ABSTRACT
Respiratory paramyxoviruses such as respiratory syncytial virus (RSV) and human parainfluenza virus type 1 (HPIV1) to HPIV4 infect virtually all children by the age of 2 to 5 years, leading to partial but incomplete protection from reinfection. Here, we used luciferase-expressing reporter Sendai viruses (the murine counterpart of HPIV1) to noninvasively measure primary infection, immune responses, and protection from reinfection by either a lethal challenge or natural transmission in living mice. Both nonattenuated and attenuated reporter Sendai viruses were used, and three inoculation strategies were employed: intramuscular (i.m.), intranasal (i.n.) at a low dose and low volume, and i.n. at a high dose and high volume. High-dose, high-volume i.n. inoculation resulted in the highest levels of antibody responses and protection from reinfection. Low-dose, low-volume i.n. inoculation afforded complete protection from contact transmission and protection from morbidity, mortality, and viral growth during lethal challenge. i.m. inoculation was inferior to i.n. inoculation at inducing antibody responses and protection from challenge. For individual mice and across groups, the levels of serum binding and neutralizing antibody responses correlated with primary infection and protection from reinfection in the lungs. Contact transmission, the predominant mode of parainfluenza virus transmission, was modeled accurately by direct i.n. inoculation of Sendai virus at a low dose and low volume and was completely preventable by i.n. vaccination of an attenuated virus at a low dose and low volume. The data highlight differences in infection and protection from challenge in the upper versus lower respiratory tract and bear upon live attenuated vaccine development.
IMPORTANCE There are currently no licensed vaccines against HPIVs and human RSV (HRSV), important respiratory pathogens of infants and children. Natural infection leads to partial but incomplete protective immunity, resulting in subsequent reinfections even in the absence of antigenic drift. Here, we used noninvasive bioluminescence imaging in a mouse model to dissect relationships among (i) the mode of inoculation, (ii) the dynamics of primary infection, (iii) consequent immune responses, and (iv) protection from high-dose, high-volume lethal challenge and contact transmission, which we find here to be similar to that of a mild low-dose, low-volume upper respiratory tract (URT)-biased infection. Our studies demonstrate the superiority of i.n. versus i.m. vaccination in protection against both lethal challenge and contact transmission. In addition to providing correlates of protection that will assist respiratory virus vaccine development, these studies extend the development of an increasingly used technique for the study of viral infection and immunity, noninvasive bioluminescence imaging.
INTRODUCTION
Human respiratory syncytial virus (HRSV), human metapneumovirus (HMPV), and human parainfluenza virus type 1 (HPIV1) to HPIV4 are leading viral causes of pediatric hospitalizations (1–3). There are currently no licensed vaccines to counter these ubiquitous respiratory pathogens of the family Paramyxoviridae. Formalin-inactivated HRSV and HPIV vaccines in the 1960s were poorly protective, and the formalin-inactivated HRSV vaccine primed for enhanced disease after subsequent HRSV infection (4, 5). Parallel strategies for HRSV and HPIV vaccines have continued during the past 5 decades, and over the last 20 years, the field has largely focused on developing live attenuated vaccines delivered to the respiratory mucosa for the pediatric population (1, 2, 6). It is possible that prefusion F protein in its native structure may be superior to previously failed subunit vaccines (7, 8), although such a vaccine might be expected, at least at first, to be best targeted to the elderly and pregnant mothers. HRSV and HPIVs are thought to transmit efficiently by contact and short-range airborne transmission (9–13), infecting nearly all children by the ages of 2 and 5 years, respectively (1–3). These viruses also reinfect people symptomatically throughout life (14), even in the absence of antigenic drift. While subversion of the host immune response may contribute to reinfection (2), it is also possible that anisotropic infections, such as those observed in smallpox infections (15), influence the magnitude and tissue tropism of both primary infection and protection from reinfection (16).
Experimentally studying respiratory virus infection and reinfection in humans is difficult because of safety, ethical, environmental, and budgetary considerations. A human challenge model has been developed for HRSV (17) but has not yet been developed for HPIVs, and early experiments have focused efforts on inoculating adults with low levels of serum antibodies in experimental drug trials. Small-animal models are attractive for basic studies of infection and immunity. However, HPIVs and HRSV are usually poor at infecting mice (18), and infection in ferrets, cotton rats, hamsters, and guinea pigs is usually asymptomatic (1). Sendai virus (SeV), the murine counterpart of HPIV1, has been used to study PIV replication, pathogenesis, and transmission in mouse models (19). HPIV1 and Sendai virus have high amino acid sequence identity (20), elicit cross-protective immunity (21–23), and have similar tissue tropisms and epidemiologies (1, 19).
To enable the visualization of Sendai virus infection and reinfection noninvasively in living mice, we previously developed both nonattenuated and attenuated luciferase-expressing reporter viruses (24). Similar bioluminescence-imaging systems have also been developed for vaccinia virus (25), herpes simplex virus 1 (HSV-1) (26), Sindbis virus (27), dengue virus (28), and influenza virus (29, 30). Wild-type Sendai virus and the nonattenuated reporter virus rSeV-luc(M-F*) have similar growth rates in cell culture and in the respiratory tracts of mice; moreover, both viruses induce similar levels of morbidity and mortality, T-cell infiltration into bronchoalveolar lavage fluid (BALF), cytokine profiles, and Sendai virus-specific antibody responses (24). Similar to HPIVs and wild-type Sendai virus (31–33), rSeV-luc(M-F*) is transmitted most efficiently by contact transmission but also by the short-range airborne route (16).
Noninvasive bioluminescence imaging of Sendai virus infection revealed a dichotomy in the respiratory tract (24). The upper respiratory tract (URT) was found to support high levels of infection (even under conditions of a low-dose inoculation, virus attenuation, or a low level of host susceptibility to pulmonary infection) and efficiently promote contact transmission even in the absence of substantial infection in the lungs. In contrast, high levels of infection in the lungs and the concomitant host response were responsible for disease severity. Thus, there appears to be a separation of transmissibility and pathogenicity for Sendai virus in mice (24). Further use of the imaging system revealed that natural infections after short-range airborne transmission are anisotropic, initiating in either the nasopharynx or trachea before either remaining localized or disseminating throughout the respiratory tract (16). Moreover, the mode of transmission and tropism of primary infection were found to determine the extent of protection from reinfection. Based on those previous findings, we hypothesized that the initiation site and dose determine the tropism and magnitude of primary infection, which in turn induce respiratory mucosal and systemic immune responses that protect from reinfection distinctly in the nasopharynx, trachea, and lungs. To test this hypothesis, we used both nonattenuated and attenuated Sendai reporter viruses inoculated either intramuscularly (i.m.) or intranasally (i.n.) at a high or low dose and volume to initiate primary infections of varying penetration into the branching and terminal airways of the lower respiratory tract (LRT). We then probed how the dynamics of primary infection determined mucosal and peripheral immune responses in addition to protection from reinfection by either natural transmission or lethal challenge. Novel aspects of these studies include (i) the demonstration that the kinetics and magnitude of Sendai virus infection after contact transmission are well approximated by a direct intranasal inoculation of a low dose and a low volume (70 PFU in 5 μl), (ii) a correlation of local and systemic immune responses and protective capacities in individual animals that were directly observed to have equivalent primary infections, and (iii) the revelation that primary Sendai virus infection by a highly attenuated virus delivered in a low dose and a low volume affords complete protection from natural contact transmission and a high level of protection from reinfection throughout the respiratory tract in a lethal challenge model.
MATERIALS AND METHODS
Viruses and cells.
Working stocks of recombinant Enders strain Sendai viruses rSeV-luc(M-F*) and rSeV-luc(P-M) were generated and characterized in vitro and in vivo previously (16, 24). In brief, the viruses were rescued by reverse genetics in LLC-MK2 cells, propagated twice in the allantoic cavities of 10-day-old embryonated eggs, plaque purified by LLC-MK2 cells, and confirmed to contain no mutations by reverse transcription-PCR (RT-PCR) and sequencing. Monolayer cultures of LLC-MK2 cells for virus plaque titration and microneutralization assays were grown in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum, l-glutamine (0.05 mg/ml), penicillin (100 U/ml), and streptomycin (0.05 mg/ml) at 37°C in 5% CO2.
Animals.
Eight-week-old female 129x1/SvJ mice (Jackson Laboratories) or 129S2/SvHsd mice (Harlan Sprague Dawley) were anesthetized by using isoflurane (Baxter Health Care Corporation) and inoculated i.n. or i.m. with phosphate-buffered saline (PBS) or virus. Control groups were inoculated i.n. with 30 μl PBS containing Ca2+ and Mg2+ or i.m. into the right thigh with 1 × 106 PFU rSeV-luc(M-F*) in 50 μl. Experimental groups were inoculated i.n. with rSeV-luc(M-F*) or rSeV-luc(P-M) at a low dose and a low volume (70 PFU in 5 μl) or a high dose and a high volume (7,000 PFU in 30 μl). Animals were monitored daily for weight loss, morbidity, and mortality. All animal studies were approved by the Animal Care and Use Committee of St. Jude Children's Research Hospital and performed in compliance with relevant institutional policies; Association for the Accreditation of Laboratory Animal Care guidelines; National Institutes of Health regulations; and local, state, and federal laws.
Tissue virus loads and noninvasive bioluminescence imaging.
On the indicated days, nasal, tracheal, and lung tissues were excised, homogenized, and resuspended in 1 ml PBS containing Ca2+ and Mg2+. Virus loads were determined by plaque titration in LLC-MK2 cells as described previously (34). Prior to imaging, mice were injected intraperitoneally (i.p.) with luciferin (Xenogen Corp.) at a dose of 150 mg/kg of body weight and anesthetized with isoflurane for 5 min. In vivo images were acquired with an Ivis charge-coupled-device (CCD) camera system (Xenogen Corp.) and analyzed with Living Image 4.2 software (Xenogen Corp.). Camera settings and image processing methods were described previously (16).
