ABSTRACT
Hepatitis C virus (HCV) efficiently infects only humans and chimpanzees. Although the detailed mechanisms responsible for this narrow species tropism remain elusive, recent evidence has shown that murine innate immune responses efficiently suppress HCV replication. Therefore, poor adaptation of HCV to evade and/or counteract innate immune responses may prevent HCV replication in mice. The HCV NS3-4A protease cleaves human MAVS, a key cellular adaptor protein required for RIG-I-like receptor (RLR)-dependent innate immune signaling. However, it is unclear if HCV interferes with mouse MAVS function equally well. Moreover, MAVS-dependent signaling events that restrict HCV replication in mouse cells were incompletely defined. Thus, we quantified the ability of HCV NS3-4A to counteract mouse and human MAVS. HCV NS3-4A similarly diminished both human and mouse MAVS-dependent signaling in human and mouse cells. Moreover, replicon-encoded protease cleaved a similar fraction of both MAVS variants. Finally, FLAG-tagged MAVS proteins repressed HCV replication to similar degrees. Depending on MAVS expression, HCV replication in mouse liver cells triggered not only type I but also type III IFNs, which cooperatively repressed HCV replication. Mouse liver cells lacking both type I and III IFN receptors were refractory to MAVS-dependent antiviral effects, indicating that the HCV-induced MAVS-dependent antiviral state depends on both type I and III IFN receptor signaling.
IMPORTANCE In this study, we found that HCV NS3-4A similarly diminished both human and mouse MAVS-dependent signaling in human and mouse cells. Therefore, it is unlikely that ineffective cleavage of mouse MAVS per se precludes HCV propagation in immunocompetent mouse liver cells. Hence, approaches to reinforce HCV replication in mouse liver cells (e.g., by expression of essential human replication cofactors) should not be thwarted by the poor ability of HCV to counteract MAVS-dependent antiviral signaling. In addition, we show that mouse MAVS induces both type I and type III IFNs, which together control HCV replication. Characterization of type I or type III-dependent interferon-stimulated genes in these cells should help to identify key murine restriction factors that preclude HCV propagation in immunocompetent mouse liver cells.
INTRODUCTION
Hepatitis C virus (HCV) infection is associated with chronic liver disease, including hepatic steatosis, fibrosis, cirrhosis, and hepatocellular carcinoma (1). Recent licensing of directly acting antivirals (DAAs) has considerably improved therapeutic options, and novel drug combinations reach cure rates of more than 90% (2). However, natural or treatment-induced virus elimination does not prevent reinfection by HCV. Moreover, many of ca. 160 million infected individuals are not diagnosed, and the vast majority of HCV patients have not been treated (3). Therefore, development of a prophylactic vaccine that efficiently prevents virus transmission is a major challenge for global control of hepatitis C. However, advances in HCV vaccine research are hampered by a lack HCV-permissive, immunocompetent animal models.
HCV, a plus-strand RNA virus and member of the family Flaviviridae, has a narrow species tropism. Besides humans, only chimpanzees are naturally susceptible to chronic HCV infection. However, high costs and ethical concerns severely limit utilization of chimpanzees for research purposes. Thus, understanding of the mechanisms that limit HCV replication in other animals, for instance, mice, is an important step to ultimately develop a robust animal model for HCV. Encouragingly, recent advances have highlighted key prerequisites for HCV infection of murine liver cells. First, occludin and CD81 have been recognized to determine HCV species tropism at the level of viral cell entry (4). Importantly, this blockade can be overcome by engineering mice to express human occludin and CD81 (5) or by adaptation of HCV to usage of mouse CD81 (6). Second, HCV replication in murine embryonic fibroblasts (7, 8), in mouse liver-derived cells (9), and in mouse livers in vivo (10) is heavily impaired by innate immune signaling, since inactivation of host molecules involved in viral RNA sensing, innate immune signaling, or responsiveness to interferons substantially increases HCV replication. Therefore, ablation of distinct innate immune signaling molecules combined with overexpression of essential human entry factors has emerged as a valid strategy to allow HCV propagation in mouse cells in vitro and in vivo (9, 10). However, this environment is only partially immunocompetent, thus limiting utility for immunological studies. Moreover, the efficiency of HCV propagation remains modest either because additional immune control mechanisms curtail HCV replication or because essential human replication cofactors are lacking.
In human cells, the HCV protease NS3-4A interferes with innate immune signaling by cleaving TRIF (TIR domain-containing adaptor-inducing beta interferon [IFN-β]) (11) and MAVS (mitochondrial antiviral signaling protein; also known as IPS-1, VISA, or Cardif) (12), two critical adaptor proteins that link cellular pattern recognition receptors with production of interferons. Nevertheless, viral interference in human cells is not complete, as HCV infection of human liver cells triggers production of both type I and III interferons which partially control HCV replication (13–16). Moreover, distinct human IFN-induced effector proteins relevant for control of HCV replication have been identified (17–19). In contrast, little is known about murine IFN-induced antiviral programs that limit HCV replication. Moreover, the interferon-stimulated genes (ISGs) that establish antiviral defenses against HCV replication in mouse cells are unknown. Finally, the level of interference of HCV with murine innate immune signaling cascades is incompletely defined. Given the importance of innate immunity for control of HCV replication in both the human and murine systems, in this study, we wished to better define the relevance of innate immune control and HCV interference for propagation of HCV in mouse liver cells.
MATERIALS AND METHODS
Reagents.
Mouse IFN-α and IFN-λ3 were purchased from eBioscience and R&D Systems, respectively. Boceprevir and 2′C-methyladenosine (2′CMA) were gifts from Marc Windisch (Institute Pasteur Korea, Seongnam, South Korea) and Tim Tellinghuisen (The Scripps Research Institute, FL), respectively. High-molecular-weight (HMW) poly(I·C) was purchased from InvivoGen. HCV subgenomic replicon (HCV-SGR) RNA was generated in-house by in vitro transcription as described previously (9).
Cell culture and generation of cell lines.
All cells were cultured in Dulbecco's modified Eagle medium (DMEM; Invitrogen) supplemented with 2 mM l-glutamine, nonessential amino acids, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 10% fetal calf serum (FCS) at 37°C and 5% CO2. MLT-MAVS−/− and MLT-IFNAR−/− cells were generated in a previous study (9). Stable cell lines expressing miR122 were created by lentiviral gene transfer as described earlier (9). MLT-MAVS−/− miR122 cells with stable restoration of human MAVS (hMAVS) or mouse MAVS (mMAVS) were created through lentiviral gene transduction followed by passaging in the presence of antibiotic pressure (5 μg/ml of blasticidin) as described previously (9).
The generation of HEK 293T-MAVS−/− cells was done by targeting the critical exons of the MAVS gene in 293T cells using the following transcription activator-like effector nuclease (TALEN) target site: 5′-TTGCTGAAGACAAGACCTAT/AAGTATATCTGCCGC/AATTTCAGCAATTTTTGCAA-3′. TALEN plasmids were assembled as described in reference 3. HEK 293T cells were plated at a density of 2 × 104 cells per well of a 96-well plate. The next day, TALEN plasmids were transfected using GeneJuice transfection reagent (Merck Millipore) at a ratio of 1:1 (100 ng of each plasmid). Subsequently, all-allelic knockout clones were isolated. In short, 14 days after limiting dilution cloning, growing monoclones were selected by bright-field microscopy. Identified clones were trypsinized and expanded in two separate wells. One well was used to recover genomic DNA, and subsequently, the target region of interest was amplified in a two-step PCR and subjected to deep sequencing. Knockout cell clones were identified as cell clones harboring all-allelic frameshift mutations using the OutKnocker webtool at http://www.outknocker.org/. To further confirm the knockout, Western blotting was performed. Note that the faint band visible in Western blotting (see Fig. 2E) is a result of a nonspecific cross-reaction of the antibody used. This was confirmed by further Western blotting and genotyping, which verified proper knockout of MAVS (data not shown). Genotypes of the respective knockout cell lines are available upon request.
