ABSTRACT
By recruiting the host protein XPO1 (CRM1), the HIV-1 Rev protein mediates the nuclear export of incompletely spliced viral transcripts. We mined data from the recently described human nuclear complexome to identify a host protein, RBM14, which associates with XPO1 and Rev and is involved in Rev function. Using a Rev-dependent p24 reporter plasmid, we found that RBM14 depletion decreased Rev activity and Rev-mediated enhancement of the cytoplasmic levels of unspliced viral transcripts. RBM14 depletion also reduced p24 expression during viral infection, indicating that RBM14 is limiting for Rev function. RBM14 has previously been shown to localize to nuclear paraspeckles, a structure implicated in retaining unspliced HIV-1 transcripts for either Rev-mediated nuclear export or degradation. We found that depletion of NEAT1 RNA, a long noncoding RNA required for paraspeckle integrity, abolished the ability of overexpressed RBM14 to enhance Rev function, indicating the dependence of RBM14 function on paraspeckle integrity. Our study extends the known host cell interactome of Rev and XPO1 and further substantiates a critical role for paraspeckles in the mechanism of action of Rev. Our study also validates the nuclear complexome as a database from which viral cofactors can be mined.
IMPORTANCE This study mined a database of nuclear protein complexes to identify a cellular protein named RBM14 that is associated with XPO1 (CRM1), a nuclear protein that binds to the HIV-1 Rev protein and mediates nuclear export of incompletely spliced viral RNAs. Functional assays demonstrated that RBM14, a protein found in paraspeckle structures in the nucleus, is involved in HIV-1 Rev function. This study validates the nuclear complexome database as a reference that can be mined to identify viral cofactors.
INTRODUCTION
Since the HIV-1 genome encodes only 15 proteins, the virus must exploit the function of host cofactors at every step in its replication cycle (1). A meta-analysis of genome-wide small interfering RNA (siRNA) screens suggests that more than 2,410 proteins, or 9.5% of human genes, may be involved in HIV-1 replication (2). While data from siRNA screens provide insight into virus-host interactions and provide an opportunity to identify “druggable” targets to inhibit virus replication, these screens have limitations. Comparison of different genome-wide siRNA screens reveal minimal overlap in the host genes involved in HIV-1 replication, and these screens may frequently yield false-positive results due to siRNA off-target effects (3).
In the present study, we utilized a novel strategy to identify host factors involved in HIV-1 replication. The human nuclear “complexome” was recently described in a high-throughput immunoprecipitation/mass spectrometry (IP/MS) study (4). This complexome describes endogenous protein complexes defined by identification of proteins that coimmunoprecipitated in more than 3,000 immunoprecipitations of HeLa cell nuclear extracts. We mined the nuclear complexome to identify cellular proteins found in complexes with XPO1 (CRM1), a cellular factor that mediates the HIV-1 Rev protein's function of nuclear export of incompletely spliced viral RNAs. XPO1 is a karyopherin adaptor protein that is involved in the transport of certain cellular proteins and a selective set of cellular RNAs, including snRNAs and some mRNAs (5). The XPO1 export pathway is distinct from that used by most cellular mRNAs or simple retroviruses such as Mason-Pfizer monkey virus (MPMV). MPMV encodes a constitutive transport element (CTE) RNA element in incompletely spliced viral transcripts that is bound by the proteins Tap/NXT and accesses an export pathway utilized by the majority of cellular mRNAs (6–8).
The HIV-1 Rev protein regulates viral posttranscriptional gene expression by directing the nuclear export of incompletely spliced viral transcripts in an XPO1-dependent process (9). The ∼9-kb unspliced viral transcript serves as genomic RNA and mRNA for the Gag and GagPol polyproteins, while a 4-kb class of singly spliced transcripts encode the Env, Vpr, Vif, and Vpu proteins (10). The nuclear export and expression of the unspliced transcripts are inhibited due to inefficient splicing and the presence of cis-acting RNA elements termed INS (instability elements) in the unspliced transcripts (10). Distinct nuclear structures termed paraspeckles appear to be involved in retaining INS-containing transcripts in the nucleus. Paraspeckles range in size from 0.5 to 1 μm in diameter, with generally between 5 and 20 paraspeckles per nucleus (11). The heterodimeric paraspeckle marker proteins p54nrb/PSF bind to INS RNAs both in vitro and in vivo, and overexpression of PSF inhibits the nuclear export and expression of INS-containing viral RNA, suggesting that these proteins retain INS RNAs in the nucleus (12). The formation of paraspeckles is dependent on the presence of a long noncoding RNA (lncRNA) termed NEAT1 (11). Depletion of NEAT1 enhances HIV-1 replication by a mechanism that involves an increase in Rev-XPO1-dependent nuclear export of INS-containing viral RNAs, further suggesting that paraspeckles retain INS RNAs in the nucleus (13). Additional studies have extended Rev's role beyond viral RNA export into facilitating efficient encapsidation of viral genomic RNA into virions that assemble at the plasma membrane, as well as in promoting translation of viral mRNAs through poorly understood mechanisms (14–16).
To mediate the export of unspliced viral transcripts, Rev binds and multimerizes via an arginine-rich amino-terminal domain on an RNA element encoded in the env gene, termed the Rev response element (RRE). Multimerized Rev interacts with XPO1 through the Rev nuclear export sequence (NES) in its leucine-rich carboxyl terminus, facilitating the assembly of a ribonucleoprotein complex (17–20). XPO1 directs this ribonucleoprotein complex through the nuclear pore by interacting with nuclear pore proteins, specifically nucleoporins such as Nup214 (21) and additional cellular proteins such as the helicase DDX3 (22). The directionality of XPO1-dependent transport is governed by differential concentrations of the Ran protein bound to GTP (Ran-GTP) or GDP (Ran-GDP) in the nucleus or cytoplasm, respectively (5). In the nucleus, XPO1 binds to Ran-GTP and accompanies the Rev-RNA complex to the cytoplasm, where Ran-GTP is hydrolyzed to Ran-GDP due to the actions of Ran-GAP and RanBP1 proteins. This leads to dissociation of XPO1 and Rev from the viral RNA. To engage in a new transport cycle, Rev binds another karyopherin protein, importin-β, through its nuclear localization signal (NLS), which imports Rev into the nucleus by interacting with Ran-GDP. Additional host proteins that may be involved in the recycling of Rev into the nucleus are unknown. In the nucleus, Ran-GDP is converted into Ran-GTP by the action of Ran-GEF, releasing Rev from importin-β and allowing another round of nuclear export (5).
Using the nuclear complexome database, we identified an XPO1-associated protein, RBM14, which is involved in Rev function. RBM14 (RNA binding motif protein 14) is an hnRNP-like nuclear protein with two RNA binding (RNA recognition motifs [RRMs]) domains in its amino terminus, and it is found in nuclear paraspeckles along with p54nrb/PSF (23). We investigated the role of RBM14 in Rev activity due to its localization in nuclear paraspeckles. These subnuclear structures have been previously implicated in Rev function (12). Depletion of RBM14 inhibited the activity of Rev-dependent reporter plasmids and reduced the levels of incompletely spliced viral transcripts in the cytoplasm. Overexpression of RBM14 stimulated Rev function, while depletion of RBM14 inhibited Rev function during viral infection, indicating that RBM14 is likely necessary for productive viral replication. RBM14 stimulation of Rev function was found to be dependent on NEAT1 RNA, the lncRNA essential for the structural integrity of nuclear paraspeckles. Our data establish that RBM14 is a novel Rev cofactor and demonstrate the utility of the nuclear complexome database for the unbiased discovery of viral cofactors.