Histopathology.
Tissues were fixed with 10% neutral buffered formalin, embedded in paraffin, and then sliced into 4-μm-thick sections for histology. Sections were deparaffinized with xylene and then rehydrated with ethanol washes. Slides were incubated in heat-induced epitope retrieval (HIER) buffer at 95°C for 1 h and then removed from heat and allowed to cool at room temperature (RT) for 30 min to unmask antigenic sites. Slides were treated with Bloxall (Vector Laboratories) according to the manufacturer's protocol, washed with PBS, and then blocked with 2.5% normal horse serum. Following the block, slides were incubated with goat anti-PIV1 serum at a 1:600 dilution in 1% bovine serum albumin (BSA)–PBS in a humidified chamber overnight at 4°C. On the following day, slides were washed, incubated with ImmPress reagent (Vector Laboratories) for 30 min, and washed again. Finally, slides were incubated with the ImPACT 3-amino-9-ethylcarbazole (AEC) peroxidase (horseradish peroxidase [HRP]) substrate (Vector Laboratories), monitored for the development of staining, and then rinsed with water and counterstained with Hematoxylin QS (Vector Laboratories). Upon drying, coverslips were mounted by using VectaMount AQ (Vector Laboratories).
ELISA and microneutralization assays.
Sera were collected 30 days after primary infection. To measure Sendai virus-specific binding antibodies in peripheral blood, 96-well enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with disrupted Sendai virus particles. Plates were blocked with PBS containing 1% BSA and then incubated with sera. After incubation, plates were washed, probed with HRP-goat anti-mouse IgG, and washed again. 3,3′,5,5′-tetramethylbenzidine (TMB) substrate buffer (Thermoscientific) was added to the wells, followed by the addition of stop solution, and the absorbance was measured (450 nm). Sendai virus neutralizing antibody levels were measured by using a microneutralization assay. Briefly, LLC-MK2 cells were plated at 2 × 105 cells/ml in 96-well flat-bottomed tissue culture plates. On the following day, diluted serum samples were plated in replicate at 50 μl/well in a separate 96-well plate. Virus was then added at ∼200 50% tissue culture infective doses (TCID50)/well. After a 3-h incubation at 37°C in 5% CO2, medium was removed from LLC-MK2 cell culture plates and replaced with the antibody-virus mixtures. Cells were incubated overnight. Supernatants were then removed and replaced with DMEM with 0.1% BSA, glutamine, gentamicin, and 3 μg/ml acetylated trypsin, and the mixture was incubated for 3 days at 37°C. Next, 50 μl of supernatant/well was overlaid onto 96-well plates containing 50 ml chicken red blood cells (RBCs) (0.5%) in PBS and incubated for 1 h at RT. The endpoint readout was determined as the dilution of serum capable of neutralizing the agglutination of at least two of the triplicate wells of RBCs.
Antibody-forming cell (AFC) ELISPOT assay.
Enzyme-linked immunosorbent spot (ELISPOT) assay plates (Multiscreen-IP; Millipore, Billerica, MA) were coated with 1 μg/100 μl/well of disrupted purified Sendai virus particles and incubated overnight at 4°C. Wells were washed 6 times with PBS and blocked with CTM (complete tumor medium) containing 10% fetal calf serum (FCS). A total of 1 × 105 lung or nasal turbinate cells prepared from whole tissues were then applied to the wells and incubated for 3 h at 37°C. Plates were washed 3 times with PBS and 3 times with PBS-Tween 20. Subsequently, 100 μl of alkaline phosphatase-conjugated goat anti-mouse IgG or IgA antibodies (Southern Biotechnologies Associates, Birmingham, AL) in 1% BSA was added to each well. After overnight incubation at 4°C, antibody was removed, and plates were developed with 1 mg/ml 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium (NBT) (Sigma-Aldrich). The plates were incubated at RT and monitored for spot appearance. Exposure was stopped by rinsing plates with water. Plates were dried, and spots were counted by using a Nikon dissecting scope.
Flow cytometry.
Bronchoalveolar lavage fluid (BALF) and whole lungs were collected from euthanized animals 10 days after inoculation. BALF samples were centrifuged to collect the cellular fraction. Whole lung tissue was homogenized by using a gentleMACs dissociator and digested by using a lung dissociation kit according to the manufacturer's protocol (MACs; Miltenyi Biotec). All cells were pretreated with a 1:200 dilution of Fc block CD16/CD32 in PBS plus 1% FCS prior to membrane staining. Whole lung tissue and BALF cells were stained with CD3-fluorescein isothiocyanate (FITC), CD4-phycoerythrin (PE), and CD8-peridinin chlorophyll protein (PerCP) antibodies (BD Pharmingen) and analyzed for the percentage of CD4+ and CD8+ T cells by flow cytometry. Cellular populations were gated and quantified by using Flowjo version 7.6.5.
Contact transmission.
Donor mice were inoculated i.n. with 7,000 PFU of rSeV-luc(M-F*) in 30 μl and placed into individual cages. After 24 h, either naive contact mice or previously vaccinated mice were placed into cages containing inoculated donor mice. Bioluminescence imaging was performed daily.
Statistics.
Animal numbers and the number of times that experiments were repeated are included in the figure legends. Statistical significance was evaluated by Student's t test using GraphPad PRISM software.
RESULTS
Inoculation method that mimics contact transmission with a low dose and a low volume.
In addition to inoculating 129 strain mice with a relatively high dose and high volume (7,000 PFU in 30 μl) to induce viral pneumonia (24), we also sought to devise an inoculation method that mimics the dynamics of infection after contact transmission. Contact transmission by Sendai virus occurs on average after 3.4 days, is 100% efficient, initiates in the nasopharynx, typically spreads 1 day later to the trachea and lungs, remains URT biased, results in mild to nonapparent clinical symptoms, and induces protective immunity (16, 24). Our comparison of direct i.n. inoculation to contact transmission was performed by using the firefly luciferase-expressing virus rSeV-luc(M-F*), a reporter virus that enables quantitative measurement of Sendai virus infection by bioluminescence in living mice while being indistinguishable from wild-type Sendai virus in the following ways: (i) similar replication kinetics in LLC-MK2 cells; (ii) similar viral load kinetics in the nasal turbinates, trachea, and lungs of 129 strain mice; (iii) similar induction of weight loss and mortality, lymphocyte infiltration in bronchoalveolar lavage fluid (BALF), and Sendai virus-specific antibodies; and (iv) similar cytokine profiles in BALF (24). Pilot experiments to mimic contact transmission included i.n. inoculations of 129 strain mice under isoflurane anesthesia with doses of rSeV-luc(M-F*) ranging from 70 to 7,000 PFU in volumes ranging from 5 to 50 μl. As shown in Fig. 1, rSeV-luc(M-F*) infection in 129 strain mice appeared similar for contact transmission and low-dose, low-volume (70 PFU in 5 μl) i.n. inoculation. The timing of transmission was similar to that reported previously (16, 24). Both groups took ∼1 day to spread from the nasopharynx to the trachea and lungs, and both groups had bioluminescence curves with similar shapes and magnitudes for the nasopharynx, trachea, and lungs. Infection by both methods resulted in URT-biased infection that resulted in only low levels of infection in the lungs (∼107 photons/s), minimal weight loss (<5%), and no mortality. From the pilot studies, inoculation with 30 μl (instead of 5 μl) resulted in a simultaneous initiation of infection in the nasopharynx, trachea, and lungs, which is consistent with data from previous studies (35, 36) but does not reflect the dynamics of infection after contact transmission. Inoculation with 700 PFU instead of 70 PFU resulted in higher bioluminescence values than those obtained for contact transmission. Overall, from this experiment, we concluded that i.n. inoculation of 70 PFU virus in 5 μl (low dose/volume) was sufficient to mimic infection dynamics after contact transmission.
FIG 1.

Similar dynamics of primary infection by the wild-type-like reporter virus rSeV-luc(M-F*) after contact transmission and after i.n. inoculation with a low dose and a low volume. (A) Timing of the spread of infection from the nasopharynx (N) to the trachea (T) and lungs (L) after contact transmission (trans.) or a low-dose, low-volume infection (low d/v). (B to D) Noninvasive bioluminescence imaging of infection in the nasopharynx (B), trachea (C), and lungs (D) was performed daily for two groups of mice. In the transmission group, donor mice were inoculated intranasally with rSeV-luc(M-F*), and recipient contact transmission mice were cohoused with the donors 1 day later. In the low-dose, low-volume group, mice were directly inoculated by i.n. inhalation of 70 PFU of rSeV-luc(M-F*) in a 5-μl volume. Values are reported as means ± standard deviations (n = 18 for contact transmission and n = 30 for i.n. inoculation with a low dose and a low volume). The bottom of the y axis is 5.5 × 105 photons/s, the limit of detection of bioluminescence. The reported values of time are normalized to the day when bioluminescence was first detected in the nasopharynx, which always preceded or coincided with first detection in the trachea and lungs.
Four i.n. inoculation groups were used to investigate the relationship among primary infection, host responses, and reinfection.
