ABSTRACT
The cases of human infections with H10N8 viruses identified in late 2013 and early 2014 in Jiangxi, China, have raised concerns over the origin, prevalence, and development of these viruses in this region. Our long-term influenza surveillance of poultry and migratory birds in southern China in the past 12 years showed that H10 influenza viruses have been introduced from migratory to domestic ducks over several winter seasons at sentinel duck farms at Poyang Lake, where domestic ducks share their water body with overwintering migratory birds. H10 viruses were never detected in terrestrial poultry in our survey areas until August 2013, when they were identified at live-poultry markets in Jiangxi. Since then, we have isolated 124 H10N8 or H10N6 viruses from chickens at local markets, revealing an ongoing outbreak. Phylogenetic analysis of H10 and related viruses showed that the chicken H10N8 viruses were generated through multiple reassortments between H10 and N8 viruses from domestic ducks and the enzootic chicken H9N2 viruses. These chicken reassortant viruses were highly similar to the human isolate, indicating that market chickens were the source of human infection. Recently, the H10 viruses further reassorted, apparently with H5N6 viruses, and generated an H10N6 variant. The emergence and prevalence of H10 viruses in chickens and the occurrence of human infections provide direct evidence of the threat from the current influenza ecosystem in China.
IMPORTANCE After the outbreak of avian-origin H7N9 influenza viruses in China, fatal human infections with a novel H10N8 virus were reported. Utilizing data from 12 years of influenza surveillance in southern China, we showed that H10 viruses were regularly introduced by migratory ducks to domestic ducks on Poyang Lake, a major aggregative site of migratory birds in Asia. The H10 viruses were maintained and amplified in domestic ducks and then transmitted to chickens and reassorted with enzootic H9N2 viruses, leading to an outbreak and human infections at live-poultry markets. The emergence of the H10N8 virus, following a pathway similar to that of the recent H7N9 virus, highlights the role of domestic ducks and the current influenza ecosystem in China that facilitates influenza viruses moving from their reservoir hosts through the live-poultry system to cause severe consequences for public health.
INTRODUCTION
Direct interspecies transmissions of avian influenza viruses (AIVs) to humans repeatedly occurred in the last 2 decades, especially in regions where AIVs have become established or enzootic in poultry (1–4). Lowly pathogenic AIVs do not usually cause explicit symptoms in poultry, and unless ongoing surveillance is in place, human infections may occur before an outbreak in poultry is recognized, as was the case with the recent H7N9 viruses (5, 6).
It was through hospital-based surveillance of patients with severe pneumonia in China that a novel H10N8 AIV was identified in Nanchang, Jiangxi, with three cases occurring between November 2013 and February 2014 (7, 8). The virus isolated from the initial patient was a reassortant, with its internal gene complex derived from the H9N2 viruses enzootic in chickens in China and its surface genes related to those found in ducks and wild birds (7, 9). This is similar to the H7N9 viruses that emerged in early 2013 (5, 6). The patients with H10N8 infections were known to have a history of visiting live-poultry markets (LPMs) or exposure to live poultry before disease onset (7, 8), suggesting that market chickens may be the source of human infections (9, 10).
H10 subtype influenza viruses from waterfowl have occasionally infected mammals. H10N7 viruses were found to cause sporadic human infection cases in Egypt in 2004 (11) and in Australia in 2010 (12). An H10N4 outbreak in mink was observed in 1984 (13), and an H10N5 virus was detected in swine in 2008 (14). All of these were short-term and isolated events. H10 viruses have never become established and caused continuous infections in mammalian species.
Continuing human infections with H7N9 AIVs (5, 6) and the enzootic status of H5N1 (15) and H9N2 (16) AIVs indicate that the influenza ecosystem in China can facilitate the emergence and persistence of influenza viruses that are able to infect humans. Whether the H10N8 viruses infecting humans will persist remains to be explored. The findings of our systematic surveillance in six provinces of southern China from 2002 to 2014 showed that H10 viruses were regularly found in wild and domestic ducks but were never identified in terrestrial poultry until August 2013. Since then, and prior to the appearance of human infections, H10N8 viruses were frequently isolated from chickens at LPMs in Nanchang.
How H10 viruses evolved in wild and domestic birds over the past decades, how the current influenza ecosystem in China led to the genesis of the H10N8 virus infecting humans, and how these H10 viruses are continuing to develop remain to be fully addressed. To examine these questions and the potential consequences of the emergence of H10 viruses in Nanchang, detailed analyses of the genomic sequences of 219 H10 and 124 related viruses isolated in our surveillance network during the last 12 years were undertaken. The H10N8 virus that infected a human resulted from the interaction of wild birds with domestic ducks in the farming system of China, leading to the transfer of H10 and N8 genes to the markets and reassortment with enzootic H9N2 viruses. The transfer of the chicken viruses to humans was coincident with H10 virus outbreaks in LPMs. The continuing presence of the H10 virus in markets led to it acquiring an N6 neuraminidase (NA), again demonstrating the role of LPMs and the current influenza ecosystem of China in generating and perpetuating influenza viruses that pose threats to public health.
MATERIALS AND METHODS
Active surveillance in southern China.
Ongoing surveillance of influenza viruses in Jiangxi province has been conducted since 2002 at live-poultry markets (LPMs) in the capital city Nanchang (primarily from either the major wholesale or retail market), two sentinel duck farms (farms raising only domestic ducks, which share their water body with migratory waterfowl), and uninhabited islets in Poyang Lake, a major overwinter aggregation site for migratory waterfowl in Asia (Table 1). Simultaneous sampling in islets and sentinel duck farms at Poyang Lake can be used to monitor the transmission of viruses between migratory waterfowl and domestic ducks.