FIG 2.
Comparison HCV NS3-4A-dependent cleavage of and interference with hMAVS and mMAVS in mouse cells. (A and B) Human (A) or mouse (B) MAVS was expressed in MLT-MAVS−/− miR122 cells in the presence or absence of GFP or the HCV NS3-4A protease. Expression of proteins was monitored by Western blotting using GFP-, actin-, mMAVS-, or hMAVS-specific antibodies. One day after lentiviral gene transduction, the medium was changed and the indicated cells were treated with addition of 10 μM boceprevir, a protease inhibitor (P.I). Twenty-four hours later, the cells were lysed for specific Western blot analysis. The cleaved (Δ) products of hMAVS and mMAVS are indicated, and β-actin expression was used as a loading control. Images are representative of two individual experiments. (C and D) Effect of HCV NS3-4A protease expression on human (C) or mouse (D) MAVS-dependent type I IFN induction. One day after the cells were transduced, the cells were either mock transfected or transfected with 10 μg/ml of poly(I·C). Four hours later, total RNA was extracted and the relative levels of mIFN-β mRNA expression were determined by qRT-PCR. Data were normalized to the mGAPDH mRNA data. Asterisks show a significant difference between the indicated data. Mean values ± SDs from three independent experiments are given.
Mice.
Normal C57BL/J6 and BALB/c mice were purchased from Charles River Labs. C57BL/6 mice lacking functional receptors for type I and III IFN (20) were bred at University Medical Center Freiburg. Mouse experiments were carried out using animals between 6 and 12 weeks of age. Mice were housed under specific-pathogen-free conditions at the Twincore animal facility, and all experiments were conducted according to institutional guidelines for animal care and use and in compliance with regulations of German animal welfare law.
In vivo immortalization and generation of tumor cell lines.
Intrahepatic tumors were induced in IFNAR−/− IFNLR1−/− double-knockout mice (20) by retrograde liver transduction with oncogenic transposon plasmids using hydrodynamic injection. For this purpose, mice received 30 μg of total plasmid DNA (8 μg of pT/KRas-G12V, 8 μg of pT3/EF1α-myrAkt1, 8 μg of pT3/EF1α-shRp53, and 6 μg of pPGK-SB13). A more detailed description of the used plasmids and plasmid construction can be found elsewhere (9). The pPGK-SB13 plasmid for expression of Sleeping Beauty SB13 was kindly provided by David A. Largaespada (University of Minnesota). For tumor induction, oncogenic transposon plasmids were injected into mice in 0.9% saline at a final volume of 10% of the animal's body weight via tail vein injection within 5 s. Once tumors became palpable (5 to 12 weeks postinjection), mice were sacrificed and tumors were harvested for subsequent isolation of immortalized cell lines. Isolated tumors were dissected and incubated for 30 min at 37°C in RPMI 1640 plus GlutaMAX supplemented with 200 μg/ml of collagenase IA, collagenase IV, and hyaluronidase IV, 300 μg/ml of dispase, and 50 μg/ml of DNase I (Sigma-Aldrich). Separated cells were purified using a 40-μm strainer, washed once, and then cultivated in DMEM plus GlutaMAX with 10% FCS and penicillin-streptomycin at 37°C in 5% CO2. Cells were then cultivated as described previously (9). Deletions of IFNAR and IFNLR1 genes were confirmed by quantitative reverse transcription-PCR (qRT-PCR).
PMH isolation.
Primary mouse hepatocytes (PMHs) were isolated from BALB/cJ and/or C57BL/6J mice by a modified 2-step Liberase perfusion (21, 22). Briefly, mice were anesthetized using ketamine (Albrecht) and Rompun (Bayer). The body cavity was opened and a catheter was placed into the portal vein and connected to a flow pump, which pumped media prewarmed at 37°C from a water bath into the catheter. The liver was first perfused with Earle's balanced salt solution (EBSS) (GIBCO) solution containing 0.5 mM EGTA (Sigma-Aldrich) and 10 mM HEPES buffer (Sigma). Subsequently, EBSS supplemented with 10 mM HEPES buffer and Liberase (Roche) at 100 μg/liter was applied for enzymatic digestion of the tissue at 37°C. After digestion for 10 to 12 min, the liver was carefully disconnected and tissue was manually disrupted with a sterile scissors and scalpel in DMEM (GIBCO Germany) containing 10% FCS. The suspended hepatocytes were passed through a 100-μm nylon filter into 50-ml Falcon tubes. The cell suspensions were centrifuged twice at 300 rpm for 5 min at 4°C, and the cell pellet was resuspended in ice-cold DMEM containing 10% FCS (PAN Biotech). Cell viability was tested by trypan blue (Fluka).
Transient HCV luciferase replication assay.
Methods for transfection of HCV-SGR RNA are described elsewhere (9). Luciferase activity of replicating HCV-SGR was analyzed as described elsewhere (9, 23).
Lentiviral pseudoparticle production.
Lentiviral, HIV-based pseudotypes were created essentially as described previously (9). Briefly, HEK 293T cells were transfected with envelope glycoprotein expression construct pcz-VSV-G, lentiviral gag-pol expression construct pCMVΔR.74, and lentiviral genomic backbone for the desired gene expression using a polyethyleneimine (PEI) method (Carl Roth).
Plasmids.
The lentiviral plasmid pWPI-JFH1-NS3-4A-BLR encodes the NS3-4A protease of HCV strain JFH1. The lentiviral plasmids pWPI-hMAVS-BLR, pWPI-mMAVS-BLR, pWPI-HA-hMAVS-GUN, and pWPI-HA-mMAVS-GUN encode human or mouse MAVS variants with or without a hemagglutinin (HA) tag. Lentiviral plasmid pWPI-mIFNLR1-BLR encodes the mouse ortholog of a high-affinity type III IFN receptor (NM174851.3). All pWPI constructs were cloned in-house with detailed cloning strategies that are available upon request. The plasmid pTRIP-pre-miR-122-puro was described previously and given as a kind gift from Matthew Evans (Mount Sinai School of Medicine, NY) (24). The IFN-β promoter reporter plasmid (pGL3b-IFN-β promoter-Luc) was kindly provided by Stefan Lienenklaus (HZI, Braunschweig, Germany).
Western blotting.