Having established i.n. inoculation methods using the wild-type-like reporter virus rSeV-luc(M-F*) to induce viral pneumonia (7,000 PFU in 30 μl) or to mimic contact transmission (70 PFU in 5 μl), we also sought to study two additional groups with identical inoculation procedures but used a live attenuated Sendai virus. The rationale for selecting a live attenuated reporter virus in addition to wild-type-like rSeV-luc(M-F*) was to include primary infections with reduced dissemination into or growth within the lungs that might also bear upon live attenuated vaccine virus development. We selected the reporter virus rSeV-luc(P-M), which contains the firefly luciferase gene and an additional gene junction inserted between the P and M genes of Sendai virus. Previously, we demonstrated that rSeV-luc(P-M) has 2- to 3-fold reduced expression levels of Sendai virus genes downstream of the insertion, 24-h-delayed and 50-fold-lower levels of growth after infection of LLC-MK2 cells at a low multiplicity of infection (MOI), 50-fold-lower viral loads in the lungs of mice at peak titer, 10-fold reduced induction of cytokines and lymphocytes in BALF, reduced weight loss (10% compared to 25%), and no mortality in 129 strain mice, compared to 80% mortality for mice infected with wild-type Sendai virus and rSeV-luc(M-F*) (24). Simultaneously, the attenuated virus rSeV-luc(P-M) had a peak titer in the nasopharynx similar to that of rSeV-luc(M-F*), promoted 100% efficient contact transmission, induced levels of serum antibody responses similar to those induced by rSeV-luc(M-F*), and protected mice from any discernible reinfection after a 7,000-PFU challenge of rSeV-luc(M-F*). Overall, the four groups selected for the present study were treated with (i) attenuated rSeV-luc(P-M) inoculated at a low dose and a low volume, (ii) rSeV-luc(P-M) inoculated at a high dose and a high volume, (iii) wild-type-like rSeV-luc(M-F*) inoculated at a low dose and a low volume, and (iv) rSeV-luc(M-F*) inoculated at a high dose and a high volume. The low dose was 70 PFU in 5 μl, and the high dose was 7,000 PFU in 30 μl. i.n. inoculation of a 30-μl volume distributes approximately half of the virus to the URT and half to the lungs, while a low inoculation volume, such as 5 μl, remains in the nasal mucosa instead of spreading throughout the whole respiratory tract (35, 36).
Dynamics of primary infection based on replicative fitness, dose, and volume.
To assess the abilities of the four inoculation protocols to cause disease in 129 strain mice, groups of mice were inoculated with either rSeV-luc(P-M) or rSeV-luc(M-F*) at a low or high dose and volume as described above and were subsequently monitored for weight loss and mortality. After inoculation with a high dose and a high volume, mice infected with wild-type-like rSeV-luc(M-F*) lost an average of 21% of their body weight, and 5/29 succumbed to infection, while all of those infected with attenuated rSeV-luc(P-M) survived and had an average maximum weight loss of 15% (Fig. 2A and B). Inoculation of a low dose and a low volume of either virus resulted in 100% survival and an average weight loss of 9%. Overall, high-dose, high-volume inoculation had a greater clinical impact than the relative replicative fitness of the viruses. The combination of high dose and high volume was required for relatively high levels of weight loss (>15%), as a low dose and a high volume (70 PFU in 30 μl) or a high dose and a low volume (7,000 PFU in 5 μl) resulted in low levels of infection in the LRT and little weight loss (24).
FIG 2.
Clinical severity, magnitude, and tropism of primary infection by wild-type-like rSeV-luc(M-F*) and attenuated rSeV-luc(P-M) based on the method of inoculation. (A and B) Body weight (A) and mortality (B) were monitored daily for mice mock inoculated with PBS, intramuscularly inoculated with 1 × 106 PFU of rSeV-luc(M-F*) (M-F* IM), or intranasally inoculated with a low dose and a low volume (low d/v) (70 PFU in 5 μl) or a high dose and a high volume (high d/v) (7,000 PFU in 30 μl) of either rSeV-luc(M-F*) or rSeV-luc(P-M) [n = 29 for the high-dose and high-volume rSeV-luc(M-F*) group; n = 30 for the other groups]. (C to J) In a separate experiment, groups of mice intranasally inoculated with a low or high dose and volume of rSeV-luc(M-F*) or rSeV-luc(P-M) were noninvasively imaged after 3, 5, or 7 days of infection (C to F) before nasal, tracheal, and lung tissues were harvested so that viral loads could be measured by plaque titration (G to J). Values reported in panels C to J are means ± standard deviations (n = 5). In panel A, the peak percent weight loss values were separated into four groups based on statistical significance (P < 0.05): rSeV-luc(M-F*) at a high dose and a high volume > rSeV-luc(P-M) at a high dose and a high volume > i.m. and the two low-dose, low-volume groups > PBS control.
To compare in vivo bioluminescence in living mice to ex vivo viral loads from recovered tissues, four groups of mice were inoculated intranasally as described above. On days 3, 5, and 7 after inoculation, one-third of each group was imaged for bioluminescence before respiratory tissues were harvested for plaque titration assays. In some cases, the trend for bioluminescence values with respect to time deviated with the trend for viral load (Fig. 2C to J). For example, in the rSeV-luc(P-M) group inoculated at a high dose and a high volume, nasal and lung bioluminescence was reduced substantially between days 5 and 7, while viral loads remained similar. An earlier reduction in bioluminescence may reflect quicker T-cell-mediated clearance of infected cells than B-cell-mediated neutralization of infectious virions. Compared to high-dose, high-volume inoculation, low-dose and low-volume inoculation resulted in delayed and reduced infection whether assessed by viral load or bioluminescence. For both viruses, the lower-inoculum protocol resulted in ∼2-, 3-, and 7-fold reductions in maximum bioluminescence in the nasopharynx, trachea, and lungs, respectively. With respect to viral loads in recovered respiratory tissues, the lower-inoculum protocol resulted in ∼2-, 3-, and 30-fold reductions for wild-type-like rSeV-luc(M-F*) and ∼7-, 30-, and 600-fold reductions for attenuated rSeV-luc(P-M) in the nasopharynx, trachea, and lungs, respectively. Thus, inoculation with a low dose and a low volume resulted in a greater reduction in infection in the lungs than in the nasopharynx and trachea, presumably because the infection was initiated in the nasopharynx at a lower level and later disseminated to the LRT. The reduction in lung infection by virus titer was greater than the reduction in bioluminescence because of the lower-dose inoculation protocol, perhaps because bioluminescence depends on reporter gene expression, which occurs earlier in the replication cycle than the production of progeny virus (24). Compared to a high-dose, high-volume inoculation, low-dose, low-volume inoculation of rSeV-luc(M-F*) reduced the lung viral load from 4,500,000 to 150,000 PFU/lung, while the lung viral load for rSeV-luc(P-M) was reduced from 500,000 to 860 PFU/lung, resulting in a rather small amount of virus in the lung.
To study the dynamics of primary infection in the four groups, we performed noninvasive bioluminescence imaging daily until clearance of the virus and performed histopathological analyses of tracheal and lung sections recovered 2, 5, and 7 days after i.n. inoculation. For mice inoculated at a low dose and a low volume, nasal bioluminescence first reached a detectable level on day 2, while tracheal and lung bioluminescence was not observed until day 3 (Fig. 3A). Similarly, Sendai virus-infected cells were not detected in tracheal sections at day 2 but were readily apparent on days 5 and 7 (Table 1 and Fig. 3B). Compared to the attenuated virus rSeV-luc(P-M), when inoculated at a low dose and a low volume, the wild-type-like virus rSeV-luc(M-F*) spread extensively in tracheal sections on days 5 and 7 and had tracheal bioluminescence values that were 2.5 and 3.5 times higher, respectively. At a low dose and a low volume, both viruses were not detected in bronchi and bronchioles in lung sections until day 7, approximately the peak of lung bioluminescence. Detection of infection in the lungs by bioluminescence was more sensitive than that by histopathology, perhaps because bioluminescence integrates the entirety of pulmonary infection, while histopathological analysis in this study was limited to a small number of lung sections. Overall, both low-dose, low-volume groups had relatively low levels of infection in the lungs, and lung infection was limited to a few cells in the bronchi and bronchioles but did not extend into the peribronchiolar alveoli or alveolar parenchyma (Fig. 4B). In contrast, both high-dose, high-volume groups had significantly higher levels of infection in the lungs, where infection was also observed to spread to peribronchiolar alveoli. Compared to the attenuated virus rSeV-luc(P-M), when inoculated at a high dose and a high volume, the wild-type-like virus rSeV-luc(M-F*) caused extensive infection in the lungs, which started to clear only after 8 days compared to 4 days (Fig. 3A) and disseminated broadly in the alveolar parenchyma (Fig. 4B). Consequently, alveolitis and interstitial pneumonitis were more prominent for rSeV-luc(M-F*) than for rSeV-luc(P-M) and were not observed for the low-dose, low-volume groups (Table 2). Overall, the timing and magnitude of infection in the trachea and lungs were generally consistent for both techniques (bioluminescence imaging and histopathology) and showed that low-dose, low-volume inoculation limited infection to the bronchi and bronchioles; high-dose, high-volume inoculation of rSeV-luc(P-M) allowed limited spread to peribronchiolar alveoli; and high-dose, high-volume inoculation of rSeV-luc(M-F*) resulted in extensive spread throughout the alveolar parenchyma.
FIG 3.
Differential dissemination of viruses during primary infection based on the dose and volume of inoculation. Groups of mice were intranasally inoculated with a low or high dose and volume (d/v) of rSeV-luc(M-F*) or rSeV-luc(P-M) as described in the legend of Fig. 2 and then noninvasively imaged for infection daily by bioluminescence (A) or euthanized after 2, 5, or 7 days so that tracheal and lung sections could be stained by immunohistochemistry and photographed (B). In panel A, bioluminescence is reported for the nasopharynx, trachea, and lungs as means ± standard deviations (n = 15). In panel B, representative sections of trachea (magnification, ×40) and lungs (magnification, ×20) were selected from groups of two mice.
TABLE 1.
Immunohistochemical staining for virus antigen in the trachea and lungs after primary infection
| Virusa | Inoculumb | Staining resultc |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trachea on day: |
Bronchi and bronchioles on day: |
Peribronchiolar alveoli on day: |
Alveolar parenchyma on day: |
||||||||||
| 2 | 5 | 7 | 2 | 5 | 7 | 2 | 5 | 7 | 2 | 5 | 7 | ||
| P-M | Low | − | + | + | − | − | + | − | − | − | − | − | − |
| M-F* | Low | − | ++ | +++ | − | − | + | − | − | − | − | − | − |
| P-M | High | ++ | +++ | + | ++ | ++ | ++ | − | + | ++ | − | − | − |
| M-F* | High | +++ | +++ | + | +++ | ++ | ++ | − | ++ | +++ | − | + | +++ |
P-M denotes the attenuated virus rSeV-luc(P-M), and M-F* denotes the wild-type-like virus rSeV-luc(M-F*).