MATERIALS AND METHODS
Plasmids, siRNAs, and antibodies.
The pCMVGagPol-RRE (CMV stands for cytomegalovirus) and pCMV-RevFlag plasmids have been described previously and were a kind gift from Marie-Louise Hammarskjöld (University of Virginia). The p54nrb-Flag plasmid has also been described previously and was kindly provided by Xuesen Dong (University of British Columbia). The pHMRLuc (Luc stands for luciferase) and RemGFP (GFP stands for green fluorescent protein) plasmids were kindly provided by Jaquelin Dudley (University of Texas, Austin).
RBM14 (sc-96838) and control (sc-37007) small interfering RNAs (siRNAs) were obtained from Santa Cruz Biotechnology and are a pooled mixture of 3 to 5 siRNAs specific for the RBM14 gene. siRNAs against NEAT1 long noncoding RNA (lncRNA) were those described previously and were obtained from Sigma-Aldrich (13). The RBM14 antiserum (ab70636) and PACS1 antiserum (ab56072) were obtained from Abcam. The anti-actin antibody (catalog no. 05-384) and Flag antibody were obtained from Millipore and Sigma-Aldrich, respectively.
siRNA and plasmid transfections.
293T cells were transfected with 10 pmol of siRNAs in 24-well culture dishes using Lipofectamine RNAimax (Life Technologies) according to the manufacturer's directions. At 24 h after the siRNA transfections, the cultures were transfected with 100 ng of pCMVGagPol-RRE plasmids and 50 ng of pCMV-RevFlag plasmid. Culture supernatants were harvested 24 h after the plasmid transfection for p24 enzyme-linked immunosorbent assays (ELISAs) using the Zeptometrix ELISA kit; for luciferase assays, cells were lysed in RLB (reporter lysis buffer) using the luciferase assay system from Promega according to the manufacturer's instructions. Luciferase activities were normalized to total protein in the cell lysates.
Immunoprecipitations and immunoblotting.
Immunoprecipitations were performed using anti-Flag M2 affinity gel (catalog no. A2220) from Sigma-Aldrich following the manufacturer's protocol. Briefly, cultures transfected with Flag-tagged plasmids were lysed 48 h after transfection with Pierce immunoprecipitation (IP) lysis buffer with added protease inhibitor cocktail (catalog no. P8340; Sigma-Aldrich); 40 μl of the anti-Flag M2 affinity gel was washed with lysis buffer and incubated with the cell lysates overnight at 4°C. Following the incubation, beads were washed with lysis buffer, and bound proteins were eluted by mixing and heating the beads in sample loading buffer for 7 min at 95°C. Samples were spun in a microcentrifuge, and immunoprecipitates were loaded on a 7 to 12% Tris gradient gel (Bio-Rad). The gels were transferred to nitrocellulose membranes and blocked with 5% nonfat dry milk (NFDM) for an hour and probed with the appropriate antibodies.
RNA immunoprecipitations.
For RNA immunoprecipitations, transfected cells were lysed with Pierce IP lysis buffer containing protease inhibitor cocktail and 100 u/ml RNase inhibitor (Promega). To bind the antibodies to Dynabeads protein A (Life Technologies), the beads (40 μl) were washed three times with lysis buffer and incubated with 5 μg of antisera in 200 μl of lysis buffer for 1 h at 4°C. Unbound antibodies were removed by washing with lysis buffer. The cell lysates were incubated with the Dynabeads protein A coated with the antisera overnight at 4°C. The beads were washed vigorously two times with 1 ml of lysis buffer containing protease inhibitor cocktail and RNase inhibitor to remove nonspecifically bound proteins. RNA was eluted from the protein bound to beads using TRIzol reagent (Life Technologies), treated with DNase, and amplified by quantitative reverse transcription-PCR (qRT-PCR) using primers specific for the unspliced GagPol transcripts.
Lentiviral shRNA packaging and transduction and HIV-1 infections.
RBM14 and control short hairpin RNAs (shRNAs) were purchased from the Baylor College of Medicine C-BASS (Cell-Based Assay Screening Service) Core laboratory. To package the shRNAs into lentiviral particles, the RBM14 or control shRNAs were transfected with psPAX2 packaging plasmid and a plasmid encoding the envelope protein. Supernatant containing the lentiviral particles was collected and used to transduce 293T cells.
Pseudotyped HIV-1 viruses containing the luciferase reporter gene were produced by transfecting 293T cells with the pNL4-3.Luc.R-E- and the vesicular stomatitis virus glycoprotein (VSV-G) expression plasmids. Cellular supernatants were harvested at 48 h posttransfection and used to infect cells transduced with the shRNA lentiviral particles.
FISH.
Gag-specific Stellaris RNA fluorescent in situ hybridization (FISH) probes labeled with Quasar 670 fluorophore were obtained from Biosearch Technologies; the Gag probes are a pool of 40 individual probes. For FISH, the cells were fixed with fixation buffer (3.7% formaldehyde in phosphate-buffered saline [PBS]) for 20 min at room temperature followed by permeabilization with 70% ethanol for 48 h. The cells were washed once with wash buffer (10% formamide in 2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) and incubated with the probe in hybridization buffer (100 mg/ml dextran sulfate and 10% formamide in 2× SSC) for 4 h at 37°C. Nonspecifically bound probes were removed by incubating the cells with wash buffer for 30 min at room temperature. Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) and fixed for microscopy using Vectashield HardSet mounting medium (Vector Laboratories). Cells were analyzed using the DeltaVision (deconvolution) image restoration microscope in the Baylor College of Medicine Integrated Microscopy Core laboratory.
RESULTS
Identification of XPO1-associated proteins.