Cells were washed with phosphate-buffered saline (PBS) and lysed in RIPA buffer (0.3 M NaCl, 20 mM Tris-HCl [pH 8], 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 1% Triton X-100) for 30 min on ice. Total protein content was determined by the Bradford assay. Equal protein amounts for each sample were mixed with 2× denaturing protein sample buffer (200 mM Tris-HCl [pH 8.8], 5 mM EDTA, 0.1% bromophenol blue, 10% sucrose, 3.3% SDS, 2% 2-mercaptoethanol [2-ME]), heated for 5 min at 98°C, loaded onto an 11% SDS gel, and resolved by electrophoresis. Subsequently, proteins were transferred to a polyvinylidene difluoride membrane which was then blocked with 5% milk in PBS containing 0.5% Tween (PBS-T) for 1 h at room temperature. The membrane was then incubated with either anti-NS3337 tag (1:300), anti-HA tag (1:1,000; Covance), anti-FLAG tag (1:1,000; Sigma-Aldrich), anti-hMAVS or anti-mMAVS (both at 1:300; Santa Cruz), anti-green fluorescent protein (anti-GFP, 1:1,000; Santa Cruz), or anti-β-actin (1:1,000; Sigma-Aldrich), followed by incubation with secondary antibody coupled to horseradish peroxidase (Sigma-Aldrich). Bound antibodies were detected with the ECL Plus detection system (GE Healthcare).
Quantitative reverse transcription-PCR.
Total RNA was extracted using the Nucleospin RNA II kit (Macherey-Nagel) by following the manufacturer's protocol. Subsequently, 2 μl was reverse transcribed into cDNA using the PrimeScript First Strand cDNA synthesis kit (TaKaRa), and PCR was carried out using a 400 nM concentration of primers together with SYBR Premix Ex Taq (TaKaRa) and was quantified with a LightCycler480 (Roche). Murine glyceraldehyde-3-phosphate dehydrogenase (mGAPDH) mRNA levels were always quantified in parallel. Primers used this study are as follows: for mouse IFN-β, CTGCGTTCCTGCTGTGCTTCTCCA (sense) and TTCTCCGTCATCTCCATAGGGATC (antisense) (25); for mouse IFN-λ2/3, AGCTGCAGGCCTTCAAAAAG (sense) and TGGGAGTGAATGTGGCTCAG (antisense) (26); for mouse GAPDH, TTCACCACCATGGAGAAGGC (sense) and GGCATCGACTGTGGTCATGA (antisense) (25); for mouse viperin, AACCCCCGTGAGTGTCAACTA (sense) and AACCAGCCTGTTTGAGCAGAA (antisense) (27); for mouse ISG15, AGCAATGGCCTGGGACCTAAA (sense) and AGCCGGCACACCAATCTT (antisense) (28); and for mouse IFIT1, CAGAAGCACACATTGAAGAA (sense) and TGTAAGTAGCCAGAGGAAG (antisense) (29).
Immunofluorescence.
HCV replication was visualized by indirect immunofluorescence as described previously (23). HCV antigen-expressing cells were detected using the mouse monoclonal NS5A-9E10 antibody (kindly provided by Charles M. Rice, Center for the Study of Hepatitis C, The Rockefeller University, NY) at a dilution of 1:1,000. Bound primary antibodies were detected using goat anti-mouse IgG-specific secondary antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 546 (Sigma-Aldrich) at a dilution of 1:1,000. Nuclear DNA was stained using 4′,6-diamidino-2-phenylindole (DAPI) at a dilution of 1:3,000.
Statistical methods.
GraphPad Prism 6 software was used for data analysis using a one-way analysis of variance (ANOVA) adjusted with Bonferroni's multiple-comparison test. P values of <0.05 (single asterisks in figures) were considered statistically significant, whereas P values of <0.01 (double asterisks) and <0.001 (triple asterisks) were considered highly significant.
RESULTS
mMAVS restricts HCV replication in MLT cells.
Previously, we have reported that HCV replicons and full-length RNAs efficiently propagate in transfected mouse liver-derived tumor (MLT) cells which lack endogenous expression of MAVS (MLT-MAVS−/− miR122 cells) (9). These cells therefore provide a unique environment to test the importance of mouse MAVS (mMAVS) for restricting HCV replication and to explore the ability of HCV to interfere with mMAVS function.
To this end, we confirmed the absence of endogenous mMAVS expression in these cells and subsequently restored mMAVS expression by lentiviral gene transfer. Murine Hep56.1D cells and human Huh-7.5 cells served as controls for specific detection of mMAVS in our protein expression analysis (Fig. 1A). Parental MLT-MAVS−/− miR122 cells were not responsive to transfection of poly(I·C), a mimic of double-stranded RNA which triggers RIG-I-like receptor (RLR [30])-dependent signaling via MAVS to produce of IFN-β mRNA (Fig. 1B). In contrast, restoration of mMAVS expression in these cells (Fig. 1A) resulted in upregulation of IFN-β mRNA upon transfection of poly(I·C) to a level similar to the one observed in poly(I·C)-transfected Hep56.1D cells (Fig. 1B). Thus, we concluded that repair of mMAVS expression in MLT-MAVS−/− miR122 cells restored the sensitivity of these cells to RLR-dependent recognition of viral double-stranded RNA comparable to that of cells expressing endogenous levels of mMAVS (e.g., Hep56.1D). Next, we analyzed the influence of reintroducing mMAVS on permissiveness for HCV replication. To this end, cells were transfected with a subgenomic JFH1 replicon expressing luciferase or a control luciferase replicon with an inactivating mutation in the viral NS5B polymerase. HCV replication in transfected cells was monitored by luciferase assays (Fig. 1C) and indirect immunofluorescence (Fig. 1D). As reported previously (9), parental MLT-MAVS−/− miR122 cells sustained vigorous HCV RNA replication, as evidenced by rapid accumulation of luciferase activity (Fig. 1C) and by detection of numerous NS5A-expressing cells (Fig. 1D). In contrast, reintroduction of mMAVS ablated HCV replication to background levels, with luciferase activity indistinguishable from that of cells transfected with the replication-incompetent viral RNA (Fig. 1C) and with no NS5A-positive cells detectable (Fig. 1D). Therefore, repair of mMAVS expression in MLT-MAVS−/− miR122 cells efficiently restored innate immune signaling and restricted HCV RNA replication.
FIG 1.
mMAVS suppresses HCV replication in transfected mouse liver-derived tumor (MLT) cells. (A) Detection of mMAVS and hMAVS proteins in given human and murine cell lines using MAVS-specific antibodies. Detection of β-actin expression was used as a loading control. (B) Responsiveness of MLT-MAVS−/− miR122 cells with or without mMAVS expression to transfection of poly(I·C). Hep56.1D mouse cells expressing endogenous levels of mMAVS served as a control. A day after seeding, the indicated cells were either left untreated, mock transfected, or transfected with 10 μg/ml of poly(I·C). Four hours later, total cellular RNA was collected and the expression of mIFN-β mRNA was determined by RT-PCR. Expression of IFN-β mRNA was normalized to endogenous levels of mGAPDH and is given relative to the basal expression in Hep56.1D cells. Asterisks show a significant difference between the indicated data. (C) Transient replication of HCV-JFH1 subgenomic replicon with luciferase reporter with an active NS5B polymerase (HCV-SGR) or a ΔGDD polymerase mutant [HCV-SGR (−) Polymerase] in given cell lines. Transfected cells were harvested at the indicated time points, and HCV RNA replication was determined by luciferase assays. RLU, relative light units; h.p.t, hours posttransfection. (D) Detection of HCV NS5A expression (red) in transfected cells by indirect immunofluorescence (IF) analysis 48 h posttransfection. Nuclei were stained with DAPI (blue). All graph data are shown as mean values ± SDs from three independent experiments, and images are representative of three individual experiments.
HCV interferes with mouse and human MAVS to similar levels.