For these i.n. inoculations, the low inoculum is 70 PFU in 5 μl and the high inoculum is 7,000 PFU in 30 μl.
The numbers 2, 5, and 7 refer to the number of days after infection when trachea and lungs were extracted, fixed, stained, and photographed. The extent of virus antigen within a section is indicated as follows: −, negative; +, a few cells; ++, multifocal positive (individual or small clusters of infected cells separated by larger areas of uninfected cells); +++, multifocal extensive (large confluent areas of infected cells).
FIG 4.
Differential magnitude and pulmonary spread of primary infection based on the dose and volume of inoculation. Mice were inoculated as described in the legend of Fig. 2. (A) AUC values of bioluminescence were determined by integration of daily measurements of total flux with respect to time. Background AUC values are also reported for control groups of uninfected mice (PBS) or mice intramuscularly inoculated with 1 × 106 PFU of rSeV-luc(M-F*) (IM). Values are means ± standard deviations (n = 15). Statistically significant differences between the intranasally inoculated groups were determined by using the Student t test and are marked with asterisks (****, P < 0.0001). (B) Lung sections were obtained after 2, 5, or 7 days of infection, and immunohistochemical staining of infected cells was performed as described in the legend of Fig. 3B. Representative micrographs from groups of two mice are shown (magnification, ×4). Arrows identify infected bronchioles, and asterisks identify the spread of infection into peribronchiolar alveoli and alveolar parenchyma.
TABLE 2.
Histopathology in the trachea and lungs after primary infection
| Virusa | Inoculumb | Severity gradec |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bronchiolitisd on day: |
Peribronchiolitise on day: |
Perivasculitisf on day: |
Alveolitisg on day: |
Interstitial pneumonitish on day: |
||||||||||||
| 2 | 5 | 7 | 2 | 5 | 7 | 2 | 5 | 7 | 2 | 5 | 7 | 2 | 5 | 7 | ||
| P-M | Low | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M-F* | Low | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| P-M | High | 0 | 1.5 | 2.5 | 0 | 1 | 2.5 | 0 | 2 | 2.5 | 0 | 1 | 2 | 0 | 1 | 2 |
| M-F* | High | 1 | 2 | 2 | 1 | 2 | 2.5 | 2 | 2 | 3 | 1 | 1 | 3 | 1 | 1.5 | 3 |
P-M denotes the attenuated virus rSeV-luc(P-M), and M-F* denotes the wild-type-like virus rSeV-luc(M-F*).
For these i.n. inoculations, the low inoculum is 70 PFU in 5 μl and the high inoculum is 7,000 PFU in 30 μl.
The numbers 2, 5, and 7 refer to the number of days after infection when trachea and lungs were extracted, fixed, stained, and photographed. Severity grades were as follows: 0, within normal limits; 1, minimal with rare or inconspicuous lesions; 2, mild with multifocal or small, focal, or widely separated but conspicuous lesions; 3, moderate with multifocal prominent lesions; 4, marked with extensive to coalescing lesions or areas of inflammation with some loss of structure; 5, severe with diffuse lesions and effacement of normal structure.
Including necrosis, inflammation, and plugging.
Including edema and inflammation.
Including margination and cuffing.
Including necrosis and inflammation.
Including septal thickening and inflammation.
Mucosal and peripheral immune responses to URT-biased low-dose, low-volume and pulmonary disseminated high-dose, high-volume infections.
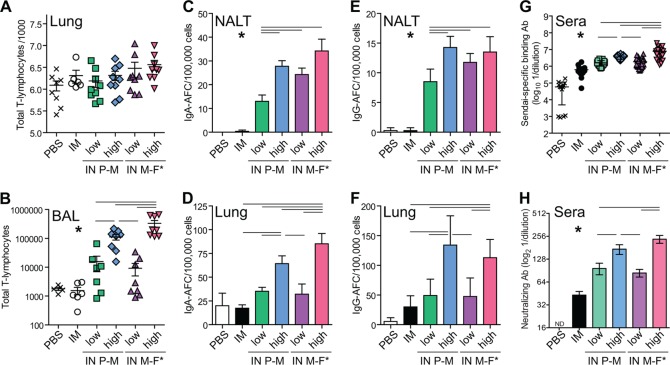
To examine the magnitude of total T-lymphocyte influx into the LRT as a function of the inoculation method and virus, groups of mice were inoculated i.n. or i.m. and then euthanized after 11 days to measure the total influx of CD3+ T lymphocytes into whole lungs or BALF (Fig. 5A and B). After 11 days of infection, Sendai virus was cleared (Fig. 3A), and the percent positive and number of T lymphocytes in BALF were near their peak levels (37). As expected, peripheral infection following i.m. injection resulted in no increase in T-lymphocyte influx compared to mock infection by PBS inoculation. All four i.n. inoculated groups experienced statistically significantly greater T-lymphocyte influx into BALF than did the i.m. and PBS groups. Moreover, the low-dose, low-volume groups had similar intermediate levels of T-lymphocyte influx, the group i.n. inoculated with rSeV-luc(P-M) at a high dose and a high volume had significantly higher levels, and the group i.n. inoculated with rSeV-luc(M-F*) at a high dose and a high volume had significantly higher levels than all other groups (Fig. 5B). Further characterization of mucosal immunological responses of the various groups was done by measuring the amount of mucosal IgA and IgG antibody-forming cells (AFCs) in nasal-associated lymphoid tissue (NALT) and in lungs 28 days after inoculation, a time when all such levels are relatively high (38). Peripheral infection after i.m. injection did not stimulate IgA and IgG AFC trafficking to NALT, whereas i.n. inoculation did (Fig. 5C and E). The levels of IgA and IgG AFCs in NALT after i.n. inoculation followed the following trend for both IgA and IgG: rSeV-luc(P-M) at a low dose and a low volume < rSeV-luc(M-F*) at a low dose and a low volume < rSeV-luc(P-M) at a high dose and a high volume ∼ rSeV-luc(M-F*) at a high dose and a high volume. In general, similar trends were seen for IgA and IgG AFC levels in the lungs (Fig. 5D and F), with two notable exceptions. First, i.m. infection resulted in a low-level induction of IgG AFCs in the lungs that was not statistically significantly different from that of rSeV-luc(P-M) at a low dose and a low volume (Fig. 5F). Second, after high-dose, high-volume i.n. inoculation, the levels of IgA AFCs in the lungs were statistically significantly higher for rSeV-luc(M-F*) than for rSeV-luc(P-M) (Fig. 5D). In peripheral blood, the levels of binding and neutralizing antibodies followed the trend i.m. < low-dose, low-volume i.n. infection for either virus < high-dose, high-volume i.n. infection for rSeV-luc(P-M) < high-dose, high-volume i.n. infection for rSeV-luc(M-F*). Overall, both mucosal and peripheral immunological responses were significantly enhanced for i.n. inoculation compared to i.m. injection, and higher levels of primary infection in the URT and LRT were associated with higher levels of mucosal and peripheral responses.
FIG 5.
Differential mucosal and systemic immunological responses to primary infection based on the inoculation method. Groups of mice were mock inoculated with PBS, intramuscularly inoculated with 1 × 106 PFU of rSeV-luc(M-F*) (IM), or intranasally (IN) inoculated with a low dose and a high volume (70 PFU in 5 μl) or a high dose and a high volume (7,000 PFU in 30 μl) of either rSeV-luc(M-F*) or rSeV-luc(P-M). (A and B) After 11 days of infection, mice were euthanized to determine the numbers of CD3+ T lymphocytes in whole lungs (A) or bronchoalveolar lavage fluid (B) by flow cytometry. Each data point for infiltrating T lymphocytes represents an individual mouse from triplicate experiments (PBS group, n = 8; i.m. group, n = 6; i.n. groups, n = 9), and horizontal bars show the group means. (C to F) For other groups, mice were euthanized after 28 days of infection to recover NALT and whole lungs to quantify IgA (C and D) and IgG (E and F) antibody-forming cells by an ELISPOT assay. Mucosal antibody-forming cell experiments were done in triplicate, and a representative data set is shown (n = 3 with duplicate wells for each ELISPOT assay). (G and H) After 30 days of infection, peripheral blood was collected so that Sendai virus-specific binding (G) and neutralizing (H) antibody (Ab) titers in sera could be determined by reciprocal endpoint dilutions in ELISAs and microneutralization assays, respectively. Panel G displays individual values and means ± standard deviations; panel H displays means ± standard deviations [n = 13 for the PBS group, n = 20 for the i.m. group, n = 24 for i.n. rSeV-luc(M-F*) at a high dose and volume, and n = 30 for the remaining three i.n. groups]. Significance was determined by using the Student t test. For panels A, D, and F, there was no statistically significant difference between the i.m. and low-dose and low-volume i.n. treatment groups. For panels B, C, E, G, and H, there is a statistically significant difference (*, P < 0.05) between the i.m. group and each of the four i.n. groups. Statistically significant differences (P < 0.05) between individual i.n. groups are shown by horizontal bars above the data, and asterisks are omitted for clarity.
Superiority of primary respiratory infection compared to peripheral i.m. injection in protection from reinfection.