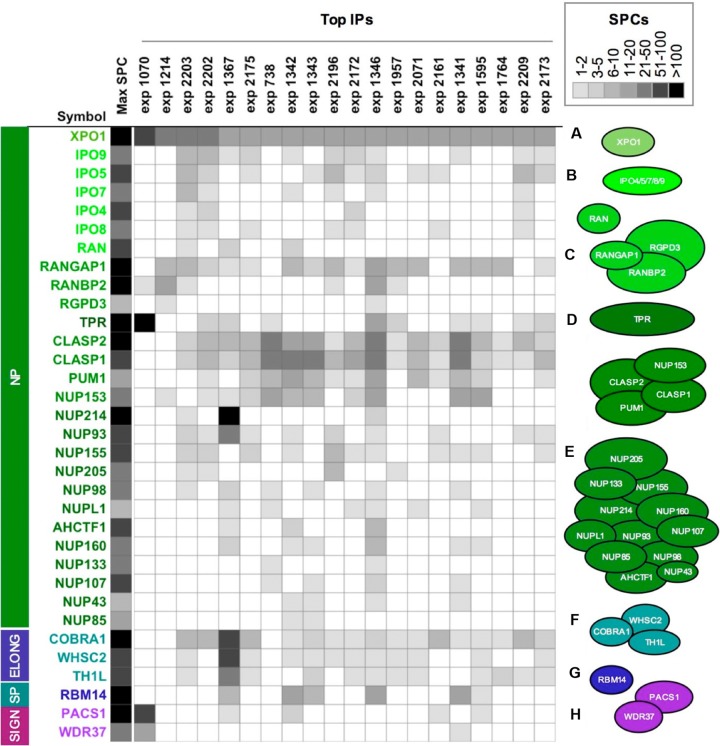
We mined the human nuclear complexome database to identify novel interacting partners of the HIV-1 Rev cofactor XPO1 (Fig. 1). The core complexes were defined as previously described using an integrative immunoprecipitation/mass spectrometry (IP/MS) approach followed by complex-complex interaction analysis. Briefly, multiple IPs that eluted the seed protein, in this case XPO1, at the highest peptide spectral counts (SPCs) as measured by MS were selected irrespective of the target antigen in the original IP. To be qualified as a legitimate interacting partner of XPO1, the interactor was required to coprecipitate multiple times. In this way, cross-reacting proteins were eliminated from the analysis by the use of reciprocal IPs (4). As seen in Fig. 1, multiple XPO1 complexes were identified, including previously known XPO1 interactors such as NUP214, Ran, and RanBP2 (24, 25).
FIG 1.
XPO1-associated proteins identified from the human nuclear complexome. Integrative MS analysis of a high-throughput immunoprecipitation (HT IP) study delineated the representative XPO-1-associated protein complexes as indicated. Identification of peptide spectral counts (SPCs) by mass spectrometry is indicated for each protein by the gray scale. Based on the SPC intensities, the top XPO1 IPs were clustered as indicated (the color for the maximum SPC is shown in the Max SPC column). The numbers after exp (for experiment) above the figure refer to the antisera used in the IPs (4). Complexes A to H are shown to the right of the figure. NP, nuclear pore; ELONG, elongation; SP, splicing; SIGN, signaling.
Our initial experimental strategy was to use siRNA depletions of XPO1-associated proteins and evaluate effects on Rev activity in reporter plasmid assays. Many XPO1-associated proteins shown in Fig. 1 are components of nuclear pores, and we reasoned that siRNA depletions of nuclear-pore-associated proteins were likely to have pleotropic effects and confound interpretation of experiments. In this study, we therefore chose to focus on non-nuclear-pore-associated proteins.
siRNA depletions of XPO1-associated proteins inhibit HIV-1 Rev function.
In initial experiments, we examined three non-nuclear-pore proteins from the complexome data, RBM14, PACS1, and NELF-A. RBM14 (complex G [Fig. 1]) is a nuclear protein, and it has been shown to function in transcription activation and pre-mRNA splicing (26). RBM14 localizes in nuclear paraspeckles, nuclear structures implicated in regulating transport of HIV-1 unspliced transcripts (12, 13). PACS1 (complex H [Fig. 1]) is predominantly a cytoplasmic protein and is believed to play a role in localization of proteins with an acidic cluster motif to the trans-Golgi network (TGN). Of interest to HIV-1 infection, PACS1 directs the TGN localization of furin (27), a cellular protease that cleaves the HIV-1 gp160 envelope protein into the gp120 and gp41 subunits (28). Additionally, the HIV-1 Nef protein has been shown to hijack PACS1 function to downregulate major histocompatibility complex class I (MHC-I) molecules (29). NELF-A (also named WHSC; complex F [Fig. 1]) is a negative regulator of RNA polymerase II (RNAPII) transcription elongation that is overcome by the action of the HIV-1 Tat protein (30, 31).
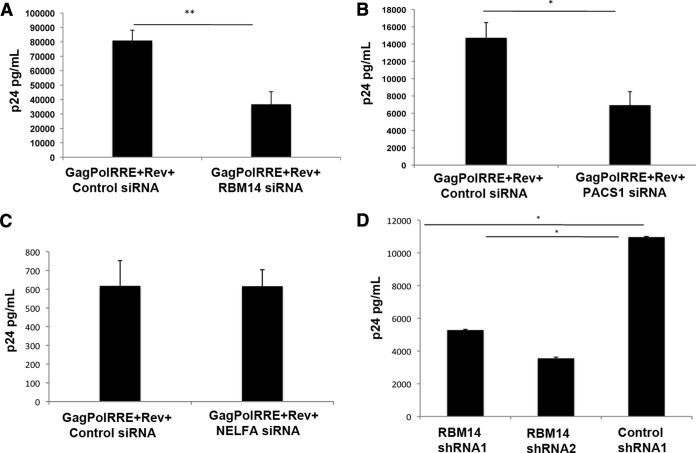
We conducted siRNA depletion experiments for RBM14, PACS1, and NELF-A and examined effects on expression of a Rev-dependent reporter plasmid pCMVGagPol-RRE. Expression of p24 from the (pCMVGagPol-RRE) plasmids is driven by the cytomegalovirus (CMV) immediate-early promoter (6, 32). In the pCMVGagPol-RRE plasmid, the HIV-1 GagPol coding sequence is placed in an intron followed by the Rev response element (RRE) sequence, thus rendering nuclear export and expression of the mRNAs Rev dependent. As shown in Fig. 2, siRNA depletions were effective in reducing the protein levels of RBM14, PACS1, and NELF-A. To exclude the possibility that any effects of RBM14 or PACS1 depletion on reporter plasmid expression were due to reduced Rev protein expression or cytotoxicity, we examined Rev expression levels and cell viability after depletion of RBM14 and PACS1 (Fig. 2B). Rev expression levels were not reduced by the depletion of RBM14 and PACS1. In contrast, the level of Rev was slightly increased upon depletion of RBM14 and especially PACS1. However, the significance of these slight increases is not clear. Additionally, as quantified by trypan blue exclusion, there were no significant differences in the viability of cells transfected with RBM14 or PACS1 siRNAs compared to control siRNA-treated cells (data not shown).
FIG 2.
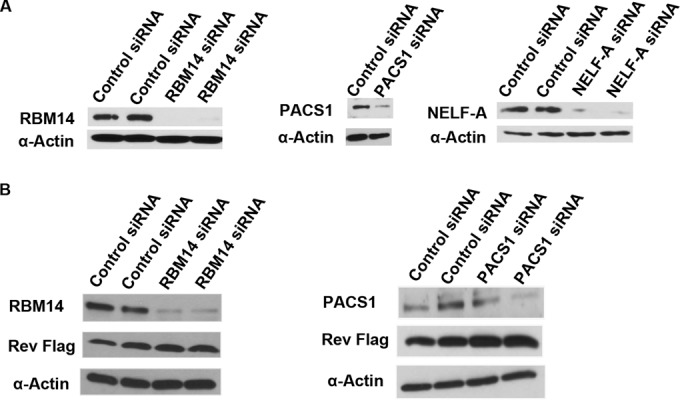
XPO1-associated protein depletions and their effect on Rev expression. (A) 293T cells were transfected with control siRNAs, RBM14 siRNAs (in duplicate), PACS1 siRNAs, or NELF-A siRNAs (in duplicate). Cell lysates were prepared 48 h posttransfection, and expression of RBM14, PACS1, NELF-A, and loading control anti-actin antibody (α-Actin) were examined in immunoblots. (B) 293T cells were transfected with Rev-Flag expression plasmids and the indicated siRNAs (in duplicate). Cell lysates were prepared 48 h posttransfection, and expression of indicated proteins was examined on immunoblots.
