenesis to insert an NheI site and a StuI site flanking the RPS17 insertion within the Kernow C-1 P6 HEV, creating the HEV P6 NheI/StuI strain. These unique restriction sites allowed us to directly insert artificial gene sequences engineered with NheI and StuI sites rather than doing multiple rounds of PCR-based mutagenesis. The Kernow-C1 P6 HEV infectious clone contained an NheI site within the pBluescript vector. This site was removed by linearizing the vector by use of NheI and then further incubating it with Klenow reagent, followed by blunt-end ligation and selection for clones which had lost the NheI site. This NheI-deficient plasmid was subsequently used as the template for overlap extension PCR mutagenesis. Mutagenic PCR was conducted using primers spk416 (5′-Gttcagcgctggtactctg-3′), spk528 (5′-gtaaggggcacGCTAgctgcatc-3′), spk527 (5′-gatgcagcTAGCgtgccccttac-3′), and spk415 (5′-gaccaggccggcgt-3′), followed by another round of amplification using primers spk416 and spk415. These amplicons along with the NheI-deficient plasmid were then digested with the NdeI and BamHI restriction enzymes, followed by ligation and transformation into E. coli. Clones were screened for gain of an NheI site, which was verified by sequencing. Clones containing the HVR NheI site were then used as the template for a second overlap extension PCR mutagenesis, using primers spk416 and spk526 (5′-cgtgtagGCctcctcggaggG-3′) and primers spk415 and spk525 (5′-CcctccgaggagGCctacacg-3′), followed by another round of PCR using primers spk416 and spk415. Amplicons were then digested using NdeI and BamHI and cloned into the Kernow C-1 P6 HEV infectious clone plasmid. Clones were screened for a gain of NheI and StuI restriction sites and subsequently verified via sequencing. The NheI site was introduced silently, producing no amino acid changes in the ORF1 protein, although the StuI site resulted in a single cysteine-to-alanine mutation at ORF1 amino acid residue 751.
Insertion of RPS17 lysine-to-alanine mutations or heterologous sequences in place of the RPS17 insertion.
gBlock gene fragments (IDT, Coralville, IA) encoding RPS17 amino acids 21 to 76, with either K44/45A, K49A, K32/R33/K44/45/49A, or K32/R33/K49A changes, were inserted into the HEV P6 NheI/StuI infectious clone by using NheI and StuI restriction sites engineered at the 5′ and 3′ ends of the gBlock. Similarly, gBlocks encoding Rous sarcoma virus (RSV) nucleocapsid (NC) amino acids 15 to 73, RSV NC amino acids 15 to 73 with cysteines 21, 23, 47, and 50 and histidines 29 and 55 changed to glycine (36), yeast poly(A) binding protein amino acids 359 to 414 (37), and ribosomal protein L3 (RibL3; GenBank accession no. CAA51839.1) amino acids 18 to 73 were inserted into the HEV P6 NheI/StuI infectious clone by using NheI and StuI restriction sites.
In vitro transcription and transfection of Huh7 liver cells.
Plasmids were linearized at the 3′ terminus by use of an MluI restriction site. Capped RNA transcripts were generated using a Ribomax T7 in vitro transcription kit (Promega) with anti-reverse cap analog (Tri-Link Biotechnologies). The fidelity of transcripts was assessed by gel electrophoresis on 0.8% agarose gels containing ethidium bromide, with visualization via UV illumination. Transcripts were normalized, and typically 11 μl of the 50-μl reaction mixture was used for transfection, using Trans-IT mRNA transfection reagent (Mirus). RNA transcripts were added to 633 μl of Optimem medium (Invitrogen), and 13.25 μl of mRNA boost reagent was added, followed by the addition of 13.25 μl of Trans-IT reagent. The RNA/Trans-IT complexes were then added to a T25 flask of Huh7 cells at >80% confluence. At 2 days posttransfection, the cells were split 1:2 into two new T25 flasks and allowed to grow for another 3 to 5 days.
Infection of HepG2C3A and BHK-21 cells by HEV.
Huh7 human liver cells are routinely used for transfection with HEV infectious clones and mutants. However, HepG2C3A cells are used for the HEV infection assay, since Huh7 cells cannot readily be infected by HEV. HepG2C3A or BHK-21 cells were seeded into 48-well collagen-coated plates (HepG2C3A) or uncoated plates (BHK-21) 1 day prior to HEV infection. T25 flasks of transiently transfected Huh7 cells were frozen and thawed three times to release virus. Cell debris was clarified by centrifugation at 1,000 × g for 5 min. A 200-μl aliquot of clarified Huh7 lysate containing HEV was transferred to the HepG2C3A or BHK-21 cells and incubated at 37°C for 1 h. The medium was then replaced with Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). At 2 days postinfection, the infected cells were split 1:2 into another 48-well plate and allowed to incubate for 3 additional days.
Flow cytometry of Huh7 cells transfected with in vitro-transcribed capped HEV RNA or of HepG2C3A or BHK-21 cells infected with HEV.
Transfected or infected cells were removed from the plate by use of 0.25% trypsin and pelleted at 600 × g for 5 min. Cells were resuspended in 1 ml of 100% methanol and left at 4°C for 15 min. Fixed cells were then stored at −80°C in methanol until processed. For processing, cells were spun out of methanol at 600 × g for 5 min and resuspended in phosphate-buffered saline (PBS) for 5 min. Cells were again pelleted and then blocked in 1 ml of blocking solution (10% Odyssey blocking buffer [LiCor Biosciences], 5% nonfat dried milk, 0.1% Triton X-100 in PBS) for 30 min at 37°C. Cells were subsequently probed with rabbit anti-ORF2 serum (1:50) in PBS for 1 h at 37°C. After washing twice in PBS, cells were incubated with goat anti-rabbit–phycoerythrin (PE) (Life Technologies) at a 1:300 dilution in PBS for 1 h at 37°C. Cells were washed twice more in PBS and finally resuspended in 100 μl of PBS. Fluorescence was analyzed for 10,000 events by use of a FACSAria flow cytometer (Becton Dickson) and analyzed using FlowJo software.
Predictions of posttranslational modifications of HEV HVR insertions.