The above-described findings suggested that HCV replication is efficiently restricted by mMAVS potentially because the HCV NS3-4A protease fails to efficiently cleave and interfere with mMAVS function. In turn, inefficient HCV interference with mMAVS in mouse cells may explain why HCV is unable to efficiently replicate in mouse cells with active innate immune signaling.
To test these hypotheses, we compared HCV interference with hMAVS and mMAVS. First, we stably expressed either human or mouse MAVS in MLT-MAVS−/− miR122 cells (Fig. 2), followed by transient expression of the NS3-4A protease via lentiviral gene transduction. This method was chosen to mimic the expression of the protease in viral invasion independent of constraints on HCV RNA replication (e.g., due to lack of HCV replication-assisting cofactors). Both hMAVS (Fig. 2A) and mMAVS (Fig. 2B) were partially cleaved upon transient coexpression of NS3-4A in MLT-MAVS−/− miR122 cells. This is either due to incomplete transduction of the cell populations with the HCV protease or due to incomplete cleavage of overexpressed MAVS proteins under these experimental conditions. Cleavage was specifically mediated by the viral protease, since coexpression of GFP did not induce cleavage and as addition of a protease inhibitor reduced cleavage of both hMAVS and mMAVS (Fig. 2A and B). When these cells were transfected with poly(I·C), coexpression of the HCV NS3-4A protease clearly lowered both hMAVS- and mMAVS-dependent production of IFN-β (Fig. 2C and D). Since the extents to which IFN-β production was suppressed were comparable between NS3-4A coexpressed with hMAVS and mMAVS, we conclude that in this system the HCV protease interferes with the function of these two MAVS proteins to similar levels. To exclude that species-specific signaling activity of hMAVS in mouse cells may mask differential HCV interference, we conducted principally similar cotransfection experiments with human HEK 293T cells, which upon transcription activator-like effector nuclease (TALEN)-mediated knockout of hMAVS lack endogenous expression of the adaptor protein (Fig. 3A). Like in the mouse cells, we noted HCV NS3-4A-dependent cleavage of both MAVS species (Fig. 3A and B). The appearance of partial cleavage of MAVS was mainly caused by the cotransfection efficiency issue, as not all cells were equally transfected with equal amounts and ratios of plasmids. Finally, using an IFN-β promoter-dependent luciferase reporter plasmid, we observed 5- to 10-fold-greater suppression of poly(I·C)-dependent IFN-β promoter activity by expression of the NS3-4A protease in the case of mMAVS than with hMAVS (Fig. 3C and D). Collectively, these results indicate that the HCV NS3-4A protease cleaves and interferes with the function of both mMAVS and hMAVS in human and mouse cells. Moreover, interference with the murine MAVS ortholog was not inferior to that with the human MAVS protein.
FIG 3.
Comparison HCV NS3-4A-dependent cleavage of and interference with hMAVS and mMAVS in human cells. (A and B) Appearance of human (A) or mouse (B) MAVS expression in the presence of HCV protease in human HEK 293T-MAVS−/− miR122 cells. The cells were reverse transfected with reporter plasmids (100 ng) together with vector expressing each indicated gene (100 ng). A day after transfection, the medium was changed and the indicated cells were treated with addition of 10 μM boceprevir, a protease inhibitor (P.I). Twenty-four hours later, the cells were lysed and specific Western blotting detection using the indicated antibodies was performed. The cleaved (Δ) products of hMAVS and mMAVS are indicated, and β-actin expression was used as a loading control. Images are representative of two individual experiments. (C and D) Effect of HCV protease presence on human (C) or mouse (D) MAVS-dependent IFN-β promoter regulation. HEK 293T-MAVS−/− miR122 cells were reverse transfected with reporter plasmids (100 ng) together with the each indicated expression vector (100 ng). A day after the cells were reverse transfected, the medium was changed and the indicated cells were transfected with either water (mock) or 10 μg/ml of poly(I·C) for 4 h. After that, the cells were treated with addition of water or 10 μM P.I. The cells were then lysed and assayed for luciferase activities 24 h later. Values were then normalized to those for MAVS-GFP-transfected cells. Asterisks show a significant difference between the indicated data. All graph data are shown as mean values ± SDs from three independent experiments, and Western blotting images are representative of two individual experiments.
To investigate whether NS3-4A expressed from an autonomously replicating HCV RNA is sufficient to overcome restriction by hMAVS or mMAVS in MLT-MAVS−/− miR122 cells, we transfected a subgenomic HCV luciferase replicon into parental MLT-MAVS−/− miR122 cells or derivative cell lines stably expressing these MAVS variants. Irrespective of whether hMAVS or mMAVS was present, luciferase activity did not rise over the background level defined by the replication-inactive replicon mutant (Fig. 4A). In parallel, only very inefficient cleavage of both MAVS variants was observed (Fig. 4B), which likely results from initial production of viral protease translated from the input of transfected viral RNA. Taken together, these results indicate that HCV replication was suppressed to background levels in cells overexpressing either hMAVS or mMAVS.
FIG 4.
Control of HCV replication in mouse liver cells by human and mouse MAVS. (A) Replication of HCV in in MLT-MAVS−/− miR122 cells stably expressing hMAVS or mMAVS. Luciferase replicon RNA was transfected into the given cell lines and RNA replication was determined at the given time points by way of luciferase assays. A replicon with inactive polymerase transfected into MLT-MAVS−/− miR122 cells served as a control. (B) Cleavage of hMAVS and mMAVS by replicon-encoded NS3-4A protease. MAVS cleavage was monitored 48 h after mock transfection (N) or HCV-SGR transfection (S) into MLT-MAVS−/− miR122 cells stably expressing hMAVS and mMAVS. (C) Schematic representation of the experimental setup used to titrate MAVS abundance. The HCV replicon (HCV-SGR) was transfected into target cells (MLT-MAVS−/− miR122 cells) to establish HCV replication. Twenty-four hours later, cells were reseeded into 12-well plates. At 48 h posttransfection, the cells were transduced with equal titers of lentiviruses carrying the gene of interest. +, low-titer dose; ++, middle-titer dose; +++, high-titer dose. After another 48 h, expression of the gene of interest and HCV RNA replication were measured. (D) Dose-dependent expression of FLAG-tagged MAVS variants. hMAVS C508R and mMAVS C470R represent hMAVS and mMAVS variants resistant to cleavage by the HCV NS3-4A protease (E) Dose-dependent repression of HCV RNA replication by FLAG-tagged MAVS variants. Given MAVS proteins were introduced into MLT-MAVS−/− miR122 cells carrying a luciferase replicon.as described for panel C. HCV RNA replication was measured by luciferase activity and normalized to the data for cells that were transduced to express GFP. Asterisks show a significant difference between the indicated data. All graph data are shown as mean values ± SDs from three independent experiments, and Western blotting images are representative of two individual experiments.