Depletion of RBM14 or PACS1 resulted in a statistically significant ∼2-fold reduction in Rev stimulation of p24 expression from the pCMVGagPol-RRE reporter plasmid relative to the cells transfected with control siRNA (Fig. 3A and B). Since the Rev-independent p24 expression from pCMVGagPol-RRE plasmid was below the limits of detection by ELISAs, we could not determine the effects of RBM14, PACS1, or NELF-A depletion on basal p24 expression. Depletion of NELF-A did not affect Rev function (Fig. 3C), and we did not therefore further investigate this XPO1-associated protein or other proteins found in complex F with NELF-A (COBRA1/NELF-B and TH1L/NELF-C/D). Our initial siRNA depletions of the WDR37 protein found in complex H with PACS1 were ineffective in reducing the level of WDR37, and we did not investigate this XPO1-associated protein. Given the absence of an effect of NELF-A depletion on Rev activity, it is likely that not all XPO1-associated proteins shown in Fig. 1 have roles in Rev function. While PACS1 depletion decreased p24 expression, suggesting that it may be involved in regulating Rev function, we focused our subsequent experiments on RBM14, since it is found in paraspeckles, a nuclear structure implicated in Rev function (12). As will be presented and discussed below (see Fig. 8), overexpression of an RBM14-GFP protein stimulated p24 expression from the pCMVGagPol-RRE reporter plasmid, further indicating that RBM14 is a cellular factor involved in Rev function.
FIG 3.
Depletion of RBM14 or PACS1 inhibits Rev function. (A to C) 293T cells were transfected with control siRNAs, RBM14 siRNA (A), PACS1 siRNA (B), or NELFA siRNA (C) for 24 h followed by transfection with the pCMVGagPol-RRE and the pCMVRev-Flag or parental Flag expression vector as indicated. Supernatants were collected 48 h later and analyzed for p24 expression by ELISAs. (D) Cultures of 293T cells were transduced with lentiviral vectors (with GFP reporter) for the indicated shRNAs. Cells transduced with the shRNA vectors were infected with VSV-G pseudotyped HIV-1 NL4-3-Luciferase virus, and cellular supernatants were analyzed for p24 expression by ELISAs. Values that were significantly different by a paired Student's t test are indicated by a bar and asterisks as follows: *, P < 0.05; **, P < 0.005.
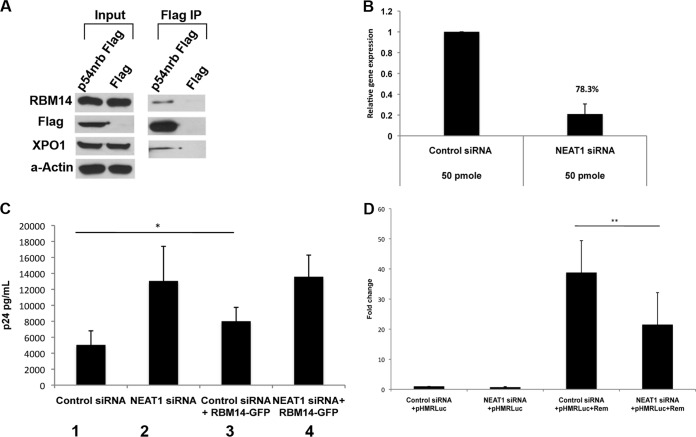
FIG 8.
RBM14 associates with the components of paraspeckles and requires paraspeckle integrity for its function. (A) Cell lysates were prepared from 293T cells transfected with p54nrb-Flag or parental Flag vector, and immunoprecipitations were performed with anti-Flag-coated agarose beads. Products of immunoprecipitates and whole-cell lysates were analyzed in immunoblots using the indicated antisera. (B) RNA was extracted and quantified by qRT-PCR in cells treated with control or NEAT1 siRNA. (C) 293T cells were treated with control or NEAT1 siRNA and transfected with expression plasmids for pCMVGagPol-RRE, Rev-Flag, and empty GFP vector or RBM14-GFP; p24 levels in the cellular supernatants were measured 48 h after transfection by ELISAs. (D) 293T cells treated with control siRNA or NEAT1 siRNA were transfected with expression plasmids for Rem, pHMRLuc, and empty firefly luciferase plasmids as indicated. Fold change was calculated as described in the legend to Fig. 5. Values that are significantly different by a paired Student's t test, are indicated by a bar and asterisks as follows: *, P < 0.05; **, P < 0.005.
The data presented in Fig. 3A show that RBM14 is required for Rev stimulation of reporter plasmids. As a further test for an important role for RBM14 in the HIV-1 life cycle, we examined the effects of RBM14 depletions on p24 expression during viral infection. We transduced cells with shRNA lentiviral vectors expressing green fluorescent protein (GFP) against RBM14 or control shRNA. Cultures of transduced cells were infected with a VSV-G pseudotyped NL4-3 luciferase virus, and viral p24 levels were measured in the supernatant. Immunoblot analysis indicated that the shRNAs were effective in depleting RBM14 levels (data not shown). A representative experiment shown in Fig. 3D demonstrates that cells transduced with RBM14 shRNA 1 and shRNA 2 showed a 48% and 32% decrease in p24 levels compared to the control shRNA-treated cells. Additionally, flow cytometry analysis also confirmed the decrease in intracellular p24 expression upon HIV-1 infection of 293T cells previously transduced with RBM14 shRNA lentiviral vectors (data not shown). These data show that RBM14 has a role in p24 expression during viral infection, likely due to its role in mediating nuclear export of unspliced viral RNA.
RBM14 associates with XPO1 and Rev in vivo.
We used coimmunoprecipitations to verify that RBM14 associates with XPO1 and to determine whether it also associates with Rev in cells. Cultures of 293T cells were transfected with an expression vector for Flag-tagged XPO-1, Flag-Rev, Flag-p54nrb, parental Flag CMV vector, or a negative-control Flag-PPM1A, and anti-Flag immunoprecipitations were performed. p54nrb is a marker protein for paraspeckles and was used as a positive control for an RBM14 coimmunoprecipitating protein (11). PPM1A is a phosphatase that negatively regulates HIV-1 gene expression through dephosphorylation of the CDK9 T-loop (33). As seen in Fig. 4, endogenous RBM14 coimmunoprecipitated with XPO1, Rev, and Flag-p54nrb, but not with PPM1A, demonstrating specificity of its interaction with XPO1, Rev, and p54nrb in cells.
FIG 4.