To examine resistance to reinfection, we performed lethal challenge experiments on the same living animals for which we previously measured primary infection and subsequent serum immunological responses. After i.n. challenge with 1 × 106 PFU rSeV-luc(M-F*), PBS mock-inoculated mice lost >25% of their initial starting weight and succumbed to infection within 10 days (Fig. 6). Mice challenged 63 days after i.m. vaccination lost an average of 16% of their starting weight, and 10% did not survive. In contrast, i.n. inoculated mice that had primary infection in the respiratory tract fared challenge much better, with 100% surviving and average weight losses of <7% for the low-dose, low-volume groups and <4% for the high-dose, high-volume groups (Fig. 6). As we observed previously after contact or short-range airborne transmission of Sendai virus (16), higher levels of primary respiratory infection were generally associated with higher levels of protection from reinfection, especially for comparisons across groups (Fig. 7 and 8). However, when individual animals within a group were compared, we noticed in some cases that one or two mice with relatively low levels of primary infection in the lungs had greater protection from reinfection than others that had up to 10-fold-higher levels of primary infection in the lungs (Fig. 7B). Exceptions to the overall trend were also seen when peak primary and secondary infection levels between individual mice within a group were compared (Fig. 7C). For entire groups, relatively high viral loads were measured throughout the respiratory tract 3 days after challenge for PBS and i.m. vaccinated groups, while no infectious virus was recovered from i.n. inoculated groups (Fig. 8G). i.n. inoculated groups had minimal levels of reinfection bioluminescence in the URT and relatively mild reinfection in the lungs that was cleared within 4 days (Fig. 8C to F). The group with the highest level of primary infection in the lungs and the greatest mucosal and peripheral immune responses, the group inoculated with rSeV-luc(M-F*) at a high dose and a high volume, also had the greatest protection from reinfection in the lungs, barely being detected (Fig. 8F). In contrast, the low-dose, low-volume i.n. inoculated groups, with limited primary infection in the lungs and lower levels of immune responses, had higher levels of reinfection bioluminescence in the lungs (Fig. 8C and E). For i.m. vaccinated mice, lung reinfection took longer to clear (6 days), and URT reinfection was more prominent (Fig. 8B). The poorer protection of i.m. vaccinated mice in the URT than in the LRT is consistent with undetectable IgA and IgG AFCs in the NALT but low levels of IgG AFCs in the lungs (Fig. 5C to F). To address the nonspecific effect of i.n. infection on protection from SeV secondary challenge, we i.n. inoculated groups of five mice with 30 μl containing either PBS or 2.4 × 107 50% egg infective doses (EID50) of cold-adapted influenza A/Puerto Rico/8/1934 (H1N1) (PR8) virus and then i.n. challenged mice 63 days later with 1 × 106 PFU rSeV-luc(M-F*) in 30 μl. While vaccination with cold-adapted PR8 reduced peak challenge SeV bioluminescence 4-fold in the lungs compared to PBS vaccination, SeV challenge bioluminescence in the nasopharynx and trachea was indistinguishable for the PBS- and cold-adapted PR8-inoculated groups (data not shown). Even when accounting for nonspecific effects on protection from SeV rechallenge, i.n. vaccination (even with a low dose and a low volume of an attenuated virus) afforded a substantially higher level of protection than did i.m. vaccination, especially in the nasopharynx and trachea. Overall, i.m. vaccination was poorer at inducing immunity in the NALT than in the lungs, as judged by AFC induction and challenge bioluminescence, and was much poorer at inducing protective immunity throughout the respiratory tract than even the mildest primary respiratory infection, a low-dose, low-volume i.n. inoculation of rSeV-luc(P-M).
FIG 6.

Clinical symptoms after reinfection (secondary challenge) with a lethal dose of rSeV-luc(M-F*). Mice were inoculated on day 0 intramuscularly with 1 × 106 PFU of rSeV-luc(M-F*) (IM), intranasally with PBS, or intranasally with a low (70 PFU in 5 μl) or high (7,000 PFU in 30 μl) dose and volume (d/v) of either rSeV-luc(M-F*) or rSeV-luc(P-M). On day 63 after the primary inoculation, i.n. secondary challenge was performed with 3 × 106 PFU of rSeV-luc(M-F*) in 30 μl, and mice were monitored for 21 days for changes in body weight (A) and survival (B). For both panels, the day of secondary challenge is reported as day 0 (which is day 63 of the overall experiment since primary inoculation). Percent starting weight values are expressed as means for the groups [for both panels, n = 9 for the PBS group, n = 10 for the i.m. group, n = 13 for i.n. rSeV-luc(M-F*) at a high dose and volume, and n = 15 for the remaining three i.n. groups].
FIG 7.
Primary infection and reinfection after secondary challenge with a lethal dose of rSeV-luc(M-F*), imaged in individual, living mice. Primary inoculation on day 0 and secondary challenge on day 63 with 3 × 106 PFU of rSeV-luc(M-F*) in 30 μl were performed as described in the legend of Fig. 6. (A and B) Nasal (A) and lung (B) bioluminescence was monitored from days 1 to 10 during the primary infection and from days 64 to 68 during the secondary challenge. Bioluminescence traces are shown for each individual mouse and are color coded based on the magnitude of peak challenge bioluminescence as follows: black, below the detection level; blue, lower one-third of reinfected mice; orange, middle one-third of reinfected mice; red, top one-third of reinfected mice. (C) Peak challenge bioluminescence values in the nasopharynx and lungs for individual mice are graphed versus peak primary bioluminescence values. Each value shown represents an individual mouse. The limit of detection is 5.5 × 105 photons/s [n = 13 for i.n. vaccination of rSeV-luc(M-F*) at a high dose and a high volume; n = 15 for the remaining three groups].
FIG 8.
Average viral loads and bioluminescence after reinfection (secondary challenge) with a lethal dose of rSeV-luc(M-F*). Primary inoculation on day 0 and secondary i.n. challenge on day 63 with 3 × 106 PFU of rSeV-luc(M-F*) in 30 μl were performed as described in the legend of Fig. 6. (A to F) Average bioluminescence values in the nasopharynx, trachea, and lungs for each group. The bottom of the y axis is 5.5 × 105 photons/s, the limit of detection [for the bioluminescence curves, n = 9 for the PBS group, n = 10 for the i.m. group, n = 13 for i.n. rSeV-luc(M-F*) at a high dose and a high volume, and n = 15 for the remaining three i.n. groups]. (G) In parallel, groups of 5 mice each were euthanized 3 days after challenge (day 66 of the experiment) so that viral loads from nasal, tracheal, and lung tissues could be measured by plaque titration. All values are shown as means ± standard deviations. ND, not detectable.
Correlations among primary infection, peripheral serum responses, and protection from reinfection.
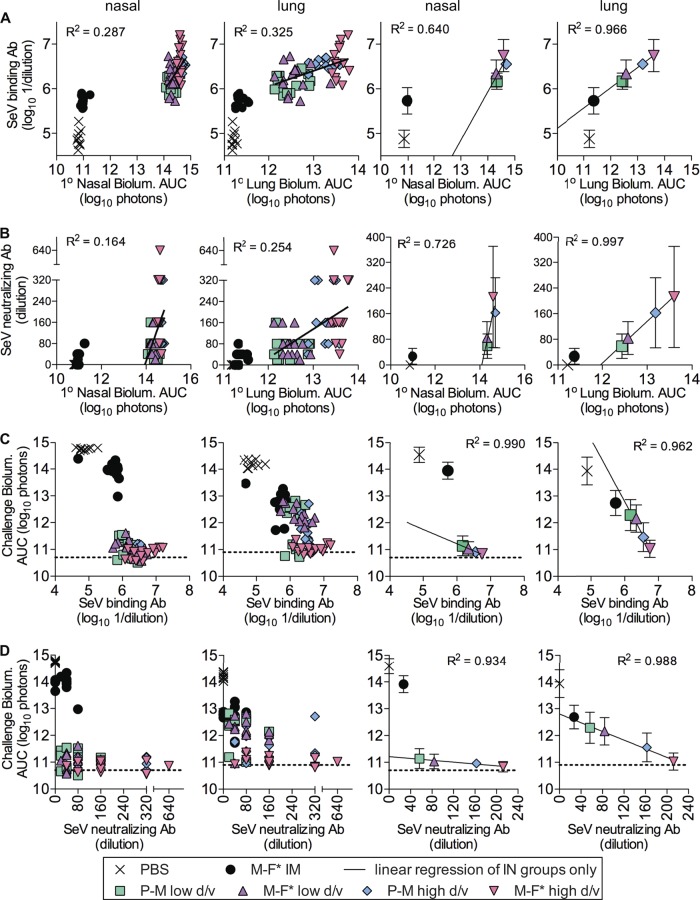
A major aim of this work was to compare Sendai virus primary infection, immune responses, and reinfection in individual mice. We first examined Sendai virus-specific serum binding and neutralizing antibody levels (Fig. 9A and B, respectively) as a function of primary infection (based on bioluminescence area under the curve [AUC] values) in the nasopharynx and lungs. Primary infection AUC values in both nasal and lung tissues correlated with serum binding and neutralizing antibody responses albeit with relatively low R2 values. Comparison of group averages of serum antibody responses versus primary infection in the lungs revealed a high correlation (R2 > 0.95) for the four i.n. inoculated groups, a correlation which could be extrapolated to the i.m. inoculated group in a plot of binding antibodies versus lung bioluminescence but not in plots of binding antibodies versus nasal bioluminescence or neutralizing antibodies versus nasal or lung bioluminescence (Fig. 9A and B). We next examined the relationship between serum antibody responses and protection from reinfection in the nasopharynx and lungs (Fig. 9C and D). For all four intranasally vaccinated groups, only low levels of reinfection in the nasopharynx were observed; in fact, numerous individual animals had bioluminescence levels below the limit of detection. Thus, despite average serum binding and neutralizing antibody levels in the four i.n. groups varying substantially between groups, reinfection bioluminescence values were universally low, and the slopes of challenge nasal bioluminescence versus serum antibody responses were small (albeit highly correlated). Comparison of group averages for reinfection bioluminescence for the i.n. inoculated groups as a function of serum neutralizing antibody responses revealed a high correlation between serum antibody levels and protection from reinfection in the lungs (R2 = 0.988), a relationship that extended to the i.m. inoculated groups in analyses of reinfection in the lungs but not in the nasopharynx. In fact, the i.m. vaccinated groups suffered from relatively high levels of reinfection in the nasopharynx despite having partial protection in the lungs.