Considering that overexpression of MAVS may preactivate cellular innate immune responses prior to transfection, thus ablating HCV replication, we chose an alternative experimental setting: MAVS proteins were introduced into MLT-MAVS−/− miR122 cells engineered to express a replicating HCV luciferase replicon (Fig. 4C). In these experiments, the degree of MAVS overexpression was modulated by serially diluting the lentiviral stocks used for transduction of MAVS proteins (Fig. 4D). Interestingly, both mMAVS and hMAVS restricted HCV replication in a dose-dependent fashion (Fig. 4E), as evidenced by ca. 85% reduction of luciferase activity at the highest dose of MAVS expression compared with the overexpression of GFP. Notably, the two MAVS proteins were overexpressed to similar levels, as was evident from our protein expression analysis utilizing FLAG-specific antibodies to detect the FLAG-tagged hMAVS and mMAVS proteins transfected in these experiments (Fig. 4D). Congruently, transfection of wild-type, untagged hMAVS and mMAVS proteins resulted in comparable and dose-dependent repression of HCV replication in MLT-MAVS−/− miR122 replicon cells (data not shown). Finally, FLAG-tagged hMAVS and mMAVS variants that are resistant to cleavage by the HCV NS3-4A protease due to mutation of the cleavage site exerted a further increased repression on HCV replication compared to that with the parental MAVS proteins that are susceptible to cleavage by HCV (ca. 3-fold) (Fig. 4E). These results indicate that the ability of HCV to cleave MAVS facilitates HCV replication. Furthermore, since the degrees of restriction exerted by parental MAVS and the cleavage-resistant MAVS variant were in each case indistinguishable between the human and the mouse orthologs, we conclude that HCV is capable of interfering with mouse MAVS to a level similar to that of the human variant. As a consequence, it is unlikely that inefficient viral cleavage of mouse MAVS is the critical limitation that prevents HCV from propagating in mouse liver cells with intact innate immune signaling.
HCV replication in mouse liver tumor cells elicits production of both type I and III IFNs in a MAVS-dependent fashion.
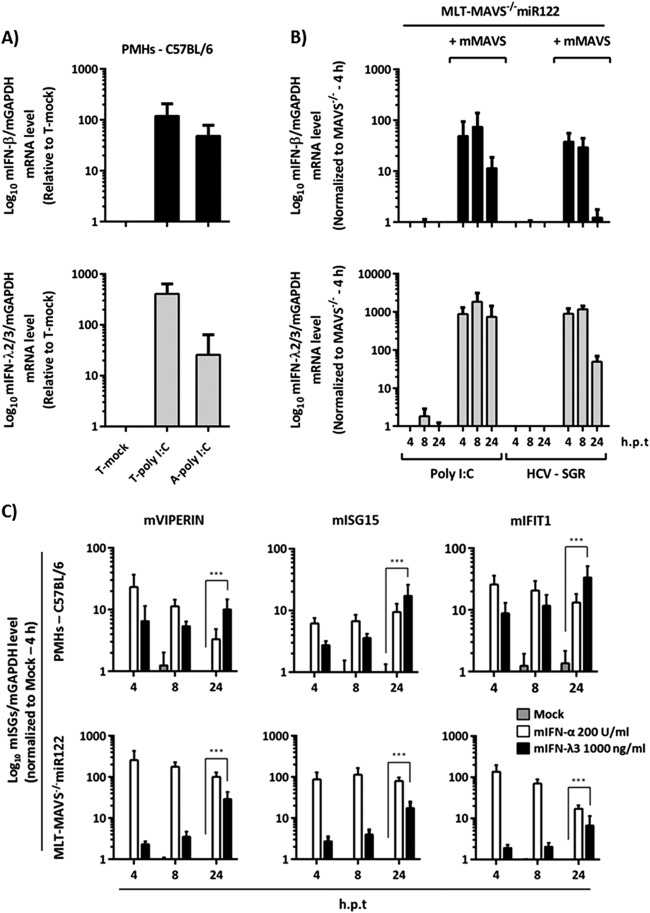
Next, we dissected the mMAVS-regulated downstream signaling events responsible for limiting HCV replication in mouse cells. To this end, we first explored which type of IFN is produced upon administration of poly(I·C) or after transfection of HCV RNA. Using mIFN-β (type I IFN)-specific and mIFN-λ (type III IFN)-specific mRNA detection systems, we observed that both types of IFNs were vigorously upregulated upon extracellular addition or transfection of poly(I·C) to primary mouse hepatocytes (Fig. 5A). In the case of MLT-MAVS−/− miR122 cells, repair of mMAVS expression led to rapid and strong induction of mIFN-β and mIFN-λ mRNA upon transfection of poly(I·C) (Fig. 5B). Likewise, transfection of HCV replicon RNA also induced both types of IFNs (Fig. 5B), indicating that HCV replication in MLT-MAVS−/− miR122 elicits type I and III IFN production in a mMAVS-dependent fashion.
FIG 5.
mMAVS-dependent induction of type I and III IFN mRNA in mouse liver cells. (A) Relative mRNA expression of mouse type I (mIFN-β) and III IFN (mIFN-λ2/3) in wild-type C57BL/6 mouse-derived primary hepatocytes. The primary cells were treated with 50 μg/ml of poly(I·C) either by transfection (T-poly I:C) or by direct addition to the culture medium (A-poly I:C). Eight hours later, total RNA from the each group of treated cells was extracted. (B) Induction of mIFN-β and mIFN-λ2/3 mRNA in MLT-MAVS−/− miR122 cells with or without mMAVS. The indicated cells were transfected with 50 μg/ml of poly(I·C) or with 10 μg of HCV replicon RNA. At the indicated time points, transfected cells were collected and total RNA was extracted. The level of IFN mRNA expression was normalized to that of mGAPDH mRNA as determined by qRT-PCR. h.p.t, hours posttransfection. (C) Induction of representative mouse interferon-stimulated genes (mISGs) in wild-type C57BL/6 mouse PMHs and in MLT-MAVS−/− miR122 cells treated with the indicated doses of exogenous mouse type I (mIFN-α) or III (mIFN-λ3) IFN. At the indicated time points, cells were harvested and total RNA was extracted. mISG mRNA levels are expressed relative to mGAPDH mRNA as determined by qRT-PCR. Asterisks show a significant difference between the indicated data. All graph data are shown as mean values ± SDs from three independent experiments.
Both type I and III IFNs control HCV replication in mouse liver tumor cells.
To assess if both types of IFN contribute to control of HCV replication in these cells, we first investigated if both primary mouse hepatocytes and MLT-MAVS−/− miR122 cells are responsive to these IFNs. To this end, we incubated these cells with exogenously added mouse IFN-α or mouse IFN-λ3 and monitored upregulation of the mRNAs of typical interferon-stimulated genes (ISGs). All three ISGs tested by us were clearly induced upon administration of either mIFN-α or mIFN-λ, indicating that these cells are responsive to both type I and III IFNs (Fig. 5C). To explore the relevance of both types of IFNs for control of HCV replication in mouse liver-derived cells, we established a novel MLT cell line lacking both type I and III IFN receptors (MLT-IFNAR−/− IFNLR1−/− miR122) (Fig. 6A). When transfected with an HCV luciferase replicon, these cells sustained vigorous HCV RNA replication similar to that with MLT-MAVS−/− miR122 cells and somewhat greater than with MLT cells lacking endogenous expression of the type I IFN receptor alone (MLT-IFNAR−/− miR122 [9]) (Fig. 6B). This observation was also corroborated by analysis of NS5A expression in the transfected cells showing larger numbers of NS5A-positive cells in the case of MAVS knockout and IFNAR plus IFNLR1 double knockout than with the IFNAR knockout alone (Fig. 6C). Therefore, inactivation of MAVS or both type I and III IFN receptors together renders MLT cells highly permissive to HCV RNA replication. To confirm that the latter cells lack functional type I and III IFN receptors, we treated them with exogenous mIFN-α or different doses of mIFN-λ3 and used the nucleoside polymerase inhibitor 2′C-methyladenosine (2′CMA) as a control. As expected, the polymerase inhibitor reduced HCV replication in all three above-mentioned cell lines (Fig. 6D). In contrast, mIFN-λ3 was antiviral in MLT-MAVS−/− miR122 and MLT-IFNAR−/− miR122 cells, and mIFN-α was active only in MLT-MAVS−/− miR122 cells. Importantly, HCV replication in MLT-IFNAR−/− IFNLR1−/− miR122 cells was fully resistant to exogenous administration of both type I and III IFN, confirming the absence of both IFN receptors (Fig. 6D). Finally, we repaired expression of IFNLR1 in the MLT cells with double knockout of type I and III IFN receptors using lentiviral gene transfer and monitored HCV replication in the presence or absence of mIFN-λ3 (Fig. 6E). Reintroduction of the type III IFN receptor chain reduced HCV replication ca. 3-fold. Moreover, it also restored responsiveness of these cells to mIFN-λ3, as addition of this type of IFN further repressed HCV replication to a level ca. 10-fold lower than in mIFN-λ3-treated cells in the absence of IFNLR1 expression (Fig. 6E). Collectively, these observations indicate that HCV replication in MLT cells is susceptible to suppression by both exogenous type I and III IFNs.