The amino acid sequences from the RPS17 insertion in the Kernow C-1 P6 HEV infectious clone or the heterologous sequences from the RSV NC, yeast poly(A) binding protein, and RibL3 protein insertions were analyzed using in silico methods to determine the likelihood of posttranslational modifications via sequence information. Acetylation prediction was performed using prediction of acetylation on internal lysines (PAIL), SUMOylation prediction was performed using SUMOplot (Abgent), ubiquitination prediction was performed using UBpred (38), and methylation prediction was performed using BPB-PPMS (39).
Western blotting to determine the methylation state of the HEV HVR.
BHK-21 cells growing in 6-well cell culture plates were transiently transfected with plasmid DNA encoding ORF1 amino acids 587 to 875 in frame with triple yellow fluorescent protein, with or without the indicated point mutations, or triple YFP alone, using Lipofectamine LTX (Invitrogen). At 24 h posttransfection, cells were lysed in RIPA buffer (Bioworld Inc.) with 1% glycerol, 1,000 U/10 ml Pierce universal nuclease for cell lysis (Thermo), and protease inhibitors. Insoluble proteins were removed via centrifugation at 10,000 × g for 10 min. A 25-μl aliquot of the clarified lysate was mixed with 20 μl of 3× Laemmli denaturing sample buffer and boiled for 10 min, and 45 μl was loaded onto a 4 to 20% Precise protein gel (Thermo) for analysis. Proteins were transferred to nitrocellulose (LiCor Bioscience), blocked with Odyssey blocking buffer (LiCor Biosciences), and probed with mouse anti-green fluorescent protein (anti-GFP) (GFP01; Thermo), followed by goat anti-mouse–IRDye680 (LiCor Bioscience). Blots were then reprobed with rabbit anti-methyl lysine (StressMarq Biosciences) followed by donkey anti-rabbit–IRdye800 (LiCor). Blots were imaged using an Odyssey CLx infrared imaging system (LiCor).
RESULTS
RPS17 and RPS19 insertions within the HEV HVR contain NLS motifs.
Two unique strains of HEV that were found to have enhanced virus growth and expanded species tropism in cell culture contain amino acids 93 to 131 of RPS19 and amino acids 21 to 76 of RPS17, respectively (Fig. 1). It has been demonstrated that amino acid glycine 127 is necessary for nucleolar localization and that amino acids 120 to 142 contribute to nuclear localization of the RPS19 protein (32), and a portion of this sequence is contained within the RPS19 insertion of the HEV strain. Concomitantly, amino acids 32 to 50 of RPS17 were demonstrated to be sufficient for active nuclear import of green fluorescent protein (our unpublished data).
FIG 1.
Insertion of human RPS17 and RPS19 sequences within the hypervariable region (HVR) in the ORF1 protein of HEV. The figure shows a schematic diagram of HEV ORF1 putative functional domains, including the HVR. Amino acids 93 through 131 of RPS19 and amino acids 21 through 76 of RPS17 are inserted into the HVR of HEV at the indicated positions in two unique strains of HEV recovered from infected patients. Glycine 127 in the RPS19 insertion and lysines 32, 44, 45, and 49 from RPS17, which are known to be involved in either nucleolar or nuclear targeting, are underlined and shown in bold.
Subcellular localization of full-length ORF1 proteins of the Kernow C-1 P1 and P6 HEV strains.
We were unable to detect endogenous levels of HEV ORF1 protein during infection by utilizing antibody staining with rabbit polyclonal antibodies raised against recombinant proteins from the HEV methyltransferase, helicase, or RdRp domain (data not shown). To determine the localization of ORF1 proteins from the P1 and P6 HEV strains, the full-length sequence of the ORF1 protein from either P1 or P6 HEV was inserted in frame with EYFP (Fig. 2A). Whether the HEV ORF1 polyprotein is processed during virus infection remains highly controversial. It is now believed that the ORF1 protein is not processed at all when expressed from cloned HEV cDNA (40, 41). Despite low levels of expression, we were able to observe fluorescence in the nuclei of Huh7 cells transfected with HEV P6 ORF1 YFP (containing RPS17) but not in those of cells transfected with HEV P1 ORF1 YFP (lacking RPS17) (Fig. 2A, white arrows), suggesting that the observed fluorescence resulted from the HVR-containing nonstructural protein.
FIG 2.

Subcellular localization of full-length ORF1 protein and HVRs from P1 and P6 strains of HEV tagged with YFP. (A) (Top) Schematic diagram depicting the full-length ORF1 proteins from the P1 and P6 strains of HEV. The RPS17 insertion is highlighted in the P6 strain, and amino acid numbers are displayed above the diagrams. (Bottom) Confocal microscopy of Huh7 liver cells transiently expressing the ORF1 protein from P1 HEV tagged with yellow fluorescent protein (a) or the ORF1 protein from P6 HEV tagged with yellow fluorescent protein (b). Arrows depict the nucleus of interest in each panel. (B) (Top) Schematic diagram depicting amino acids 587 to 818 (HEV P1) or 875 (HEV P6), encompassing the HVR of P1 or P6 HEV attached to three copies of yellow fluorescent protein. (Bottom) Confocal imaging of Huh7 liver cells transiently expressing either the HEV P1 HVR containing amino acids tagged with triple YFP or the HEV P6 HVR containing amino acids tagged with triple YFP.
The RPS17 sequence insertion in the Kernow C-1 P6 HEV HVR bestows functional nuclear localization to yellow fluorescent protein.
In order to determine whether the RPS17 sequence or other amino acid differences outside the insertion were responsible for nuclear trafficking, an expression construct containing three consecutive copies of YFP was created to assess the nuclear trafficking ability of the HVRs from the Kernow C-1 P6 and P1 HEV strains. Triple YFP, at approximately 80 kDa, is above the size limit for passive diffusion through nuclear pores (50 kDa) (42). For triple YFP to be found in the nucleus, the amino acids attached to it must contain an active NLS. We inserted HEV ORF1 amino acids 587 to 818 (P1 virus) or 875 (P6 virus) in frame with triple YFP and subsequently assessed the ability of these amino acid sequences to alter the subcellular localization of YFP within the cell (Fig. 2B). When transiently expressed in Huh7 liver cells, triple YFP with no additional amino acid sequences localized predominantly to the cytoplasm (Fig. 2B, panel a). The HVR from P1 HEV also localized predominantly to the cytoplasm (Fig. 2B, panel b), suggesting that there were no active nuclear localization signals within the HVR of the P1 strain. However, the HVR from P6 HEV localized predominantly to the nuclei of Huh7 cells, suggesting that an active nuclear import signal within the inserted RPS17 sequence in the HEV P6 HVR bestows active nuclear import signals to the Kernow C-1 HEV ORF1 protein.