FIG 9.
Correlations between primary infection, serum antibody responses, and protection from reinfection (secondary challenge). (A) Peripheral serum binding antibody responses plotted as a function of the magnitude of primary infection bioluminescence areas under the curve. (B) Neutralizing antibody responses plotted as a function of bioluminescence areas under the curve. (C and D) Secondary challenge bioluminescence areas under the curve plotted as a function of peripheral serum binding antibody responses (C) and peripheral serum neutralizing antibody responses (D). Bioluminescence AUC values were determined by integration of daily measurements of total flux with respect to time. Linear regression and R2 values correspond to the four intranasally infected experimental groups only, despite the line being extended past the range of these values. Primary infection occurred during the first 2 weeks of the experiment, serum was collected on day 30, and reinfection occurred after secondary challenge on day 63. Data for individual animals are shown in the leftmost two panels, and group values (means ± standard deviations) are shown in the rightmost two panels.
Overall, our analyses revealed that serum binding antibody levels are an excellent predictor of antecedent primary Sendai virus infection in the lungs, whether primary infection or vaccination was i.n. or i.m. (Fig. 9A, right). Our work also showed that serum neutralizing antibody levels are an excellent predictor of subsequent protection from reinfection in the lungs by Sendai virus, whether primary infection or vaccination was i.n. or i.m. (Fig. 9D, right). We were unable to conduct comparisons of mucosal antibody responses to primary and secondary infections in individual mice, as our measurements of AFCs were done with tissues harvested from euthanized animals. Nevertheless, rapid protection against Sendai virus infection was previously associated with robust IgA and IgG AFC responses in the NALT and lungs (38), and for comparisons of group averages in the present study, a higher-level mucosal AFC response (Fig. 5C to F) was consistent with greater protection from reinfection in the lungs and nasopharynx (Fig. 8).
Protection from natural contact transmission after i.m. versus i.n. vaccination.
To intensively probe protection from reinfection, the above-described experiments were performed by using 3 × 106 PFU of rSeV-luc(M-F*) in a 30-μl volume as the challenge virus (Fig. 7 and 8). However, we were able to model natural infection after contact transmission at a much lower level of inoculated virus, namely, 70 PFU delivered i.n. in a 5-μl volume (Fig. 1). Therefore, we next sought to determine whether i.m. vaccination affords a level of protection from natural contact transmission that is comparable to that of i.n. vaccination. Groups of mice were vaccinated with either 3 × 106 PFU of rSeV-luc(M-F*) i.m. or 70 PFU of rSeV-luc(P-M) i.n. in a 5-μl volume. Thirty days after vaccination, the i.n. vaccinated group had higher levels of serum binding antibody responses than did the i.m. vaccinated group (Fig. 10A). On day 63 of the experiment, donor mice were i.n. inoculated with 7,000 PFU of rSeV-luc(M-F*) in a 30-μl volume, and on day 64, the donor mice were cohoused in contact with the i.m. and i.n. vaccinated mice or with naive mice. Each individual mouse that had been vaccinated i.n. with a low dose and a low volume of the attenuated virus rSeV-luc(P-M) showed no detectable reinfection bioluminescence in the nasopharynx, trachea, or lungs in the transmission model (Fig. 10B). In contrast, i.m. vaccinated mice displayed substantial levels of reinfection in the nasopharynx and trachea albeit at lower levels than for naive mice. While not fully protected in the URT, i.m. vaccinated mice were protected from reinfection in the lungs.
FIG 10.

Serum antibody responses and protection from reinfection by contact transmission after “vaccination” (primary infection) by the intramuscular (IM) or intranasal (IN) route. (A) Groups of mice were inoculated i.n. with a low dose and a low volume (70 PFU in 5 μl) of the attenuated virus rSeV-luc(P-M) or injected i.m. with 1 × 106 PFU of the wild-type-like virus rSeV-luc(M-F*), and peripheral blood sera were then collected 30 days later to measure Sendai virus-specific serum binding antibody levels. Values for individual mice are shown as fold increases over naive levels. Statistically significant differences between the i.m. and i.n. groups were determined by using the Student t test (***, P < 0.001). (B) On day 63 of the experiment, previously naive donor mice were inoculated intranasally with 7,000 PFU of rSeV-luc(M-F*) in 30 μl, and 1 day later, they were then placed individually into a cage containing two mice that had been unvaccinated (naive) or vaccinated by the i.m. or i.n. route on day 0 of the experiment. Bioluminescence was then monitored for the next 11 days (days 64 to 74 of the experiment). Lung bioluminescence levels were below the level of detection for all i.m. and i.n. vaccinated mice (i.n. values are hidden underneath i.m. values). Mean bioluminescence values ± standard deviations (n = 6) are shown for donor mice; values for the three recipient groups correspond to individual mice (n = 4 for each group).
DISCUSSION
This work aimed to use noninvasive bioluminescence imaging to study the relationships among primary Sendai virus infection, mucosal and systemic immunological responses, and susceptibility to reinfection. Groups of mice were inoculated with either low or high doses and volumes, both of which are helpful in examining the breadth of infection and immunity. We found that low-dose, low-volume inoculation (70 PFU in 5 μl) initiates infection in the nasopharynx and accurately mimics the URT-biased dynamics of infection visualized after contact transmission, the primary mode of transmission of Sendai virus (16, 31–33). Similar to other highly infectious respiratory viruses, the 50% infectious dose (ID50) of Sendai virus is rather low (<10 PFU) (24, 37), supporting low-dose, low-volume infections. In addition to the contact route, HRSV and HPIVs are also thought to be transmitted over a short range by large-particle droplets (9–13). Sendai virus is transmitted by the short-range airborne route albeit less efficiently than by contact (16, 32). We have recently shown that Sendai virus infection after short-range airborne transmission is anisotropic (16), like smallpox infection (15). Such anisotropic infections by Sendai virus initiate in the nasopharynx or trachea, and the extent of respiratory tract dissemination and the magnitude of the host response determine protection from reinfection (16). To study viral pneumonia, we also inoculated mice i.n. with a relatively high dose (7,000 PFU) and high volume (30 μl), which initiates infection throughout the respiratory tract (36). Inoculations with relatively high doses (≥200 PFU) and high volumes (20 to 50 μl) are common in Sendai virus pathogenesis and immunological studies (37, 39–44). High-dose, high-volume inoculation is valuable in inducing LRT pathogenesis, which is mostly observed clinically as croup (laryngotracheobronchitis) in children infected with HPIV1 and as viral pneumonia in those infected with HPIV3 (13). Overall, the use of both low-dose, low-volume and high-dose, high-volume inoculation strategies to mimic different infection phenotypes appears advantageous in studying respiratory virus infection and immunity.
Luciferase-expressing reporter viruses were first developed for DNA and positive-strand RNA viruses (45). Similar development for negative-strand RNA reporter viruses lagged mainly because of reporter virus attenuation and/or genetic instability (46, 47). In 2011, we developed the first nonattenuated negative-strand RNA virus, rSeV-luc(M-F*), in which we inserted the firefly luciferase reporter gene downstream of a naturally attenuated gene start sequence that we optimized in the reporter virus to mitigate the effects of foreign gene insertion (24). Subsequently, luciferase reporter viruses with near-native, albeit mildly attenuated, in vitro and in vivo fitness have been developed for RSV and influenza viruses (29, 30, 48). Thus, noninvasive bioluminescence imaging of real-time infection is expanding to many virus systems and promises a variety of advantages over traditional dissection of euthanized animals, most notably the ability to monitor infection and reinfection in the same individual animals. The present study further validates the use of in vivo bioluminescence imaging by demonstrating concordance between time courses of bioluminescence in living mice and traditional markers of infection probed by analysis of tissues extracted from euthanized mice at various times postinfection, such as viral loads and histopathological images.
Here, we inoculated mice with a wild-type-like or attenuated reporter virus at a low or high dose and volume. We were able to quantify for each individual (i) the dynamics of primary infection, (ii) peripheral serum antibody responses, and (iii) susceptibility to reinfection by lethal challenge. Individuals from all four groups had robust primary infections in the URT and were well protected against lethal challenge in the URT, suffering only low-grade nasopharyngeal reinfections that cleared within 2 days. This finding is consistent with previously reported observations that the murine nasal cavity supports robust Sendai virus infection even for an attenuated virus, at a low dose, and in a strain highly susceptible to lung infection (24). The measured levels of binding and neutralizing antibodies in peripheral blood sera varied substantially between individuals and groups, and these noninvasive immunological markers did not correlate well with infection in the URT, which uniformly reached a high level during primary infection and was low upon challenge. In contrast, primary viral infection in the lungs was a better predictor of subsequent serum antibody responses, which in turn were a better predictor of resistance to reinfection in the lungs than in the URT. For both individuals and groups, primary infection in the lungs was more highly correlated with the level of serum binding than with the level of serum neutralizing antibodies. Moreover, the link between primary lung infection and serum binding antibodies applied not only to i.n. infection but also to i.m. injection. In contrast, serum neutralizing antibody levels were a better predictor of subsequent protection from reinfection in the lungs, whether primary infection was respiratory or i.m. Overall, analysis of lung infections for entire groups revealed strong correlations between the average levels of (i) primary lung infection and serum binding antibodies and (ii) serum neutralizing antibodies and protection from reinfection in the lungs.