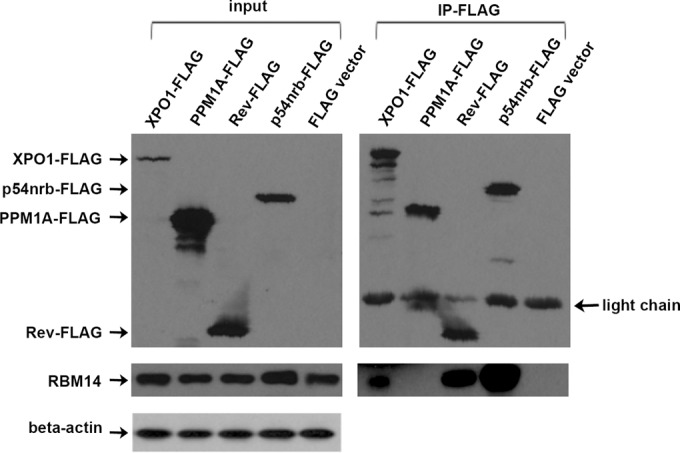
RBM14 associates with XPO1 and Rev. (A) 293T cells were transfected with Flag-tagged expression plasmids for XPO1, PPM1A, Rev, p54nrb, and parental vector (CMV-Flag) followed by immunoprecipitation with anti-Flag agarose beads (IP-FLAG). Immunoprecipitates were analyzed by immunoblotting for Flag, endogenous RBM14, and anti-actin as indicated. The position of the IgG light-chain band is indicated to the right of the blot.
RBM14 is not involved in MMTV Rem nuclear export or HIV-1-activated transcription.
We analyzed the effects of RBM14 depletions on the function of mouse mammary tumor virus (MMTV) Rem protein, which is a functional Rev homologue. Similar to Rev, it facilitates the nuclear export of incompletely spliced MMTV transcripts through an XPO1 pathway (34). To examine the effects of RBM14 depletion on Rem function, we used the pHMRLuc plasmid which contains the luciferase coding sequences in an intron and thus is Rem dependent. Depletion of RBM14 resulted in a slight increase in Rem-stimulated luciferase expression relative to control siRNA-treated cells (Fig. 5A), but this increase was not statistically significant (P value of 0.206). Thus, although RBM14 is involved in mediating HIV-1 Rev function in an XPO1-mediated process, it does not appear to play a necessary role in MMTV Rem function. As shown and discussed below (see Fig. 8), depletion of NEAT1, a long noncoding RNA (lncRNA) required for integrity of nuclear paraspeckles (11), stimulates nuclear export of HIV-1 Rev-dependent transcripts while it reduces expression of Rem-dependent transcripts. Thus, the mechanisms whereby XPO1 facilitates nuclear export of unspliced viral RNAs diverge between the HIV-1 Rev and MMTV Rem proteins.
FIG 5.
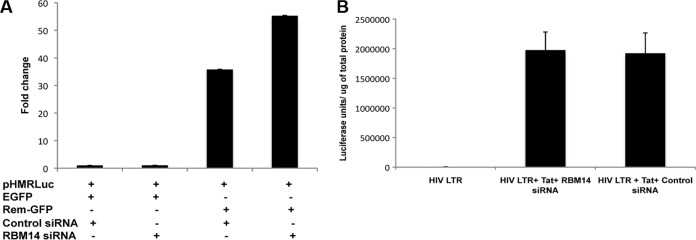
RBM14 depletion does not affect MMTV Rev function or HIV-1-activated transcription. (A) 293T cells were transfected with control siRNAs or siRNAs against RBM14, and at 24 h posttransfection, cells were transfected with pHMRLuc, containing the Rem response element, pCMV-Luc expressing firefly luciferase, and enhanced GFP (EGFP) or RemGFP expression plasmids. Renilla and firefly luciferase activities were measured in cell lysates 24 h posttransfection. Relative renilla luciferase values were calculated by normalizing to firefly luciferase activity and setting the value in control siRNA-treated cells at 1.0. (B) 293T cells were transfected with control siRNA or RBM14 siRNA for 24 h followed by transfection with HIV-1 LTR-luciferase and pCMV-Tat expression plasmids. Cells were harvested 48 h after transfection and assayed for luciferase activity and normalized to total protein.
To determine whether RBM14 has a role in HIV-1 transcription, we examined the effects of RBM14 depletion on transcription directed by the HIV-1 long terminal repeat (LTR) transactivation by the viral Tat protein. We transfected cells with control siRNAs or siRNAs against RBM14 and cotransfected cells with an HIV-1 LTR luciferase plasmid and a Tat expression plasmid (Fig. 5B). RBM14 depletion had no effect on HIV-1 Tat-mediated activation of an HIV-1 LTR-luciferase reporter plasmid. Thus, RBM14 has no apparent role in regulating HIV-1 transcription.
Depletion of RBM14 decreases the levels of cytoplasmic unspliced GagPol mRNA.
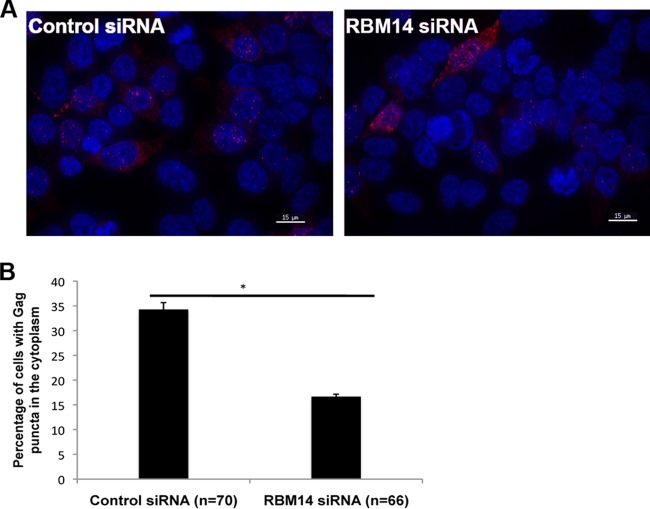
In the absence of Rev in HIV-1-infected CD4+ T cells, incompletely spliced HIV-1 transcripts fail to be exported to the cytoplasm and accumulate in the nucleus, where they are either subject to splicing or are degraded (35). Additionally, incompletely spliced transcripts accumulate in the nuclei of latently infected resting CD4+ T cells from patients treated with antiviral drugs, suggesting that a deficiency of Rev function may contribute to latent infection (36). To determine the effects of siRNA depletions on the levels of unspliced GagPol transcripts in the cytoplasm, we quantified the GagPol transcripts in cells depleted of RBM14 and transfected with the pCMVGagPol-RRE reporter and Rev expression plasmids by fluorescent in situ hybridization (FISH). We used a fluorescently labeled Gag RNA probe to determine the effect of RBM14 depletion on the in vivo localization of the unspliced Gag transcript. As seen in Fig. 6, RBM14 depletion decreased the Gag puncta in the cytoplasm by 50% compared to the control siRNA-treated cells, indicating a defect in the nuclear export of the unspliced transcripts in the absence of RBM14. These data suggest that RBM14 functions to enhance Rev-mediated nuclear export of incompletely spliced viral transcripts, consistent with the analysis of Rev function in Fig. 3.
FIG 6.
RBM14 depletion reduces the level of unspliced HIV-1 mRNA in cytoplasm. (A) Control or RBM14 siRNA-treated 293T cells were transfected with the pCMVGagPol-RRE and the Rev-Flag plasmids. At 48 h posttransfection, cells were fixed, stained with fluorescent Gag-specific RNA probes, and imaged by deconvolution microscopy. (B) Quantitation of the cells in panel A that contain Gag puncta in the cytoplasm. Percentage of cells containing Gag puncta in the cytoplasm were calculated from the total number of cells containing Gag puncta. The values were significantly different (P < 0.05) by a paired Student's t test, as indicated by the bar and asterisk.