FIG 6.
Regulation of HCV-SGR replication in mouse cells by mouse type III IFNs. (A) Phenotypic appearances of all MLT cells. (B) Transient replication of the HCV luciferase replicon without polymerase activity in MLT-MAVS−/− miR122, MLT-IFNAR−/− miR122, and MLT-IFNAR−/− IFNLR1−/− miR122 cells. At the indicated time points posttransfection, HCV RNA replication was determined by luciferase activity. (C) Indirect immunofluorescence analysis of NS5A expression in given cell lines 48 h posttransfection. The HCV-NS5A protein was detected by specific antibody (9E10), and cell nuclei were stained with DAPI. (D) Effect of exogenously added mouse type I and III IFNs (mIFNa and mIFN-λ3, respectively) on HCV replication in given MLT cells. Forty-eight hours after administration of the given drugs, HCV RNA replication was determined by luciferase assays. Luciferase activity was normalized to that measured in control cells that were mock treated. Asterisks indicate a significant difference compared to each related mock group. (E) Repair of type III IFN receptor expression restores responsiveness of MLT-IFNAR−/− IFNLR1−/− miR122 cells to antiviral effect mediated by mIFN-λ3. Given MLT cells carrying a luciferase replicon were transduced to express GFP or the mIFNLR1 cDNA. Subsequently, cells were treated as indicated with 2′CMA or mIFN-λ3. Luciferase activity was determined 48 h later and is expressed relative to that in mock-treated and untransduced cells. Asterisks show a significant difference between the indicated data. All graph data are shown as mean values ± SDs from three independent experiments; images are representative of two individual experiments.
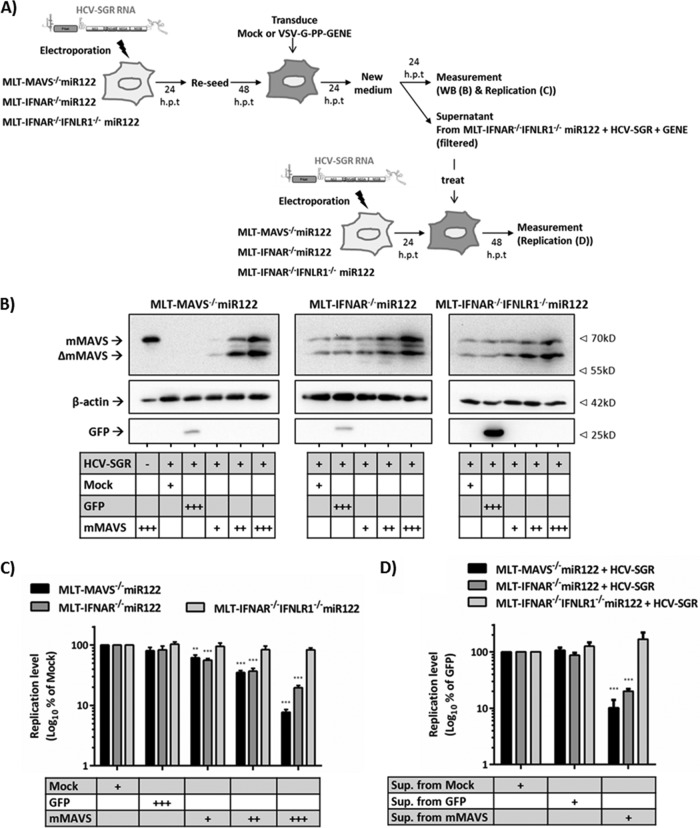
Finally, we explored if MAVS-dependent upregulation of endogenous type I and III IFNs limits HCV replication in the MLT culture system by using the experimental setup detailed in Fig. 7A. To this end, we transduced mMAVS into MLT-MAVS−/− miR122, MLT-IFNAR−/− miR122, or MLT-IFNAR−/− IFNLR1−/− miR122 cells that had been transfected with an HCV replicon. Subsequently, we monitored MAVS cleavage by HCV (Fig. 7B) and the influence of mMAVS overexpression on HCV replication by using luciferase assays (Fig. 7C). As expected, lentiviral gene transfer resulted in dose-dependent expression of mMAVS in all transduced cell lines and HCV NS3-4A partially cleaved overexpressed or endogenous mMAVS (Fig. 7B). Reintroduction of mMAVS into MLT-MAVS−/− miR122 cells, which endogenously express both functional type I and III IFN receptors (Fig. 6), suppressed HCV-dependent luciferase activity in a dose-dependent fashion down to less than 10% at the highest dose of mMAVS expression (Fig. 7C). Notably, mMAVS overexpression also suppressed HCV replication in MLT cells lacking the type I IFN receptor, albeit with modestly reduced efficacy (Fig. 7C). In contrast, in cells that lack both type I and III IFN receptors, overexpression of mMAVS no longer was antiviral, as evidenced by stable high-level luciferase expression irrespective of the level of overexpressed mMAVS (Fig. 7C). To examine if the antiviral activity was caused by mMAVS-dependent secretion of both type I and III IFNs, we assessed the bioactivity of the supernatants collected from the mMAVS-overexpressing MLT-IFNAR−/− IFNLR1−/− miR122 cells. To this end, these supernatants were filtered and passed onto MLT-MAVS−/− miR122, MLT-IFNAR −/− miR122, or MLT-IFNAR−/− IFNLR1−/− miR122 cells that had been transfected with an HCV replicon (Fig. 7D). The conditioned supernatants could suppress de novo HCV-dependent luciferase activity only in the MLT-MAVS−/− miR122 and MLT-IFNAR−/− miR122 cells and not in the MLT cells lacking both IFN receptors (Fig. 7D), confirming the secretion of bioactive type I and III IFNs. Taken together, these data indicate that HCV replication in mouse-derived liver cells triggers mMAVS-dependent signaling which upregulates both type I and III IFNs, which, in turn, suppress HCV replication.