Kernow P6, P6 NheI/StuI, and P1 strains of HEV all express detectable levels of ORF2 in Huh7 cells transfected with capped HEV RNA transcripts and in infected HepG2C3A cells, whereas swine HEV ORF2 expression is not detectable.
Despite the fact that swine HEV and the Kernow P1, Kernow P6, and Kernow P6 NheI/StuI strains of HEV all belong to genotype 3, there were marked differences in the ability to be expressed within Huh7 liver cells (Fig. 3A and B) and, concomitantly, the ability to infect HepG2C3A cells (Fig. 3B). The cell culture-adapted HEV P6 strain produced detectable ORF2 protein in 32.2% of transfected Huh7 cells after 5 days. The P6 strain possessing NheI and StuI sites was reduced to 22.25% of cells expressing ORF2 protein, although this difference was not significant (P = 0.055). The HEV P1 strain produced ORF2 protein in only 8.24% of cells, which was significantly reduced compared to the levels for both the P6 virus and the P6 NheI/StuI virus (P < 0.0001), and the ORF2 protein level in the genotype 3 swine HEV strain was below the limit of detection (Fig. 3B). Using equivalent volumes of transfected Huh7 cell lysates containing HEV to infect HepG2C3a cells in a virus infection assay led to a similar trend in data regarding the expression of HEV ORF2 protein in Huh7 cells. The HEV P6 and P6 NheI/StuI strains infected 3.86% and 7.31% of the HepG2C3a cells and were not statistically different from one another (P = 0.0625). The HEV P1 strain infected significantly fewer of the HepG2C3a cells than did the P6 or P6 NheI/StuI strain (1.18%; P = 0.024 for P6 and P < 0.0001 for P6 NheI/StuI). Again, the level for the genotype 3 swine HEV strain was below the level of detection (Fig. 3C).
FIG 3.

Transfection of Huh7 cells and infection of HepG2C3A cells with parental HEV strains. (A) Fluorescence images of Huh7 cells 5 days after either mock transfection (a) or transfection with capped RNA from the designated HEV strains (b to d). Samples were fixed and probed with rabbit anti-ORF2 antibody followed by goat anti-rabbit–fluorescein isothiocyanate (FITC) antibody. Each panel is a representative 20× field acquired using the same imaging parameters. (B) Flow cytometry quantification of Huh7 cells transfected with the capped RNA transcripts of the designated HEV strains, performed at 5 days posttransfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 separate transfections stained in parallel. Asterisks denote statistically significant differences from the P6 and P6 NheI/StuI strains (P < 0.05). The value for the P6 NheI/StuI strain was not significantly different from that for the P6 strain (P = 0.055). (C) Flow cytometry quantification of HepG2C3A cells infected with 200 μl of clarified Huh7 cell lysate transfected with the capped RNA transcripts of the designated HEV strains, performed at 5 days postinfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 replicates stained in parallel. Asterisks denote statistically significant differences from the P6 and P6 NheI/StuI strains (P < 0.05). The value for the P6 NheI/StuI strain was not significantly different from that for the P6 strain (P = 0.0626).
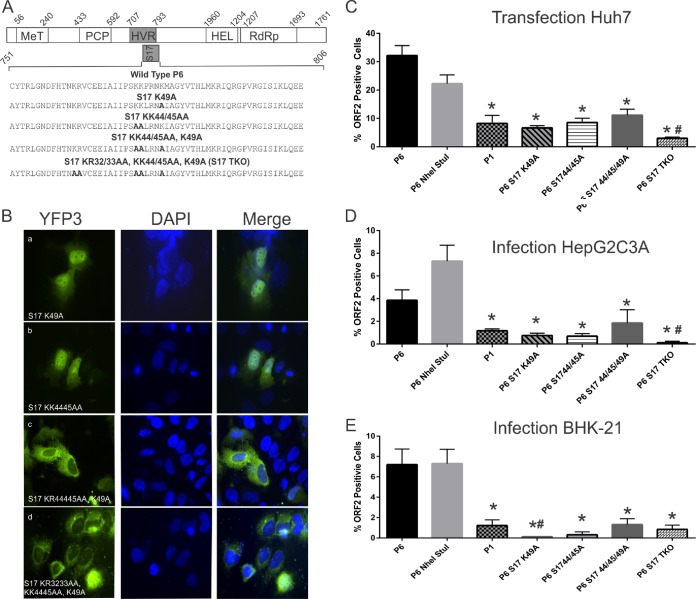
Mutation of lysine residues within the RPS17 insertion of the Kernow C-1 P6 HEV alters nuclear localization.
To map the determinants contributing to nuclear localization of the HVR of Kernow C-1 P6 HEV, we mutated the lysine amino acids 32, 44, 45, and 49 and/or arginine amino acid 33, either singly or in combination (Fig. 4A), as these basic amino acids were previously shown to be involved in nuclear import of RPS17 (our unpublished data). As expected, the K44/45A or K49A mutation singly was not sufficient to abolish nuclear localization of the P6 HEV HVR protein tagged with triple YFP (Fig. 4B). However, K44/45/49A or K32/33/44/45/49A (S17TKO) mutations were sufficient to abolish nuclear localization (Fig. 4B).
FIG 4.
Effects of mutation of lysines within the RPS17 NLS to alanine on HEV HVR protein subcellular localization, expression, and infectivity. (A) Diagram depicting the P6 HEV ORF1 protein with the functional domains labeled. The blowup at bottom depicts the amino acid sequences of the RPS17 protein inserted into the HVR of the HEV ORF1 protein. Mutant constructs possessing lysines mutated to alanine are depicted below the wild-type sequence. (B) Confocal imaging of Huh7 liver cells transiently expressing the HVR of P6 HEV with the indicated lysine residue altered to alanine, attached to three copies of yellow fluorescent protein. (C) Flow cytometry quantification of Huh7 cells transfected with capped RNA transcripts of the designated HEV strains, performed at 5 days posttransfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 separate transfections stained in parallel. (D) Flow cytometry quantification of HepG2C3A cells infected with 200 μl of clarified Huh7 cell lysate transfected with the RNA transcripts of designated HEV strains, performed at 5 days postinfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 replicates stained in parallel. (E) Flow cytometry quantification of BHK-21 cells infected with 200 μl of clarified Huh7 cell lysate transfected with RNA transcripts of the specified HEV strains, performed at 5 days postinfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 replicates stained in parallel. Asterisks indicate samples whose values were significantly lower than those for both the P6 and P6 NheI/StuI strains (P < 0.05). Octothorpes (#) indicate samples whose values were statistically lower than those for P1 (P < 0.05).