RBM14 associates with the viral unspliced transcripts in an XPO1-dependent manner.
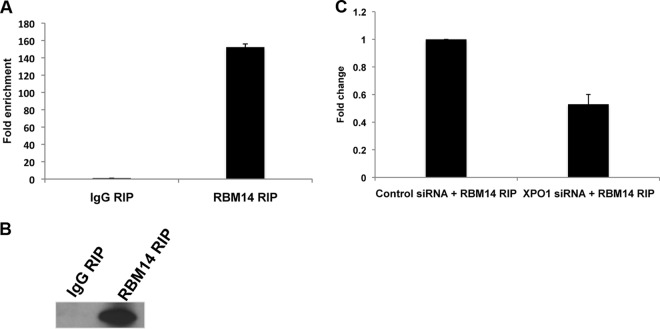
RBM14 is predicted to be an RNA binding protein, as it contains two RRMs (RNA recognition motifs) in its N-terminal domain, and it has been shown to regulate the activity of nuclear receptor transcription coactivators and to modulate splicing in a promoter-dependent manner (26). To determine whether RBM14 may enhance nuclear export of incompletely spliced HIV-1 RNA through an association with viral transcripts, we performed an RNA immunoprecipitation (RIP) from cells cotransfected with plasmids for the pCMVGagPol-RRE reporter and Rev expression. The RIP performed with the anti-RBM14 antiserum showed a >100-fold increase in the GagPol transcript bound by RBM14 relative to the control IgG RIP (Fig. 7). We confirmed the recovery of RBM14 using anti-RBM14 antibody through immunoblotting (Fig. 7B). Since XPO1 may mediate RBM14 binding to viral RNA, we used an siRNA depletion of XPO1 to determine whether it is involved in the RBM14 association with the GagPol transcript (Fig. 7C). In cells depleted of XPO1, there was an ∼40% reduction in the GagPol transcript bound by RBM14 relative to the amount of transcript present in the IgG control immunoprecipitation (Fig. 7C). These data indicate that RBM14 binding to the GagPol transcript is partially dependent on XPO1.
FIG 7.
Association of RBM14 with the GagPol transcript. (A) RNA from cell lysates of 293T cells transfected with the GagPol-RRE and Rev-Flag expression plasmids was immunoprecipitated with RBM14-specific or IgG antibody, followed by qRT-PCR with primers specific for the GagPol transcript. The GagPol transcript levels were normalized to the respective input RNA, and fold enrichment was calculated by setting the GagPol transcript levels in the IgG RNA immunoprecipitation (RIP) reaction at 1.0. Error bars refer to standard deviations. (B) Immunoprecipitates from the IgG and RBM14 RIP were eluted in loading buffer and evaluated in an immunoblot to confirm recovery of RBM14. (C) 293T cells treated with XPO1 or control siRNA were transfected with the GagPol-RRE and Rev-Flag plasmids. RNA immunoprecipitation and qRT-PCR were carried out as in panel A.
RBM14 requires nuclear paraspeckle structural integrity for regulation of Rev activity.
Nuclear paraspeckles contain a lncRNA1, NEAT1, and the longer isoform NEAT2, as well as several proteins, PSP1, RBM14, and the p54nrb/PSF heterodimer. Each of these protein components of paraspeckles contains two RRM motifs followed by a protein interaction domain, and they thus share structural similarities (26, 37). The p54nrb/PSF heterodimer and the NEAT1 lncRNA have been implicated in retaining HIV-1 unspliced transcripts in paraspeckles (12, 38). It has been proposed that the p54nrb-PSF complex may function in transferring HIV-1 unspliced transcripts to the neighboring nucleolus, where Rev-XPO1 export complexes are believed to be assembled (12). As shown in Fig. 4, p54nrb associates with RBM14. To determine whether XPO1 also associates with the paraspeckle protein p54nrb, cells were transfected with a p54nrb-Flag or parental expression plasmids, and immunoprecipitations were performed with anti-Flag beads (Fig. 8A). Endogenous RBM14 and XPO1 were found in Flag immunoprecipitates in p54nrb-Flag-transfected cells but not with parental-vector-transfected cells. These data indicate that both RBM14 and XPO1 associate with p54nrb, consistent with the notion that XPO1 interacts with paraspeckle proteins, facilitating the shuttling of viral transcripts from the paraspeckles to the nucleolus.
We were interested in determining whether the localization of RBM14 to the paraspeckles is required for RBM14 enhancement of Rev activity. As shown in Fig. 8C (compare columns 1 and 3), we observed that overexpression of a RBM14-GFP fusion protein stimulated Rev activity. We used siRNAs to deplete NEAT1 lncRNA and evaluated the effects on the ability of overexpressed RBM14-GFP to enhance Rev stimulation of the GagPol-RRE reporter plasmid (Fig. 8C). It is known that depletion of NEAT1 lncRNA results in the abolition of nuclear paraspeckles and an enhancement of unspliced viral RNA expression (13). NEAT1 depletion was confirmed by qRT-PCR (Fig. 8B). As expected, depletion of NEAT1 stimulated p24 expression. While overexpressed RBM14-GFP was able to stimulate p24 expression from the reporter plasmid in control siRNA-transfected cells (compare columns 1 and 3 in Fig. 8C), it had no stimulatory effect on p24 expression in NEAT lncRNA-depleted cells (compare columns 2 and 4). This result suggests that the structural integrity of nuclear paraspeckles, mediated by NEAT1 lncRNA, is required for the ability of overexpressed RBM14 to enhance Rev function.
Our analysis of the MMTV Rem protein indicated that RBM14 depletion does not reduce Rem function (Fig. 5A). We were therefore interested to determine the consequence of NEAT1 depletion on Rem activity. As shown in Fig. 8D, NEAT1 depletion resulted in a decrease in Rem-dependent luciferase expression. This is in contrast to NEAT1 depletions in the HIV-1 system where nuclear export of unspliced viral RNA is enhanced. These data indicate that paraspeckles have distinct mechanistic roles in nuclear export of unspliced HIV-1 transcripts versus unspliced MMTV transcripts.
DISCUSSION
In this study, we mined the human nuclear complexome (4) to identify a novel cofactor for the HIV-1 Rev protein. While previous studies have identified Rev cofactors through siRNA screens or immunoprecipitation/mass spectrometry approaches using Rev as the bait (39, 40), the characterization of the human nuclear complexome is distinctive in the comprehensive manner in which it has revealed a tiered and modular organization of XPO1 protein complexes (Fig. 1). Using this approach, we extend the Rev-XPO1 interaction network and demonstrate its potential complexity.
We used multiple assays to demonstrate that RBM14 is a Rev cofactor. Depletion of RBM14 significantly decreased Rev-mediated activation of a reporter plasmid. Additionally, overexpression of RBM14 increased p24 expression from the GagPol-RRE reporter plasmid, suggesting that this XPO1-associated protein is limiting for Rev function. Depletions of RBM14 reduced the cytoplasmic accumulation of Rev-dependent unspliced GagPol transcripts and inhibited p24 expression during viral infection. Since depletion of RBM14 had no significant effect on the function of the MMTV Rem protein, a Rev functional homologue that utilizes an XPO1-dependent pathway, it is likely that RBM14 regulates an XPO1 pathway that is different in HIV-1 and MMTV. Additionally, RBM14 depletion did not affect Tat or Tax transactivation of the HIV-1 or human T-cell leukemia virus type 1 (HTLV-1) LTRs (data not shown), respectively, indicating that RBM14's transcriptional coactivation function is not involved in transcriptional activation of these two retroviruses.