Some individual mice had low reinfection bioluminescence values (near or below the limit of detection) in the lungs despite having only low-level serum neutralizing antibody responses. These individuals were protected from reinfection at a level far higher than would have been predicted by neutralizing antibody responses in peripheral blood. Another caveat is that some individual mice within the same group that had the same level of serum neutralizing antibody became reinfected in the lungs at levels that differed 10- to 100-fold. It is unknown why two genetically matched individuals, presumably free of coinfection, that were inoculated identically (same virus, dose, and volume) and had approximately the same level of primary infection would have substantially different peripheral serum responses or reinfection levels. It is possible that individuals suffered different levels of stress (49), varied in their stage of the estrous cycle at the time of inoculation (50), inadvertently became coinfected (51), had different social or behavioral habits, or differed by another unknown factor(s). We were unable to noninvasively measure the levels of antibodies and T cells present in the respiratory mucosal surfaces of living mice before and after reinfection, yet such immunological responses lie at the heart of protective immunity (38, 43, 44, 52). Novel technologies to noninvasively measure the dynamics of innate and antibody responses as well as immune cell trafficking throughout the respiratory mucosal surface would be expected to enhance the analysis of infection, immunity, and reinfection.
Compared to i.m. vaccination, even the least robust primary infection in the respiratory tract, which resulted after i.n. inoculation of the attenuated virus at a low dose and a low volume, was superior at eliciting the following aspects of protective immunity: (i) IgA and IgG AFCs in the NALT, (ii) serum binding and neutralizing antibody titers, (iii) morbidity and mortality after lethal challenge, (iv) viral load and bioluminescence after lethal challenge, and (v) protection from natural transmission by the contact route. With respect to protection from lethal challenge, initial bioluminescence values during reinfection were 10-fold lower in the nasopharynx after i.n. infection than after i.m. infection, consistent with increased levels of AFCs and antibody at the site of reinfection. Similarly, for influenza vaccination, low-level responses of IgA AFCs to an i.m. administered inactivated influenza virus vaccine compared to an i.n. administered live attenuated vaccine have been reported (53). This is consistent with passive transfer experiments describing how systemic antibody reaches the lungs better than the URT. Rapid clearance after 2 days in the nasopharynx for i.n. vaccinated mice, compared to 7 days for i.m. inoculation, was also most likely enhanced by CD8+ T cells in the diffuse NALT, which are rapidly activated after i.n. inoculation of Sendai virus and persist for >8 months (44). i.n. vaccination of the attenuated virus at a low dose and a low volume resulted in poor penetration of primary infection into the lungs, resulting in relatively low pulmonary AFC levels that were slightly, albeit not significantly, higher than those promoted by i.m. primary infection. Upon subsequent challenge, initial pulmonary bioluminescence in the lungs was reduced 3-fold for i.n. versus i.m. vaccination, and infection was cleared 3 days earlier, presumably due to increased B- and T-cell activity at the site of infection. Compared to peripheral priming of CD8+ T cells, i.n. priming enables reactivation in lung-draining mediastinal lymph nodes (MLNs) that helps maintain antigen-specific CD8+ T cells positioned to clear reinfection (43), which most likely contributed to a quicker clearance of reinfection in the lungs. i.n. vaccinated mice were completely resistant to reinfection, within the limit of detection of bioluminescence, after contact exposure to infected donor mice, whereas i.m. vaccinated mice became reinfected in the nasopharynx and trachea by direct contact. We have shown here and previously (16, 24) that natural infection after contact transmission initiates in the nasopharynx before later disseminating to the lungs. Partial protection from reinfection in the URT, observed as 10- to 20-fold-lower bioluminescence values, for i.m. vaccinated animals may have prevented dissemination into the lungs. Moreover, our studies demonstrated that i.m. vaccination was more effective at promoting protective immunity in the lungs than in the nasopharynx. Thus, while i.m. vaccination may afford an attractive vehicle to reduce pathogenesis promoted by infection in the lungs after natural exposure, just as it does in influenza virus infection (54), it may not fully protect from reinfection and may allow limited transmission, which has been linked to virus shedding in the URT (24, 55).
In addition to providing a mouse model to study respiratory paramyxovirus dissemination and pathogenesis, Sendai virus is also a promising Jennerian vaccine candidate against HPIV1 (1, 56) and vaccine vector for preclinical HPIV2, HPIV3, and HRSV vaccines (57–63). There are no confirmed cases of pathogenic Sendai virus infection in humans, and i.n. inoculation of Sendai virus in healthy humans has been well tolerated (56). In nonhuman primates, i.n. delivered wild-type Sendai virus and Sendai virus expressing the HRSV F protein have been shown to protect against HPIV1 and HRSV challenge, with no adverse events (21, 58, 64). Results on protection from natural reinfection in the present study suggest that an i.m. delivery approach for a Sendai virus-based vaccine may protect well against viral pneumonia. However, i.n. vaccination was found in the mouse model to be superior to i.m. vaccination in every way, would be expected to better control sustained virus transmission, and is generally thought of as the safest and most appropriate mode of delivery for respiratory paramyxovirus vaccines in naive infants (1). For HRSV, administrations of live vaccines by the i.m. or i.n. route have relative advantages and disadvantages compared to each other. An advantage of live i.m. vaccination is that it does not induce vaccine-enhanced disease like i.m. vaccination with inactivated virus, and it does not result in nasal inflammation like live i.n. vaccination can (65), which could interfere with breathing. On the other hand, live i.m. vaccines may be more susceptible to suppression by preexisting antibodies than live i.n. vaccines (66), in part because live i.n. vaccines are typically more immunogenic within the respiratory mucosa than i.m. vaccines.
ACKNOWLEDGMENTS
We thank Heba Mostafa, Olga Bridges, and Lauren Jayne for assistance with bioluminescence-imaging experiments and analysis, and we thank Hassan Zaraket and Husni Elbahesh for helping to maintain cell cultures and monitor animals. We thank Sherri Surman for her assistance with flow cytometry, AFC, and challenge experiments. We thank Shelby Patrick and the Animal Resource Center (ARC) for animal care. We thank Allen Portner for kindly providing the reverse genetics system for Sendai virus as well as cell lines and antibodies.
This work was supported by grant R01AI083370 from the National Institute of Allergy and Infectious Diseases (NIAID), a Cancer Center support grant (CA 21765), the American Lebanese Syrian Associated Charities (ALSAC), and the Children's Infection Defense Center (CIDC) at St. Jude Children's Research Hospital.
REFERENCES
- 1.Karron RA, Collins PL. 2007. Parainfluenza viruses, p 1497–1526 InKnipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Collins PL, Melero JA. 2011. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res 162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, Staat MA, Iwane M, Prill MM, Williams JV, New Vaccine Surveillance Network . 2013. Burden of human metapneumovirus infection in young children. N Engl J Med 368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 89:449–463. [DOI] [PubMed] [Google Scholar]
- 5.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 89:435–448. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt AC, Schaap-Nutt A, Bartlett EJ, Schomacker H, Boonyaratanakornkit J, Karron RA, Collins PL. 2011. Progress in the development of human parainfluenza virus vaccines. Expert Rev Respir Med 5:515–526. doi: 10.1586/ers.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang G-Y, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko S-Y, Todd J-P, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall CB, Douglas RG Jr. 1981. Modes of transmission of respiratory syncytial virus. J Pediatr 99:100–103. [DOI] [PubMed] [Google Scholar]
- 10.Hall CB, Douglas RG, Schnabel KC, Geiman JM. 1981. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun 33:779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CB. 2001. Respiratory syncytial virus and parainfluenza virus. N Engl J Med 344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 12.Henrickson KJ. 2003. Parainfluenza viruses. Clin Microbiol Rev 16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schomacker H, Schaap-Nutt A, Collins PL, Schmidt AC. 2012. Pathogenesis of acute respiratory illness caused by human parainfluenza viruses. Curr Opin Virol 2:294–299. doi: 10.1016/j.coviro.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falsey AR. 2007. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med 28:171–181. doi: 10.1055/s-2007-976489. [DOI] [PubMed] [Google Scholar]
- 15.Milton DK. 2012. What was the primary mode of smallpox transmission? Implications for biodefense. Front Cell Infect Microbiol 2:150. doi: 10.3389/fcimb.2012.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke CW, Bridges O, Brown S, Rahija R, Russell CJ. 2013. Mode of parainfluenza virus transmission determines the dynamics of primary infection and protection from reinfection. PLoS Pathog 9:e1003786. doi: 10.1371/journal.ppat.1003786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagga B, Woods CW, Veldman TH, Gilbert A, Mann A, Balaratnam G, Lambkin-Williams R, Oxford JS, McClain MT, Wilkinson T, Nicholson BP, Ginsburg GS, Devincenzo JP. 2013. Comparing influenza and RSV viral and disease dynamics in experimentally infected adults predicts clinical effectiveness of RSV antivirals. Antivir Ther 18:785–791. doi: 10.3851/IMP2629. [DOI] [PubMed] [Google Scholar]
- 18.Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, Lee S, Goleniewska K, Pretto C, Williams JV, Hotard A, Sherrill TP, Peebles RS, Moore ML. 2011. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol 85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faisca P, Desmecht D. 2007. Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res Vet Sci 82:115–125. doi: 10.1016/j.rvsc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Mishin VP, Brown SA, Russell CJ, Boyd KL, Babu YS, Taylor G, Xiong X, Yan X, Portner A, Alymova IV. 2009. Effect of hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 on growth and pathogenicity of Sendai/human parainfluenza type 3 chimera virus in mice. Antimicrob Agents Chemother 53:3942–3951. doi: 10.1128/AAC.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurwitz JL, Soike KF, Sangster MY, Portner A, Sealy RE, Dawson DH, Coleclough C. 1997. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15:533–540. doi: 10.1016/S0264-410X(97)00217-X. [DOI] [PubMed] [Google Scholar]