FIG 7.
mMAVS-dependent secreted type I and III IFNs cooperatively restrict HCV replication in mouse liver cells. (A) Schematic representation of the experimental setup. First, the HCV replicon (HCV-SGR) was transfected into target cells to establish HCV replication. Twenty-four hours later, cells were reseeded into 12-well plates. Forty-eight posttransfection, the cells were mock transduced or challenged with matched titers of lentiviruses carrying either GFP or mMAVS using three different doses. +, low-titer dose; ++, middle-titer dose; +++, high-titer dose. At the desired time point, expression of the gene of interest was measured by Western blotting (B) and dose-dependent repression of HCV replication in given cell lines by mMAVS expression was measured by luciferase assays (C). Data are expressed relative to data for the mock-treated controls, and asterisks indicate a significant difference compared to each related mock treatment. (D) To test the activity of secreted IFN, the supernatants from the mock-transduced or gene-of-interest (+++)-transduced MLT-IFNAR−/− IFNLR1−/− miR122 cells with replicating HCV-SGR were collected, filtered with a 0.45-mm filter, and then used to treat de novo HCV-SGR replication in the indicated cells. Forty-eight hours later, the HCV RNA replication was measured by luciferase assays. Data are expressed relative to those for the mock-treated controls, and asterisks indicate a significant difference compared to each related mock group. All graph data are shown as mean values ± SDs from three independent experiments, and Western blotting images are representative of two individual experiments.
DISCUSSION
In this study, we showed that mMAVS-dependent innate immune signaling potently restricts HCV replication in mouse liver cells by way of inducing both type I and III IFN production. These results refine and extend previous in vitro and in vivo studies highlighting the fact that ablation of host proteins involved in innate immune signaling facilitates HCV replication in mouse cells (7–10). Notably, mMAVS does potently restrict HCV replication in mouse cells in spite of its susceptibility to cleavage by the CV NS3-4A protease, which was reported recently (31, 32). However, it remained undetermined if the efficiency of cleavage paralleled that of hMAVS and if expression of mMAVS poses a greater degree of restriction on HCV replication than its human ortholog.
Therefore, we quantitatively and qualitatively compared the cleavage efficiencies of hMAVS and mMAVS by the HCV NS3-4A protease both in human and in mouse cells. Moreover, we explored to what level HCV interferes with the signaling activities of both proteins and how efficiently these two orthologs restrict HCV replication in mouse liver cells. Overexpressed HCV NS3-4A similarly diminished hMAVS- and mMAVS-dependent signaling in mouse (Fig. 2) and human (Fig. 3) cells. Moreover, replicon-encoded protease cleaved similar fractions of overexpressed hMAVS and mMAVS, as was evident from detection of these proteins via a FLAG tag (Fig. 4). Therefore, although species-specific functioning of hMAVS and mMAVS in human and mouse cells cannot be fully ruled out, these analyses strongly suggest that in the human and mouse cells tested by us, cleavage and viral interference with hMAVS and mMAVS were comparable.
These novel findings have implications relevant to the development of immunocompetent mouse models for HCV. First, it is unlikely that inefficient mMAVS cleavage by HCV per se prevents efficient HCV replication in mouse liver cells. Therefore, MAVS function apparently does not pose an insurmountable barrier to HCV replication. Of note, in human cells, HCV NS3-4A also cleaves human TRIF, which transmits Toll-like receptor 3 (TLR-3)-dependent signaling for production of IFN (11). Since there is controversy about whether mouse TRIF is susceptible to cleavage by HCV (32, 33), it is possible that poor adaptation of HCV to interfere with TRIF-dependent signaling prevents efficient HCV replication in mouse cells. While resolving this question merits further work, it is also possible that lack of human replication cofactors limits replication and accumulation of HCV proteins to establish sufficient interference with innate immunity. If these cofactors were known, their ectopic expression in the mouse environment may reinforce HCV replication so that artificial ablation of MAVS-dependent (and/or TRIF-dependent) innate immunity is not necessary. The cell lines described in this study should be a useful platform to screen for such human replication cofactors, for instance, by cDNA library-based approaches.
An alternative approach to establish (pseudo)immunocompetent mouse models for HCV may be to selectively eliminate distinct IFN-expressed antiviral effector proteins to circumvent global elimination of central innate immunity signaling molecules. This study indicates that in the context of MLT cells, both type I- and III-dependent antiviral effector mechanisms are triggered by HCV replication which cooperate to efficiently suppress HCV replication. This conclusion is based on our observations that HCV RNA triggers both mouse IFN-β and IFN-λ mRNA expression (Fig. 5) and that HCV replication in MLT cells can be suppressed by exogenous administration of both type I and III IFNs (Fig. 6). Moreover, we observed that MAVS signaling-dependent suppression of HCV replication mediated by secreted type I and type III IFNs was most pronounced in cells expressing both type I and III receptors, was reduced when the type I IFN receptor was lacking, and was fully ablated when both IFN receptors were absent (Fig. 7). Although MAVS also directly activates interferon-stimulated genes (reference 34 and data not shown), this is apparently not sufficient to efficiently restrict HCV replication in the MLT cells lacking both IFN receptors (Fig. 7). These data indicate that to mount a strong antiviral effect, it is necessary for MAVS-dependent antiviral effector mechanisms to involve signaling through type I and III IFN receptors. Further work will be needed to find out which IFN-dependent mouse effector proteins curtail HCV propagation. Evaluation of the type I- and III IFN-dependent gene expression signatures should provide interesting clues in this direction.
To what extent both type I and III IFNs limit HCV replication in mouse livers in vivo remains to be shown. Notably, a recent study concluded that type III IFN did not impact acute infection of mouse livers by hepatotropic viruses due to the absence of expression of the type III IFN receptor (35). Given that expression of this receptor is tightly controlled by epigenetic mechanisms, it is possible that this may change in the course of a long-term (36), chronic infection. In any case, firm evidence derived from human liver cells and from chimpanzees firmly establishes that HCV induces both type I and III IFN responses which contribute to control of replication (13, 14, 16). In this regard, the novel MLT cells described here may be useful not only to delineate species-specific restrictions for HCV replication in mouse liver cells but also to model both type I and III IFN-dependent HCV control mechanisms.
ACKNOWLEDGMENTS
We are very grateful to Takaji Wakita for the gift of the JFH1 isolate, to Charles Rice for Huh-7.5 cells and 9E10 antibody, and to Matthew Evans for the miR122 expression construct. We also thank all members of the Institute for Experimental Virology at Twincore for helpful comments and discussions of this work.
This work was supported by a grant from the European Research Council, ERC-2011-StG_281473-(VIRAFRONT), and by a grant from the Helmholtz Association SO-024 to T.P.
Twincore Centre for Experimental and Clinical Infection Research is a joint venture between the Medical School Hanover and the Helmholtz Centre for Infection Research, Braunschweig.