Lysine residues in the RPS17 HVR insertion are important for efficient viral replication in cells transfected with in vitro-transcribed capped viral RNA and for infection of HepG2C3A cells.
The RPS17 K44/45A, K49A, and K32/44/45/49A (S17TKO) mutations reduced the percentage of cells expressing the HEV ORF2 protein to 6.65%, 8.57%, and 2.99%, respectively (Fig. 4C). Each lysine mutation significantly reduced the number of cells expressing the HEV ORF2 protein compared to either P6 (32.2%) or the parental P6 strain containing NheI and StuI restriction sites (22.25%) (P = 0.001, P < 0.0001, and P < 0.0001, respectively). The K44/45A and K49A mutants singly were not significantly different from the HEV P1 virus (8.24% of cells expressing ORF2 protein; P = 0.947, P = 0.69, and P = 0.24, respectively) (Fig. 4C). When the transfected Huh7 cell lysates from the K44/45A and K49A mutants were used to infect HepG2C3A cells, they infected cells to degrees similar to that with the HEV P1 strain (1.18% for P1 strain, 0.70% for KK44/45AA strain, and 0.75% for K49A strain; P = 0.14 and P = 0.093, respectively). The triple-lysine-knockout HEV mutant (S17TKO) infected significantly fewer HepG2C3A cells (0.12%) than the HEV P1 strain did (1.18%) (P = 0.0053) (Fig. 4D).
Replacing the RPS17 insertion in the P6 HEV HVR with amino acid sequences from heterologous proteins containing nuclear localization signals can functionally replace RPS17 for nuclear localization of the nonstructural protein.
In order to gain insight into the RPS17-mediated enhancement of P6 HEV replication and in vitro species tropism expansion, we chose four heterologous amino acid sequences to substitute for RPS17 (Fig. 5A). The RSV NC protein contains nucleic acid binding, protein-protein interaction, and nuclear localization functions. The RSV NC ΔCys/His sequence retains nuclear localization and protein-protein interaction functions but has greatly reduced nucleic acid binding. The yeast poly(A) binding protein has nucleic acid binding properties but does not have a known nuclear localization signal. Finally, ribosomal protein L3 is another ribosomal protein, from the 60S ribosomal subunit as opposed to the 40S ribosomal subunit (as for RPS17 and RPS19), with unknown nucleic acid binding and nuclear localization signals, but the inserted sequence is lysine rich (9 lysines compared to 6 in RPS17).
FIG 5.
Effects of substitutions of exogenous protein sequences for the RPS17 insertion within the HVR of the HEV P6 strain on HEV protein subcellular localization, expression, and infectivity. (A) Diagram depicting the P6 HEV ORF1 protein with the functional domains labeled. The blowup at bottom depicts the amino acid sequences inserted in place of the RPS17 protein in the HVR. (B) Confocal imaging of Huh7 liver cells transiently expressing the HVR of P6 HEV with the indicated lysines altered to alanine, attached to three copies of yellow fluorescent protein. (C) Flow cytometry quantification of Huh7 cells transfected with the capped RNA transcripts of the specified HEV strains, performed at 5 days posttransfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 separate transfections stained in parallel. (D) Flow cytometry quantification of HepG2C3A cells infected with 200 μl of clarified Huh7 cell lysate transfected with the capped RNA transcripts of the specified HEV strains, performed at 5 days postinfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 replicates stained in parallel. (E) Flow cytometry quantification of BHK-21 cells infected with 200 μl of clarified Huh7 cell lysate transfected with the RNA transcripts of the specified HEV strains, performed at 5 days postinfection. Samples were fixed in methanol and probed with rabbit anti-ORF2 followed by goat anti-rabbit PE antibodies. Each bar represents a minimum of 5 replicates stained in parallel. Asterisks indicate samples whose values were significantly lower than those for both the P6 and P6 NheI/StuI strains (P < 0.05). Octothorpes (#) indicate samples whose values were statistically lower than that for P1 (P < 0.05).
As expected, the substituted RSV NC sequences bestowed nuclear trafficking functions to the HEV HVR (Fig. 5B, panels b and c). The yeast poly(A) binding protein insertion did not have an active NLS, and consequently, the HEV HVR remained predominantly cytoplasmic (Fig. 5B, panel d). The RibL3 protein insertion had an active nuclear localization signal producing fluorescence both within the nucleus and within the cytoplasm (diffuse and punctate) (Fig. 5B, panel a).
P6 HEV containing a heterologous NLS from RSV NC does not recapitulate the enhanced replication of wild-type P6 HEV.
In order to further evaluate which functional property of RPS17 contributes to the enhanced expression and in vitro host range expansion of the P6 HEV strain, we inserted amino acids 15 to 73 from the RSV NC protein. The NC region of RSV has a very well-classified nuclear localization signal (36), and cysteine-histidine amino acid motifs are responsible for nucleic acid binding (43). Transfection of HEV RNA encoding either RSV NC or RSV NC with the cysteine-histidine box amino acids mutated to alanine (ΔCys/His) into Huh7 cells resulted in the number of cells expressing the viral ORF2 protein resembling that for the HEV P1 strain. Approximately 4.7% of RSV NC-transfected cells and 3.33% of RSV NC ΔCys/His-transfected cells expressed the ORF2 protein, compared to 22.25% of HEV P6 NheI/StuI-transfected cells and 8.24% of HEV P1-transfected cells. Both RSV NC and RSV NC ΔCys/His HVR HEVs yielded significantly fewer cells expressing the ORF2 protein than wild-type P6 or P6 NheI/StuI (P < 0.0001) virus did, while there was no significant difference in the number of cells expressing the ORF2 protein between P1 virus and RSV NC or RSV NC ΔCys/His HVR-expressing virus (P = 0.54 and P = 0.15, respectively) (Fig. 5C). Similarly, the RSV NC HVR insertion- and RSV NC ΔCys/His-containing viruses infected both HepG2C3A cells (0.26% and 1.62%, respectively) and BHK-21 cells (1.13% and 0.51%, respectively) with efficiencies similar to those of the HEV P1 strain (1.18% for HepG2C3A cells and 1.22% for BHK-21 cells) (Fig. 5D and E).