RBM14 was discovered as a transcriptional coactivator of nuclear hormone receptors and has been shown to regulate both alternative splicing and transcription of cellular genes, particularly steroid hormone-responsive genes (26, 41). Using a NES (nuclear export sequence) prediction algorithm, we were unable to identify a NES motif in the RBM14 protein sequence. This suggests that if RBM14 shuttles between the nucleus and cytoplasm, it may exit the nucleus through an association, either direct or indirect, with the XPO1 NES binding site.
Importantly, RBM14 localizes to nuclear paraspeckles (42). HIV-1 unspliced transcripts are believed to be sequestered in nuclear paraspeckles by the p54nrb and PSF proteins which bind the INS (instability elements) in unspliced viral transcripts (12). It has been speculated that this process likely contributes to preventing the splicing of viral transcripts. It has been shown by live-cell microscopy that XPO1 colocalizes with Rev in the nucleolus and traffics between the nucleolus and cytoplasm (38). As paraspeckles are proximal to the nucleolus, paraspeckles could act as storage sites for the unspliced viral transcripts, from where they traffic to the nucleolus to access the Rev-XPO1 export pathway in an RBM14-dependent manner (12). Additional support for a role of paraspeckles in Rev function is the identification of PSP1, a paraspeckle protein, in coimmunoprecipitations with Rev in a proteomics study (39).
In this study, we found that RBM14 stimulation of Rev function depends on NEAT1 RNA, an lncRNA that is required for the structural integrity of paraspeckles. NEAT1 RNA has previously been implicated in regulating the expression of cellular mRNAs that contain inverted Alu repeats through nuclear retention (43). In our study, depletion of NEAT1 RNA abrogated the ability of overexpressed RBM14 to enhance HIV-1 p24 production. Interestingly, NEAT1 depletion decreased Rem-dependent reporter gene expression in contrast to the increase in Rev-dependent p24 expression. This suggests that NEAT1 has multiple roles in regulating XPO1-mediated retroviral mRNA transport, and similar to cellular transcripts, nuclear retention in paraspeckles also regulates posttranscriptional viral gene expression. Intriguingly, NEAT1 lncRNA was recently reported to be induced following infections with influenza and herpes simplex viruses, leading to transcriptional activation of antiviral genes such as the interleukin 8 (IL-8) gene (44). Increased levels of NEAT1 and a corresponding increase in the numbers of paraspeckles following infection with influenza virus or herpes simplex virus was found to activate transcription of the IL-8 gene by sequestering SFPQ (splicing factor proline/glutamine rich), a transcriptional repressor that binds to the IL-8 promoter. This recent finding indicates that paraspeckles regulate innate immunity, and it is tempting to speculate that the involvement of paraspeckles in Rev function may somehow affect innate immune responses in HIV-1-infected cells.
In addition to associating with Rev and XPO1, RBM14 also associates with the paraspeckle marker protein p54nrb and binds to the GagPol transcript in a partially XPO1-dependent manner. Paraspeckles are dynamic structures whose protein constituents shuttle between paraspeckles and the nucleolus (37, 45). While we have not addressed the cycling of RBM14 between paraspeckles and the nucleolus, given its structural and functional similarities to other paraspeckle proteins, it seems likely that RBM14 is mobile in the nucleus (11). Taking all the data together, we propose that the RBM14-p54nrb-PSF complex acts as a cellular complex for the transfer of unspliced HIV-1 transcripts from the paraspeckles to the XPO1-Rev complex in the nucleolus. The depletion of RBM14 by siRNAs in this study may have reduced the trafficking of unspliced viral transcripts from paraspeckles to the nucleolus where they access the XPO1-Rev export pathway.
The potential of host cofactors to serve as therapeutic targets for HIV-1 infection is hindered by our limited understanding of their interactions with the viral replication machinery. In this study, we used a novel approach to identify XPO1-associated proteins that are novel Rev cofactors, thus extending the virus-host interactome and demonstrating the validity of mining data from the human nuclear complexome database.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI102483 and AI114335 (to A.P.R.) and P30AI1036211 (Baylor College of Medicine-University of Texas at Houston Center for AIDS Research [CFAR]). We thank Mary Marie-Louise Hammarskjöld (University of Virginia), Jacquelin Dudley (University of Texas, Austin) and Xuesen Dong (University of British Columbia) for the plasmids. We thank Fabio Stossi and Radhika Dandekar at the Baylor College of Medicine (BCM) Integrated Microscopy Core for help with imaging. We thank Jason Kimata and Ronald Javier (Baylor College of Medicine) for critical comments on the manuscript.
We declare that we have no conflicts of interest.
S.B. designed, performed experiments, and analyzed data. H.L. performed immunoprecipitation experiments in Fig. 4. J.C. performed the flow cytometry experiments and analysis for viral infection in Fig. 3D. D.E.L. analyzed flow cytometry data. J.Q. and A.M. performed the human complexome study and analyzed the XPO1 data in the complexome. A.P.R. conceived the study, designed, and analyzed experiments. S.B. and A.P.R. wrote the manuscript. We all read and approved the final manuscript.