REFERENCES
- 1.Maasoumy B, Wedemeyer H. 2012. Natural history of acute and chronic hepatitis C. Best Pract Res Clin Gastroenterol 26:401–412. doi: 10.1016/j.bpg.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Tse MT. 2013. All-oral HCV therapies near approval. Nat Rev Drug Discov 12:409–411. doi: 10.1038/nrd4036. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DL. 2013. Global control of hepatitis C: where challenge meets opportunity. Nat Med 19:850–858. doi: 10.1038/nm.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. 2009. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorner M, Horwitz JA, Robbins JB, Barry WT, Feng Q, Mu K, Jones CT, Schoggins JW, Catanese MT, Burton DR, Law M, Rice CM, Ploss A. 2011. A genetically humanized mouse model for hepatitis C virus infection. Nature 474:208–211. doi: 10.1038/nature10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitzegeio J, Bankwitz D, Hueging K, Haid S, Brohm C, Zeisel MB, Herrmann E, Iken M, Ott M, Baumert TF, Pietschmann T. 2010. Adaptation of hepatitis C virus to mouse CD81 permits infection of mouse cells in the absence of human entry factors. PLoS Pathog 6:e1000978. doi: 10.1371/journal.ppat.1000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang KS, Cai Z, Zhang C, Sen GC, Williams BR, Luo G. 2006. Replication of hepatitis C virus (HCV) RNA in mouse embryonic fibroblasts: protein kinase R (PKR)-dependent and PKR-independent mechanisms for controlling HCV RNA replication and mediating interferon activities. J Virol 80:7364–7374. doi: 10.1128/JVI.00586-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin LT, Noyce RS, Pham TN, Wilson JA, Sisson GR, Michalak TI, Mossman KL, Richardson CD. 2010. Replication of subgenomic hepatitis C virus replicons in mouse fibroblasts is facilitated by deletion of interferon regulatory factor 3 and expression of liver-specific microRNA 122. J Virol 84:9170–9180. doi: 10.1128/JVI.00559-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frentzen A, Anggakusuma, Gurlevik E, Hueging K, Knocke S, Ginkel C, Brown RJ, Heim M, Dill MT, Kroger A, Kalinke U, Kaderali L, Kuehnel F, Pietschmann T. 2014. Cell entry, efficient RNA replication, and production of infectious hepatitis C virus progeny in mouse liver-derived cells. Hepatology 59:78–88. doi: 10.1002/hep.26626. [DOI] [PubMed] [Google Scholar]
- 10.Dorner M, Horwitz JA, Donovan BM, Labitt RN, Budell WC, Friling T, Vogt A, Catanese MT, Satoh T, Kawai T, Akira S, Law M, Rice CM, Ploss A. 2013. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature 501:237–241. doi: 10.1038/nature12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M Jr, Lemon SM. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A 102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 13.Marukian S, Andrus L, Sheahan TP, Jones CT, Charles ED, Ploss A, Rice CM, Dustin LB. 2011. Hepatitis C virus induces interferon-lambda and interferon-stimulated genes in primary liver cultures. Hepatology 54:1913–1923. doi: 10.1002/hep.24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park H, Serti E, Eke O, Muchmore B, Prokunina-Olsson L, Capone S, Folgori A, Rehermann B. 2012. IL-29 is the dominant type III interferon produced by hepatocytes during acute hepatitis C virus infection. Hepatology 56:2060–2070. doi: 10.1002/hep.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheahan T, Imanaka N, Marukian S, Dorner M, Liu P, Ploss A, Rice CM. 2014. Interferon lambda alleles predict innate antiviral immune responses and hepatitis C virus permissiveness. Cell Host Microbe 15:190–202. doi: 10.1016/j.chom.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, Liang TJ. 2012. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology 142:978–988. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz P, Reuter A, Bender S, Bartenschlager R. 2013. Interferon-stimulated genes and their role in controlling hepatitis C virus. J Hepatol 59:1331–1341. doi: 10.1016/j.jhep.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Schoggins JW. 2014. Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol 6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper J, Fields JK. 1988. A hospital wellness program: where challenge meets opportunity. Home Healthc Nurse 6:10–16. [DOI] [PubMed] [Google Scholar]
- 21.Rothe M, Rittelmeyer I, Iken M, Rudrich U, Schambach A, Glage S, Manns MP, Baum C, Bock M, Ott M, Modlich U. 2012. Epidermal growth factor improves lentivirus vector gene transfer into primary mouse hepatocytes. Gene Ther 19:425–434. doi: 10.1038/gt.2011.117. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Q, Loya K, Rani B, Mobus S, Balakrishnan A, Lamle J, Cathomen T, Vogel A, Manns MP, Ott M, Cantz T, Sharma AD. 2013. MicroRNA-221 overexpression accelerates hepatocyte proliferation during liver regeneration. Hepatology 57:299–310. doi: 10.1002/hep.25984. [DOI] [PubMed] [Google Scholar]
- 23.Anggakusuma, Colpitts CC, Schang LM, Rachmawati H, Frentzen A, Pfaender S, Behrendt P, Brown RJ, Bankwitz D, Steinmann J, Ott M, Meuleman P, Rice CM, Ploss A, Pietschmann T, Steinmann E. 2014. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 63:1137–1149. doi: 10.1136/gutjnl-2012-304299. [DOI] [PubMed] [Google Scholar]
- 24.Narbus CM, Israelow B, Sourisseau M, Michta ML, Hopcraft SE, Zeiner GM, Evans MJ. 2011. HepG2 cells expressing microRNA miR-122 support the entire hepatitis C virus life cycle. J Virol 85:12087–12092. doi: 10.1128/JVI.05843-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. 2010. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol 11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommereyns C, Paul S, Staeheli P, Michiels T. 2008. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog 4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossaller L, Rathinam VA, Bonegio R, Chiang PI, Busto P, Wespiser AR, Caffrey DR, Li Q Z, Mohan C, Fitzgerald KA, Latz E, Marshak-Rothstein A. 2013. Overexpression of membrane-bound fas ligand (CD95L) exacerbates autoimmune disease and renal pathology in pristane-induced lupus. J Immunol 191:2104–2114. doi: 10.4049/jimmunol.1300341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. 2006. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fensterl V, White CL, Yamashita M, Sen GC. 2008. Novel characteristics of the function and induction of murine p56 family proteins. J Virol 82:11045–11053. doi: 10.1128/JVI.01593-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 31.Ahlen G, Derk E, Weiland M, Jiao J, Rahbin N, Aleman S, Peterson DL, Pokrovskaja K, Grander D, Frelin L, Sallberg M. 2009. Cleavage of the IPS-1/Cardif/MAVS/VISA does not inhibit T cell-mediated elimination of hepatitis C virus non-structural 3/4A-expressing hepatocytes. Gut 58:560–569. doi: 10.1136/gut.2007.147264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogt A, Scull MA, Friling T, Horwitz JA, Donovan BM, Dorner M, Gerold G, Labitt RN, Rice CM, Ploss A. 2013. Recapitulation of the hepatitis C virus life-cycle in engineered murine cell lines. Virology 444:1–11. doi: 10.1016/j.virol.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe T, Kaname Y, Hamamoto I, Tsuda Y, Wen X, Taguwa S, Moriishi K, Takeuchi O, Kawai T, Kanto T, Hayashi N, Akira S, Matsuura Y. 2007. Hepatitis C virus nonstructural protein 5A modulates the Toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol 81:8953–8966. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazear HM, Lancaster A, Wilkins C, Suthar MS, Huang A, Vick SC, Clepper L, Thackray L, Brassil MM, Virgin HW, Nikolich-Zugich J, Moses AV, Gale M Jr, Fruh K, Diamond MS. 2013. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog 9:e1003118. doi: 10.1371/journal.ppat.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermant P, Demarez C, Mahlakoiv T, Staeheli P, Meuleman P, Michiels T. 2014. Human but not mouse hepatocytes respond to interferon-lambda in vivo. PLoS One 9:e87906. doi: 10.1371/journal.pone.0087906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding S, Khoury-Hanold W, Iwasaki A, Robek MD. 2014. Epigenetic reprogramming of the type III interferon response potentiates antiviral activity and suppresses tumor growth. PLoS Biol 12:e1001758. doi: 10.1371/journal.pbio.1001758. [DOI] [PMC free article] [PubMed] [Google Scholar]