HEV strains containing amino acid sequences from yeast poly(A) binding protein and ribosomal protein L3 inserted into the HVR of HEV are unable to restore expression levels of ORF2 protein to the levels observed for P6 HEV in transfected Huh7 or infected HepG2C3A cells.
Insertion of an amino acid sequence from the yeast poly(A) binding protein which contained RNA binding ability but not nuclear targeting signals led to production of HEV ORF2 protein in 11.24% of Huh7 cells, but this number was not significantly greater than that for the HEV P1 strain (8.24%; P = 0.4) (Fig. 5C). This strain was also not capable of infecting HepG2C3A cells to a higher degree than that of the HEV P1 strain (0.52% versus 1.18%; P = 0.11) (Fig. 5D), nor was it capable of infecting BHK-21 cells to a greater extent than that of the HEV P1 strain (0.57% versus 1.22%; P = 0.1) (Fig. 5E). Finally, addition of a heterologous ribosomal sequence from the L3 protein was unable to give expression of the HEV ORF2 protein in more cells than the case for the HEV P1 strain (5.99% versus 8.24%; P = 0.75); the strain with the RibL3 HVR insertion was actually significantly worse than the P1 strain at infecting both HepG2C3A cells (0.11% versus 1.18%; P = 0.036) (Fig. 5D) and BHK-21 cells (0.33% versus 1.22%; P = 0.035) (Fig. 5E).
The RPS17 and heterologous sequences inserted within the HVR of the HEV P6 strain are predicted to be posttranslationally modified on lysines to various degrees, but these modifications do not correlate with enhanced expression or infectivity.
A predominant feature of lysine residues is their ability to be posttranslationally modified, leading to changes in protein function. We assayed four of the most common types of lysine modification in the RPS17, RSV NC, yeast poly(A) binding protein, and RibL3 HVR insertions to determine if there were differences which could explain the changes in expression of HEV ORF2 protein from P6 and each of the other HVR insertion viruses.
Acetylation is known to play a role in gene expression and metabolism. Acetylation of the HEV HVR insertions was predicted using prediction of acetylation on internal lysines (PAIL), set at medium stringency, which typically produces results with an accuracy of 87.97% (44). When each of the insertions was analyzed, numerous lysines provided positive results. The Kernow HEV P6 strain had several hits, including RPS17 lysine 44 (K775) (score of 0.66), K45 (K776) (1.0), K49 (K781) (1.12), and K59 (K802) (1.68). The yeast poly(A) binding protein insertion lysines K753 (17.39), K755 (7.12), K775 (0.41), and K793 (1.46) all had some probability of being acetylated. RSV NC lysines K754 (12.61), K762 (0.58), K764 (1.78), K768 (1.25), K773 (1.34), K800 (1.86), K803 (1.42), and K804 (1.15) were also predicted to be acetylated. Finally, lysines K773 (0.53), K774 (3.53), K776 (2.65), K795 (1.07), K799 (1.54), and K805 (18.32) from the RibL3 HVR insertion were predicted to be acetylated (Fig. 6A).
FIG 6.

Predictions of posttranslational modifications of the RPS17 insertion within the HVR of HEV P6 and of exogenous protein sequences used in place of RPS17. (A) Table showing predicted common posttranslational modifications of lysines within the sequences inserted into the HEV P6 HVR. The insert is listed in the left column, followed by acetylation prediction using PAIL (44), SUMOylation prediction using SUMOplot (Abgent), ubiquitination prediction using UBpred (38), and methylation prediction using BPB-PPMS (39). ND, none detected. (B) Western blots of BHK-21 cell lysates transiently expressing the HVRs from HEV P1, P6, and lysine-mutated P6 HVRs attached to triple YFP. The blot on the left was probed with mouse anti-GFP followed by goat anti-mouse–IRDye680 to detect expression of the fusion protein, denoted by arrows. The blot on the right is the same blot reprobed using rabbit anti-methyl lysine followed by donkey anti-rabbit–IRDye800. No lysine methylation of any of the HEV HVR constructs was evident.
Small ubiquitin-like modifiers (SUMO) are involved in numerous cell processes, including nuclear-cytosolic transport, transcriptional regulation, and protein stability. SUMOylation of the insertions in the HEV P6 HVR was predicted using SUMOplot software from Abgent. Only lysines 773 (score of 0.27) and 794 (0.44) from the RSV NC insertion and lysines 768 (0.58) and 773 (0.61) from the RibL3 insertion were predicted to be SUMOylated (Fig. 6A).
Ubiquitin is an 8.5-kDa regulatory protein which can signal for protein degradation via the proteosome, alter subcellular cellular localization, and promote or prevent protein-protein interactions. Ubiquitination of the HEV HVR insertions was assessed using UbPred software (38). The cutoff for low confidence of ubiquitination was 0.62. The scores for the HEV P6, yeast poly(A) binding protein, and RSV NC HVR insertions were all lower than the 0.62 cutoff. Only lysine 773 from the RibL3 protein insertion yielded a lysine predicted to be ubiquitinated with a medium confidence (0.82).
Protein methylation has been studied best in histone proteins. Methylation can act epigenetically to repress or activate gene expression. We used computational identification of protein methylation sites through biprofile Bayes feature extraction (BPB-PPMS) (39). Lysine 775 from the HEV P6 insertion and lysine 800 from the RibL3 protein were predicted to be methylated (scores of 0.5 and 0.51, respectively) (Fig. 6A).
Because the HEV P6 RPS17 insertion was predicted to be methylated and this methylation state differed from that for both the RSV NC and yeast poly(A) binding protein HVR insertions, whose corresponding strains were not as infectious as wild-type P6 virus, we experimentally tested whether the RPS17 insertion from P6 HEV was methylated by using an anti-methyl lysine antibody. Despite equivalent levels of P6 and P1 HVR expression, no significant lysine methylation was observed (Fig. 6B). This suggests that the amount of methylation of the lysines is very small or is dependent on expression of other viral proteins or that the lysines are not actually methylated.