REFERENCES
- 1.Arhel N, Kirchhoff F. 2010. Host proteins involved in HIV infection: new therapeutic targets. Biochim Biophys Acta 1802:313–321. doi: 10.1016/j.bbadis.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Bushman FD, Malani N, Fernandes J, D'Orso I, Cagney G, Diamond TL, Zhou H, Hazuda DJ, Espeseth AS, Konig R, Bandyopadhyay S, Ideker T, Goff SP, Krogan NJ, Frankel AD, Young JA, Chanda SK. 2009. Host cell factors in HIV replication: meta-analysis of genome-wide studies. PLoS Pathog 5:e1000437. doi: 10.1371/journal.ppat.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goff SP. 2008. Knockdown screens to knockout HIV-1. Cell 135:417–420. doi: 10.1016/j.cell.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malovannaya A, Lanz RB, Jung SY, Bulynko Y, Le NT, Chan DW, Ding C, Shi Y, Yucer N, Krenciute G, Kim BJ, Li C, Chen R, Li W, Wang Y, O'Malley BW, Qin J. 2011. Analysis of the human endogenous coregulator complexome. Cell 145:787–799. doi: 10.1016/j.cell.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutten S, Kehlenbach RH. 2007. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol 17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Bray M, Prasad S, Dubay JW, Hunter E, Jeang KT, Rekosh D, Hammarskjold ML. 1994. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci U S A 91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst RK, Bray M, Rekosh D, Hammarskjold ML. 1997. A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol Cell Biol 17:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin L, Guzik BW, Bor YC, Rekosh D, Hammarskjold ML. 2003. Tap and NXT promote translation of unspliced mRNA. Genes Dev 17:3075–3086. doi: 10.1101/gad.1155703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollard VW, Malim MH. 1998. The HIV-1 Rev protein. Annu Rev Microbiol 52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 10.Karn J, Stoltzfus CM. 2012. Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb Perspect Med 2:a006916. doi: 10.1101/cshperspect.a006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox AH, Lamond AI. 2010. Paraspeckles. Cold Spring Harb Perspect Biol 2:a000687. doi: 10.1101/cshperspect.a000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zolotukhin AS, Michalowski D, Bear J, Smulevitch SV, Traish AM, Peng R, Patton J, Shatsky IN, Felber BK. 2003. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol Cell Biol 23:6618–6630. doi: 10.1128/MCB.23.18.6618-6630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Chen CY, Yedavalli VS, Jeang KT. 2013. NEAT1 long noncoding RNA and paraspeckle bodies modulate HIV-1 posttranscriptional expression. mBio 4(1):e00596–12. doi: 10.1128/mBio.00596-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groom HC, Anderson EC, Lever AM. 2009. Rev: beyond nuclear export. J Gen Virol 90:1303–1318. doi: 10.1099/vir.0.011460-0. [DOI] [PubMed] [Google Scholar]
- 15.Brandt S, Blissenbach M, Grewe B, Konietzny R, Grunwald T, Uberla K. 2007. Rev proteins of human and simian immunodeficiency virus enhance RNA encapsidation. PLoS Pathog 3:e54. doi: 10.1371/journal.ppat.0030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blissenbach M, Grewe B, Hoffmann B, Brandt S, Uberla K. 2010. Nuclear RNA export and packaging functions of HIV-1 Rev revisited. J Virol 84:6598–6604. doi: 10.1128/JVI.02264-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer U, Huber J, Boelens WC, Mattaj IW, Luhrmann R. 1995. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell 82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 18.Fornerod M, Ohno M, Yoshida M, Mattaj IW. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051–1060. doi: 10.1016/S0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 19.Ossareh-Nazari B, Bachelerie F, Dargemont C. 1997. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 20.Wen W, Meinkoth JL, Tsien RY, Taylor SS. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell 82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 21.Hutten S, Kehlenbach RH. 2006. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Mol Cell Biol 26:6772–6785. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. 2004. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki T, Chin WW, Ko L. 2001. Identification and characterization of RRM-containing coactivator activator (CoAA) as TRBP-interacting protein, and its splice variant as a coactivator modulator (CoAM). J Biol Chem 276:33375–33383. doi: 10.1074/jbc.M101517200. [DOI] [PubMed] [Google Scholar]
- 24.Bernad R, van der Velde H, Fornerod M, Pickersgill H. 2004. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol Cell Biol 24:2373–2384. doi: 10.1128/MCB.24.6.2373-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehlenbach RH, Dickmanns A, Kehlenbach A, Guan T, Gerace L. 1999. A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. J Cell Biol 145:645–657. doi: 10.1083/jcb.145.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auboeuf D, Dowhan DH, Li X, Larkin K, Ko L, Berget SM, O'Malley BW. 2004. CoAA, a nuclear receptor coactivator protein at the interface of transcriptional coactivation and RNA splicing. Mol Cell Biol 24:442–453. doi: 10.1128/MCB.24.1.442-453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205–216. doi: 10.1016/S0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 28.Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk HD, Garten W. 1992. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360:358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- 29.Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853–866. doi: 10.1016/S0092-8674(02)01162-5. [DOI] [PubMed] [Google Scholar]
- 30.Natarajan M, Schiralli Lester GM, Lee C, Missra A, Wasserman GA, Steffen M, Gilmour DS, Henderson AJ. 2013. Negative elongation factor (NELF) coordinates RNA polymerase II pausing, premature termination, and chromatin remodeling to regulate HIV transcription. J Biol Chem 288:25995–26003. doi: 10.1074/jbc.M113.496489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterlin BM, Price DH. 2006. Controlling the elongation phase of transcription with P-TEFb. Mol Cell 23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Fang J, Kubota S, Yang B, Zhou N, Zhang H, Godbout R, Pomerantz RJ. 2004. A DEAD box protein facilitates HIV-1 replication as a cellular co-factor of Rev. Virology 330:471–480. doi: 10.1016/j.virol.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Dow EC, Liang YY, Ramakrishnan R, Liu H, Sung TL, Lin X, Rice AP. 2008. Phosphatase PPM1A regulates phosphorylation of Thr-186 in the Cdk9 T-loop. J Biol Chem 283:33578–33584. doi: 10.1074/jbc.M807495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mertz JA, Simper MS, Lozano MM, Payne SM, Dudley JP. 2005. Mouse mammary tumor virus encodes a self-regulatory RNA export protein and is a complex retrovirus. J Virol 79:14737–14747. doi: 10.1128/JVI.79.23.14737-14747.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malim MH, Cullen BR. 1993. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol 13:6180–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF. 2006. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog 2:e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M, Lamond AI. 2002. Paraspeckles: a novel nuclear domain. Curr Biol 12:13–25. doi: 10.1016/S0960-9822(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 38.Daelemans D, Costes SV, Lockett S, Pavlakis GN. 2005. Kinetic and molecular analysis of nuclear export factor CRM1 association with its cargo in vivo. Mol Cell Biol 25:728–739. doi: 10.1128/MCB.25.2.728-739.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naji S, Ambrus G, Cimermancic P, Reyes JR, Johnson JR, Filbrandt R, Huber MD, Vesely P, Krogan NJ, Yates JR III, Saphire AC, Gerace L. 2012. Host cell interactome of HIV-1 Rev includes RNA helicases involved in multiple facets of virus production. Mol Cell Proteomics 11:M111.015313. doi: 10.1074/mcp.M111.015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kula A, Gharu L, Marcello A. 2013. HIV-1 pre-mRNA commitment to Rev mediated export through PSF and Matrin 3. Virology 435:329–340. doi: 10.1016/j.virol.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 41.Auboeuf D, Dowhan DH, Dutertre M, Martin N, Berget SM, O'Malley BW. 2005. A subset of nuclear receptor coregulators act as coupling proteins during synthesis and maturation of RNA transcripts. Mol Cell Biol 25:5307–5316. doi: 10.1128/MCB.25.13.5307-5316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersen JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M, Lamond AI. 2002. Directed proteomic analysis of the human nucleolus. Curr Biol 12:1–11. doi: 10.1016/S0960-9822(01)00650-9. [DOI] [PubMed] [Google Scholar]
- 43.Chen LL, Carmichael GG. 2009. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell 35:467–478. doi: 10.1016/j.molcel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imamura K, Imamachi N, Akizuki G, Kumakura M, Kawaguchi A, Nagata K, Kato A, Kawaguchi Y, Sato H, Yoneda M, Kai C, Yada T, Suzuki Y, Yamada T, Ozawa T, Kaneki K, Inoue T, Kobayashi M, Kodama T, Wada Y, Sekimizu K, Akimitsu N. 2014. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol Cell 53:393–406. doi: 10.1016/j.molcel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Shav-Tal Y, Blechman J, Darzacq X, Montagna C, Dye BT, Patton JG, Singer RH, Zipori D. 2005. Dynamic sorting of nuclear components into distinct nucleolar caps during transcriptional inhibition. Mol Biol Cell 16:2395–2413. doi: 10.1091/mbc.E04-11-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]