- 22.Sangster M, Smith FS, Coleclough C, Hurwitz JL. 1995. Human parainfluenza virus type 1 immunization of infant mice protects from subsequent Sendai virus infection. Virology 212:13–19. doi: 10.1006/viro.1995.1448. [DOI] [PubMed] [Google Scholar]
- 23.Dave VP, Allan JE, Slobod KS, Smith FS, Ryan KW, Takimoto T, Power UF, Portner A, Hurwitz JL. 1994. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology 199:376–383. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- 24.Burke CW, Mason JN, Surman SL, Jones BG, Dalloneau E, Hurwitz JL, Russell CJ. 2011. Illumination of parainfluenza virus infection and transmission in living animals reveals a tissue-specific dichotomy. PLoS Pathog 7:e1002134. doi: 10.1371/journal.ppat.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez JF, Rodriguez D, Rodriguez JR, McGowan EB, Esteban M. 1988. Expression of the firefly luciferase gene in vaccinia virus: a highly sensitive gene marker to follow virus dissemination in tissues of infected animals. Proc Natl Acad Sci U S A 85:1667–1671. doi: 10.1073/pnas.85.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luker GD, Bardill JP, Prior JL, Pica CM, Piwnica-Worms D, Leib DA. 2002. Noninvasive bioluminescence imaging of herpes simplex virus type 1 infection and therapy in living mice. J Virol 76:12149–12161. doi: 10.1128/JVI.76.23.12149-12161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook SH, Griffin DE. 2003. Luciferase imaging of a neurotropic viral infection in intact animals. J Virol 77:5333–5338. doi: 10.1128/JVI.77.9.5333-5338.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoggins JW, Dorner M, Feulner M, Imanaka N, Murphy MY, Ploss A, Rice CM. 2012. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc Natl Acad Sci U S A 109:14610–14615. doi: 10.1073/pnas.1212379109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heaton NS, Leyva-Grado VH, Tan GS, Eggink D, Hai R, Palese P. 2013. In vivo bioluminescent imaging of influenza A virus infection and characterization of novel cross-protective monoclonal antibodies. J Virol 87:8272–8281. doi: 10.1128/JVI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran V, Moser LA, Poole DS, Mehle A. 2013. Highly sensitive real-time in vivo imaging of an influenza reporter virus reveals dynamics of replication and spread. J Virol 87:13321–13329. doi: 10.1128/JVI.02381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker JC, Reynolds RK. 1968. Natural history of Sendai virus infection in mice. Am J Epidemiol 88:112–125. [DOI] [PubMed] [Google Scholar]
- 32.van der Veen J, Poort Y, Birchfield DJ. 1972. Effect of relative humidity on experimental transmission of Sendai virus in mice. Proc Soc Exp Biol Med 140:1437–1440. doi: 10.3181/00379727-140-36691. [DOI] [PubMed] [Google Scholar]
- 33.van der Veen J, Poort Y, Birchfield DJ. 1970. Experimental transmission of Sendai virus infection in mice. Arch Gesamte Virusforsch 31:237–246. [DOI] [PubMed] [Google Scholar]
- 34.Luque LE, Bridges OA, Mason JN, Boyd KL, Portner A, Russell CJ. 2010. Residues in the heptad repeat A region of the fusion protein modulate the virulence of Sendai virus in mice. J Virol 84:810–821. doi: 10.1128/JVI.01990-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson AH, McRoberts JG, Crowe SR, London L, London SD. 1999. Optimal induction of upper respiratory tract immunity to reovirus 1/L by combined upper and lower respiratory tract inoculation. Vaccine 17:1404–1415. doi: 10.1016/S0264-410X(98)00382-X. [DOI] [PubMed] [Google Scholar]
- 36.Southam DS, Dolovich M, O'Byrne PM, Inman MD. 2002. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol 282:L833–L839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 37.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. 1992. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol 149:1319–1325. [PubMed] [Google Scholar]
- 38.Sealy R, Jones BG, Surman SL, Hurwitz JL. 2010. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine 28:6749–6756. doi: 10.1016/j.vaccine.2010.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh T, Iwai H, Ueda K. 1991. Comparative lung pathology of inbred strains of mice resistant and susceptible to Sendai virus infection. J Vet Med Sci 53:275–279. doi: 10.1292/jvms.53.275. [DOI] [PubMed] [Google Scholar]
- 40.Sangster M, Hyland L, Sealy R, Coleclough C. 1995. Distinctive kinetics of the antibody-forming cell response to Sendai virus infection of mice in different anatomical compartments. Virology 207:287–291. doi: 10.1006/viro.1995.1079. [DOI] [PubMed] [Google Scholar]
- 41.Mo XY, Sarawar SR, Doherty PC. 1995. Induction of cytokines in mice with parainfluenza pneumonia. J Virol 69:1288–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anh DB, Faisca P, Desmecht DJ. 2006. Differential resistance/susceptibility patterns to pneumovirus infection among inbred mouse strains. Am J Physiol Lung Cell Mol Physiol 291:L426–L435. doi: 10.1152/ajplung.00483.2005. [DOI] [PubMed] [Google Scholar]
- 43.Takamura S, Roberts AD, Jelley-Gibbs DM, Wittmer ST, Kohlmeier JE, Woodland DL. 2010. The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. J Exp Med 207:1153–1160. doi: 10.1084/jem.20090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. 2011. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8(+) T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology 410:429–436. doi: 10.1016/j.virol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luker KE, Luker GD. 2010. Bioluminescence imaging of reporter mice for studies of infection and inflammation. Antiviral Res 86:93–100. doi: 10.1016/j.antiviral.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasan MK, Kato A, Shioda T, Sakai Y, Yu D, Nagai Y. 1997. Creation of an infectious recombinant Sendai virus expressing the firefly luciferase gene from the 3′ proximal first locus. J Gen Virol 78(Part 11):2813–2820. [DOI] [PubMed] [Google Scholar]
- 47.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. 2010. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci U S A 107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hotard AL, Shaikh FY, Lee S, Yan D, Teng MN, Plemper RK, Crowe JE, Moore ML. 2012. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology 434:129–136. doi: 10.1016/j.virol.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheridan JF, Dobbs C, Brown D, Zwilling B. 1994. Psychoneuroimmunology: stress effects on pathogenesis and immunity during infection. Clin Microbiol Rev 7:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein SL. 2012. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays 34:1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alymova IV, Portner A, Takimoto T, Boyd KL, Babu YS, McCullers JA. 2005. The novel parainfluenza virus hemagglutinin-neuraminidase inhibitor BCX 2798 prevents lethal synergism between a paramyxovirus and Streptococcus pneumoniae. Antimicrob Agents Chemother 49:398–405. doi: 10.1128/AAC.49.1.398-405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohlmeier JE, Woodland DL. 2009. Immunity to respiratory viruses. Annu Rev Immunol 27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- 53.Sealy R, Webby RJ, Crumpton JC, Hurwitz JL. 2013. Differential localization and function of antibody-forming cells responsive to inactivated or live-attenuated influenza virus vaccines. Int Immunol 25:183–195. doi: 10.1093/intimm/dxs107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coleclough C, Sealy R, Surman S, Marshall DR, Hurwitz JL. 2005. Respiratory vaccination of mice against influenza virus: dissection of T- and B-cell priming functions. Scand J Immunol 62(Suppl 1):S73–S83. doi: 10.1111/j.1365-3083.2005.01613.x. [DOI] [PubMed] [Google Scholar]
- 55.Iida T. 1972. Experimental study on the transmission of Sendai virus in specific pathogen-free mice. J Gen Virol 14:69–75. doi: 10.1099/0022-1317-14-1-69. [DOI] [PubMed] [Google Scholar]
- 56.Slobod KS, Shenep JL, Lujan-Zilbermann J, Allison K, Brown B, Scroggs RA, Portner A, Coleclough C, Hurwitz JL. 2004. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 22:3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 57.Jones BG, Sealy RE, Surman SL, Portner A, Russell CJ, Slobod KS, Dormitzer PR, DeVincenzo J, Hurwitz JL. 2014. Sendai virus-based RSV vaccine protects against RSV challenge in an in vivo maternal antibody model. Vaccine 32:3264–3273. doi: 10.1016/j.vaccine.2014.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones BG, Sealy RE, Rudraraju R, Traina-Dorge VL, Finneyfrock B, Cook A, Takimoto T, Portner A, Hurwitz JL. 2012. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine 30:959–968. doi: 10.1016/j.vaccine.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones B, Zhan X, Mishin V, Slobod KS, Surman S, Russell CJ, Portner A, Hurwitz JL. 2009. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine 27:1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhan X, Slobod KS, Krishnamurthy S, Luque LE, Takimoto T, Jones B, Surman S, Russell CJ, Portner A, Hurwitz JL. 2008. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine 26:3480–3488. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd KL, Scroggs RA, Surman S, Portner A, Slobod KS. 2007. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine 25:8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takimoto T, Hurwitz JL, Zhan X, Krishnamurthy S, Prouser C, Brown B, Coleclough C, Boyd KL, Scroggs RA, Portner A, Slobod KS. 2005. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol 18:255–266. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- 63.Takimoto T, Hurwitz JL, Coleclough C, Prouser C, Krishnamurthy S, Zhan X, Boyd KL, Scroggs RA, Brown B, Nagai Y, Portner A, Slobod KS. 2004. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol 78:6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skiadopoulos MH, Surman SR, Riggs JM, Elkins WR, St Claire M, Nishio M, Garcin D, Kolakofsky D, Collins PL, Murphy BR. 2002. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology 297:153–160. doi: 10.1006/viro.2002.1416. [DOI] [PubMed] [Google Scholar]
- 65.Malkin E, Yogev R, Abughali N, Sliman J, Wang CK, Zuo F, Yang C-F, Eickhoff M, Esser MT, Tang RS, Dubovsky F. 2013. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One 8:e77104. doi: 10.1371/journal.pone.0077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prince GA, Jenson AB, Hemming VG, Murphy BR, Walsh EE, Horswood RL, Chanock RM. 1986. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J Virol 57:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]