DISCUSSION
Retroviruses, many DNA viruses, and some negative-strand RNA viruses utilize both the nucleus and the cytoplasm for viral replication and assembly (45, 46). In this study, we show for the first time that a strain of HEV, a single-stranded positive-sense RNA virus, can accrue insertions within its HVR that likely bestow nuclear import functions to the viral ORF1 protein, as well as cleavage products which contain the HVR. However, simple nuclear localization did not appear to correspond to enhanced viral protein expression, infectivity, or an expansion in host range in vitro, as lysine mutations which did not disrupt nuclear localization (KK44/45AA) allowed expression of the HEV ORF2 protein to levels significantly lower than those in the wild-type P6 HEV strain (Fig. 4). Additionally, heterologous protein sequences from ribosomal protein L3 and RSV NC, which also bestowed nuclear localization to the HEV ORF1 protein, also gave expression of significantly less HEV ORF2 protein than that with the wild-type strain (Fig. 5).
In this study, we attempted to use forward genetics to determine which functional aspects of the RPS17 insertion may lead to enhanced viral protein expression and infectivity. We utilized the RSV NC protein, which possesses nucleic acid binding, nuclear localization, and protein dimerization functions. Although we did not test for nucleic acid binding and dimerization capabilities, RSV NC did retain nuclear trafficking capabilities. The inability of RSV NC and RSV NC ΔCys/His insertions to restore HEV protein expression levels to those of the HEV P6 strain suggests that nucleic acid binding, nuclear localization, and dimerization attributes likely did not contribute to the enhanced viral protein expression. To further evaluate whether nucleic acid binding plays a role in the enhanced ORF2 protein expression of HEV P6, we inserted a portion of the yeast poly(A) binding protein which is known to interact with nucleic acids. The yeast poly(A) binding protein P6 virus again did not restore infectivity to wild-type levels, further suggesting that general nucleic acid binding was not responsible for the enhanced viral protein expression and infectivity seen for HEV P6. Additionally, we chose a sequence of the ribosomal protein L3 that is not a component of the 40S ribosomal subunit but is lysine rich to determine whether any ribosomal protein is capable of the enhanced viral protein expression observed with the HEV strains possessing RPS19 and RPS17 insertions. The inserted RibL3 sequence contained an NLS, as it bestowed nuclear trafficking ability to the HVR triple YFP construct, but it could not restore HEV ORF2 protein expression to the levels seen in HEV P6. This result suggested that not just any ribosomal protein is capable of enhancing HEV expression, nor is general nuclear localization a contributing factor.
Another possibility that may contribute to the enhanced expression of P6 HEV is a gain of posttranslational modifications through the RPS17 insertion in the HEV HVR. Lysines are notable for their ability to be posttranslationally modified. Lysines can be modified through acetylation, biotinylation, carboxylation, neddylation, pupylation, SUMOylation, and ubiquitination, and these modifications can modify protein function. Although we cannot fully rule out differences in posttranslational modification causing the enhanced viral protein expression and infectivity of the HEV P6 strain, we did not see a likely correlation between the predicted lysine acetylation, SUMOylation, ubiquitination, or methylation and the differences in expression of P6 and P1 viruses or any of our mutants (Fig. 6A).
The effects of the RPS17 insertion on enhancing the replication of the HEV P6 strain appear to be dependent on having lysine residues at RPS17 amino acids 49 and 44 or 45, as mutation of these residues to alanine lowered the expression and infectivity of the P6 strain to those of the P1 strain. Lysine is a basic amino acid that often participates in hydrogen bonding through the generation of salt bridges and is thus often found in active binding sites, suggesting that protein-protein interactions occurring at this position within the RPS17 protein may contribute to enhanced HEV replication.
One interesting example of a viral protein interacting with host ribosomal proteins is the case of the hantavirus nucleocapsid (N) protein, which interacts with the RPS19 protein (47–49). It has been shown that RPS19 interaction with N protein facilitates ribosome loading onto the viral RNA transcripts, leading to preferential translation of viral transcripts. Both RPS19 and RPS17 are located within the head region of the 40S ribosome, and both were found to be inserted into the HEV HVR, leading to enhanced virus replication. An interesting hypothesis is that the RPS17 and RPS19 insertions within the HVR of HEV attract and assemble the 40S ribosomal proteins as part of the HEV replication complex, essentially coupling transcription of the viral genome with translation of the viral proteins, leading to enhanced viral protein production. Although the interaction domains linking RPS17 and RPS19 to the 40S ribosome have not been mapped, it would make physiological sense that they would overlap the nuclear localization signals. Once a ribosome has assembled in the nucleus, it would no longer need to access the nucleus, preferring to remain cytosolic, where the majority of translation occurs. It is possible that by disrupting lysines within the 40S assembly domains of RPS17, we abolished 40S ribosomal protein assembly within the nonstructural protein, uncoupling HEV transcription and translation and thus reducing the expression of HEV P6 to P1 levels of replication.
Further research is warranted to uncouple functional nuclear localization from the role of lysine residues in the enhanced viral protein expression and infectivity of the HEV P6 strain. HEV may take advantage of the highly conserved amino acid sequences and function of ribosomal proteins to enhance translation of viral proteins. If this is a conserved mechanism, then insertion of ribosomal proteins may serve as a way to adapt additional strains of HEV to grow more efficiently in cell culture, allowing us to address the molecular aspects of the viral life cycle that have been difficult to study thus far due to the inability of HEV strains to grow sufficiently in cell culture. Another question that remains to be answered is as follows: since the cell culture-adapted P6 HEV strain was found to possess enhanced replication properties, why was it not the dominant strain found within the original host? It is possible that HEV has evolved to have suboptimal expression and replication to avoid the host immune response, thus prolonging viral infection and spread. It will be interesting to compare the host responses to both the HEV P6 and P1 strains to determine if there is an enhanced immune response to match the enhanced replication seen in cell culture for the HEV P6 strain.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (grants R01AI074667 and R01AI050611).
We thank Susanne U. Emerson of NIAID, NIH, Bethesda, MD, for generously providing the Kernow C-1 P1 and P6 infectious clone plasmids as well as the subclone Huh-7 cell line. We also thank Dianjun Cao from Virginia Tech, Blacksburg, VA, for providing us with his rabbit anti-ORF1 protein antibodies.
REFERENCES
- 1.Purcell RH, Emerson SU. 2008. Hepatitis E: an emerging awareness of an old disease. J Hepatol 48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. 2012. Hepatitis E. Lancet 379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 3.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, Cointault O, Esposito L, Abravanel F, Danjoux M, Durand D, Vinel JP, Izopet J, Rostaing L. 2008. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 4.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. 2011. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 5.Renou C, Lafeuillade A, Cadranel JF, Pavio N, Pariente A, Allegre T, Poggi C, Penaranda G, Cordier F, Nicand E, ANGH . 2010. Hepatitis E virus in HIV-infected patients. AIDS 24:1493–1499. doi: 10.1097/QAD.0b013e32833a29ab. [DOI] [PubMed] [Google Scholar]
- 6.Meng XJ. 2012. Emerging and re-emerging swine viruses. Transbound Emerg Dis 59(Suppl 1):85–102. doi: 10.1111/j.1865-1682.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- 7.Pavio N, Meng XJ, Renou C. 2010. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res 41:46. doi: 10.1051/vetres/2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, Takahashi M, Hoshino Y, Takahashi H, Ichiyama K, Nagashima S, Tanaka T, Okamoto H. 2009. ORF3 protein of hepatitis E virus is essential for virion release from infected cells. J Gen Virol 90:1880–1891. doi: 10.1099/vir.0.010561-0. [DOI] [PubMed] [Google Scholar]
- 9.Emerson SU, Nguyen HT, Torian U, Burke D, Engle R, Purcell RH. 2010. Release of genotype 1 hepatitis E virus from cultured hepatoma and polarized intestinal cells depends on open reading frame 3 protein and requires an intact PXXP motif. J Virol 84:9059–9069. doi: 10.1128/JVI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenney SP, Pudupakam RS, Huang YW, Pierson FW, LeRoith T, Meng XJ. 2012. The PSAP motif within the ORF3 protein of an avian strain of the hepatitis E virus is not critical for viral infectivity in vivo but plays a role in virus release. J Virol 86:5637–5646. doi: 10.1128/JVI.06711-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magden J, Takeda N, Li T, Auvinen P, Ahola T, Miyamura T, Merits A, Kaariainen L. 2001. Virus-specific mRNA capping enzyme encoded by hepatitis E virus. J Virol 75:6249–6255. doi: 10.1128/JVI.75.14.6249-6255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parvez MK, Khan AA. 2014. Molecular modeling and analysis of hepatitis E virus (HEV) papain-like cysteine protease. Virus Res 179:220–224. doi: 10.1016/j.virusres.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karpe YA, Lole KS. 2011. Deubiquitination activity associated with hepatitis E virus putative papain-like cysteine protease. J Gen Virol 92:2088–2092. doi: 10.1099/vir.0.033738-0. [DOI] [PubMed] [Google Scholar]
- 14.Purdy MA, Lara J, Khudyakov YE. 2012. The hepatitis E virus polyproline region is involved in viral adaptation. PLoS One 7:e35974. doi: 10.1371/journal.pone.0035974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koonin EV, Gorbalenya AE, Purdy MA, Rozanov MN, Reyes GR, Bradley DW. 1992. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: delineation of an additional group of positive-strand RNA plant and animal viruses. Proc Natl Acad Sci U S A 89:8259–8263. doi: 10.1073/pnas.89.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpe YA, Lole KS. 2010. NTPase and 5′ to 3′ RNA duplex-unwinding activities of the hepatitis E virus helicase domain. J Virol 84:3595–3602. doi: 10.1128/JVI.02130-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpe YA, Lole KS. 2010. RNA 5′-triphosphatase activity of the hepatitis E virus helicase domain. J Virol 84:9637–9641. doi: 10.1128/JVI.00492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrawal S, Gupta D, Panda SK. 2001. The 3′ end of hepatitis E virus (HEV) genome binds specifically to the viral RNA-dependent RNA polymerase (RdRp). Virology 282:87–101. doi: 10.1006/viro.2000.0819. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad I, Holla RP, Jameel S. 2011. Molecular virology of hepatitis E virus. Virus Res 161:47–58. doi: 10.1016/j.virusres.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pudupakam RS, Huang YW, Opriessnig T, Halbur PG, Pierson FW, Meng XJ. 2009. Deletions of the hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: evidence for attenuation of HVR deletion mutants in vivo. J Virol 83:384–395. doi: 10.1128/JVI.01854-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pudupakam RS, Kenney SP, Cordoba L, Huang YW, Dryman BA, Leroith T, Pierson FW, Meng XJ. 2011. Mutational analysis of the hypervariable region of hepatitis E virus reveals its involvement in the efficiency of viral RNA replication. J Virol 85:10031–10040. doi: 10.1128/JVI.00763-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lhomme S, Garrouste C, Kamar N, Saune K, Abravanel F, Mansuy JM, Dubois M, Rostaing L, Izopet J. 2014. Influence of polyproline region and macro domain genetic heterogeneity on HEV persistence in immunocompromised patients. J Infect Dis 209:300–303. doi: 10.1093/infdis/jit438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johne R, Reetz J, Ulrich RG, Machnowska P, Sachsenroder J, Nickel P, Hofmann J. 2014. An ORF1-rearranged hepatitis E virus derived from a chronically infected patient efficiently replicates in cell culture. J Viral Hepat 21:447–456. doi: 10.1111/jvh.12157. [DOI] [PubMed] [Google Scholar]
- 24.Shukla P, Nguyen HT, Faulk K, Mather K, Torian U, Engle RE, Emerson SU. 2012. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depends on an inserted human gene segment acquired by recombination. J Virol 86:5697–5707. doi: 10.1128/JVI.00146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen HT, Torian U, Faulk K, Mather K, Engle RE, Thompson E, Bonkovsky HL, Emerson SU. 2012. A naturally occurring human/hepatitis E recombinant virus predominates in serum but not in faeces of a chronic hepatitis E patient and has a growth advantage in cell culture. J Gen Virol 93:526–530. doi: 10.1099/vir.0.037259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla P, Nguyen HT, Torian U, Engle RE, Faulk K, Dalton HR, Bendall RP, Keane FE, Purcell RH, Emerson SU. 2011. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc Natl Acad Sci U S A 108:2438–2443. doi: 10.1073/pnas.1018878108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dresios J, Panopoulos P, Synetos D. 2006. Eukaryotic ribosomal proteins lacking a eubacterial counterpart: important players in ribosomal function. Mol Microbiol 59:1651–1663. doi: 10.1111/j.1365-2958.2006.05054.x. [DOI] [PubMed] [Google Scholar]
- 28.Filipenko ML, Iantsen EI, Muravlev AI, Kopantsev EP, Karpova GG, Mertvetsov NP. 1995. Mapping the genes for ribosomal proteins S14 and S17 on human chromosomes using cDNA from a panel of hybrid cells. Bioorg Khim 21:349–353. [PubMed] [Google Scholar]
- 29.Bommer UA, Stahl J, Henske A, Lutsch G, Bielka H. 1988. Identification of proteins of the 40S ribosomal subunit involved in interaction with initiation factor eIF-2 in the quaternary initiation complex by means of monospecific antibodies. FEBS Lett 233:114–118. doi: 10.1016/0014-5793(88)81366-8. [DOI] [PubMed] [Google Scholar]
- 30.Cmejla R, Cmejlova J, Handrkova H, Petrak J, Pospisilova D. 2007. Ribosomal protein S17 gene (RPS17) is mutated in Diamond-Blackfan anemia. Hum Mutat 28:1178–1182. doi: 10.1002/humu.20608. [DOI] [PubMed] [Google Scholar]
- 31.Aspesi A, Pavesi E, Robotti E, Crescitelli R, Boria I, Avondo F, Moniz H, Da Costa L, Mohandas N, Roncaglia P, Ramenghi U, Ronchi A, Gustincich S, Merlin S, Marengo E, Ellis SR, Follenzi A, Santoro C, Dianzani I. 2014. Dissecting the transcriptional phenotype of ribosomal protein deficiency: implications for Diamond-Blackfan anemia. Gene 545:282–289. doi: 10.1016/j.gene.2014.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Da Costa L, Tchernia G, Gascard P, Lo A, Meerpohl J, Niemeyer C, Chasis JA, Fixler J, Mohandas N. 2003. Nucleolar localization of RPS19 protein in normal cells and mislocalization due to mutations in the nucleolar localization signals in 2 Diamond-Blackfan anemia patients: potential insights into pathophysiology. Blood 101:5039–5045. doi: 10.1182/blood-2002-12-3878. [DOI] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34.Huang YW, Haqshenas G, Kasorndorkbua C, Halbur PG, Emerson SU, Meng XJ. 2005. Capped RNA transcripts of full-length cDNA clones of swine hepatitis E virus are replication competent when transfected into Huh7 cells and infectious when intrahepatically inoculated into pigs. J Virol 79:1552–1558. doi: 10.1128/JVI.79.3.1552-1558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emerson SU, Nguyen H, Torian U, Purcell RH. 2006. ORF3 protein of hepatitis E virus is not required for replication, virion assembly, or infection of hepatoma cells in vitro. J Virol 80:10457–10464. doi: 10.1128/JVI.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lochmann TL, Bann DV, Ryan EP, Beyer AR, Mao A, Cochrane A, Parent LJ. 2013. NC-mediated nucleolar localization of retroviral gag proteins. Virus Res 171:304–318. doi: 10.1016/j.virusres.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadler SG, Kapouch JL, Elliott JI, Williams KR. 1992. Shuffling of amino acid sequence: an important control in synthetic peptide studies of nucleic acid-binding domains. Binding properties of fragments of a conserved eukaryotic RNA binding motif. J Biol Chem 267:3750–3757. [PubMed] [Google Scholar]
- 38.Radivojac P, Vacic V, Haynes C, Cocklin RR, Mohan A, Heyen JW, Goebl MG, Iakoucheva LM. 2010. Identification, analysis, and prediction of protein ubiquitination sites. Proteins 78:365–380. doi: 10.1002/prot.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao J, Xu D, Tsai SN, Wang Y, Ngai SM. 2009. Computational identification of protein methylation sites through bi-profile Bayes feature extraction. PLoS One 4:e4920. doi: 10.1371/journal.pone.0004920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suppiah S, Zhou Y, Frey TK. 2011. Lack of processing of the expressed ORF1 gene product of hepatitis E virus. Virol J 8:245. doi: 10.1186/1743-422X-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sehgal D, Thomas S, Chakraborty M, Jameel S. 2006. Expression and processing of the hepatitis E virus ORF1 nonstructural polyprotein. Virol J 3:38. doi: 10.1186/1743-422X-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macara IG. 2001. Transport into and out of the nucleus. Microbiol Mol Biol Rev 65:570–594. doi: 10.1128/MMBR.65.4.570-594.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meric C, Spahr PF. 1986. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol 60:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li A, Xue Y, Jin C, Wang M, Yao X. 2006. Prediction of Nepsilon-acetylation on internal lysines implemented in Bayesian discriminant method. Biochem Biophys Res Commun 350:818–824. doi: 10.1016/j.bbrc.2006.08.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiscox JA. 2003. The interaction of animal cytoplasmic RNA viruses with the nucleus to facilitate replication. Virus Res 95:13–22. doi: 10.1016/S0168-1702(03)00160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittaker GR, Kann M, Helenius A. 2000. Viral entry into the nucleus. Annu Rev Cell Dev Biol 16:627–651. doi: 10.1146/annurev.cellbio.16.1.627. [DOI] [PubMed] [Google Scholar]
- 47.Cheng E, Haque A, Rimmer MA, Hussein IT, Sheema S, Little A, Mir MA. 2011. Characterization of the interaction between hantavirus nucleocapsid protein (N) and ribosomal protein S19 (RPS19). J Biol Chem 286:11814–11824. doi: 10.1074/jbc.M110.210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haque A, Mir MA. 2010. Interaction of hantavirus nucleocapsid protein with ribosomal protein S19. J Virol 84:12450–12453. doi: 10.1128/JVI.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mir MA, Sheema S, Haseeb A, Haque A. 2010. Hantavirus nucleocapsid protein has distinct m7G cap- and RNA-binding sites. J Biol Chem 285:11357–11368. doi: 10.1074/jbc.M110.102459. [DOI] [PMC free article] [PubMed] [Google Scholar]