TABLE 1.
H10 subtype avian influenza viruses isolated from migratory and domestic birds in six provinces of southern China from 2002 to 2014a
| Yr | No. of isolates (no. of viruses sequenced) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Migratory ducks |
Sentinel ducks in JX | Domestic ducks and geese from LPMs |
Chickens from LPMs |
Total | ||||||||
| JX | Others | JX | FJ | GD | GX | GZ | YN | JX | Others | |||
| 2002 | / | — | — | / | / | — | / | / | / | / | — | — |
| 2003 | 2 (2) | — | 1 | / | / | 3 | / | / | / | / | — | 6 (2) |
| 2004 | — | — | — | / | — | — | — | / | — | / | — | — |
| 2005 | 9 (6) | — | 3 (3) | / | — | — | 1 | — | — | / | — | 13 (9) |
| 2006 | — | — | 267 (6) | / | — | 4 | — | — | 1 | / | — | 272 (6) |
| 2007 | — | — | 3 | 8 | — | — | — | — | — | — | — | 11 |
| 2008 | 2 (1) | — | — | 39 (7) | — | 2 | — | 5b | — | — | — | 48 (8) |
| 2009 | 1 (1) | — | 30 (4) | 10 (5) | — | 1 | 1 | 1 | — | — | — | 44 (10) |
| 2010 | — | — | — | 3 | — | — | — | — | — | — | — | 3 |
| 2011 | — | — | — | 18 | — | — | — | 2 | — | — | — | 20 |
| 2012 | 1 (1) | — | — | — | — | — | — | 2c | — | — | — | 3 (1) |
| 2013 | 12 (12) | — | 103 (20) | 27 (27) | — | — | — | — | 1 | 38 (38) | — | 181 (97) |
| 2014 | — | — | — | — | — | — | — | — | — | 86 (86) | — | 86 (86) |
| Total | 27 (23) | — | 407 (33) | 105 (39) | — | 10 | 2 | 10 | 2 | 124 (124) | — | 687 (219) |
Abbreviations: JX, Jiangxi; FJ, Fujian; GD, Guangdong; GX, Guangxi; GZ, Guizhou; YN, Yunnan; Others, all except Jiangxi; LPMs, live-poultry markets; —, no isolate; /, not sampled.
Including 2 goose isolates from Guizhou LPMs.
Including 1 goose isolate from Guizhou LPMs.
Migratory bird samples were obtained from fresh fecal droppings collected at isolated, publicly accessible islets on the lake from mid-October to the next March without disturbing the birds. Cloacal samples were taken year-round from sentinel ducks, approximately every 10 days. Sampling at LPMs in Fujian, Guangdong, Guangxi, Guizhou, Jiangxi, and Yunnan was carried out weekly from 2002 to 2014. Paired oropharyngeal and cloacal swabs were taken from apparently healthy birds at the markets. Surveillance in farms and markets was conducted according to procedures and guidelines in the WHO Manual on Animal Influenza Diagnosis and Surveillance (17).
Sample swabs were collected in vials with transport medium (medium 199 with antibiotics) and kept in cool boxes before being sent to the laboratory, where they were inoculated in 9- to 11-day-old embryonated chicken eggs for 48 to 72 h at 36°C to isolate influenza viruses (5). Hemagglutinin (HA)-positive isolates were further subtyped by hemagglutinin inhibition and neuraminidase inhibition assays using a panel of reference antisera, as described previously (5).
Full-genome sequencing.
Samples were selected for sequencing as described below. Sequencing was performed by using a Roche 454 Genome Sequencer Junior with sample preparation according to the manufacturer's protocols, giving ∼150× coverage of the influenza virus genome on average. Original reads from 454 sequencing were assembled into contigs by using overlapping regions of 40 nucleotides with >90% identity with the 454 de novo assembler. Contigs were identified as influenza A virus sequences by using BLAST against the influenza A virus sequences from GenBank. The contigs were then assembled with Lasergene, version 9.0 (DNAStar). For samples having mixed infections, based on manual curation, if two copies of an internal gene segment had <97% identity in the nucleotide sequence, they were separated and named mix-a and mix-b. Otherwise, the gene was marked as “mixed” with degenerate nucleotide codes and excluded from further analysis.
Data set preparation and alignment.
Nucleotide sequences generated in this study were combined with all publicly available sequences of avian influenza A viruses in GenBank (http://www.ncbi.nlm.nih.gov/GenBank) and H10N8 and H7N9 human virus sequences from GISAID (http://gisaid.org/). Sequences with >5 ambiguous nucleotides, or <70% of the length of the coding region, or that were mosaic (18) were excluded. Sequences were aligned by using MUSCLE v3.5 (19), with manual adjustments.
Phylogenetic analysis.
A “panoramic” maximum likelihood (ML) phylogeny of each gene segment was first constructed by using the rapid hill-climbing search method and the GTRGAMMA nucleotide substitution model in RAxML v7.6.8 (20). The Eurasian lineage that contains the H10N8 human and chicken isolates was retained, except for the N8 gene, where the major North American lineage was kept. The number of sequences of each segment was reduced to ∼1,000 by keeping those sequences most closely related to the H10N8 viruses. They were then built into ML phylogenies by 100 independent tree searches using RAxML v7.6.8. The robustness of the topologies was evaluated with the Shimodaira-Hasegawa approximate likelihood ratio test (21) on the best trees. The smaller ML trees shown in Fig. 2 and 3 were created with PhyML v3.0 (22), with bootstrap support values obtained from 1,000 pseudoreplicates.
FIG 2.
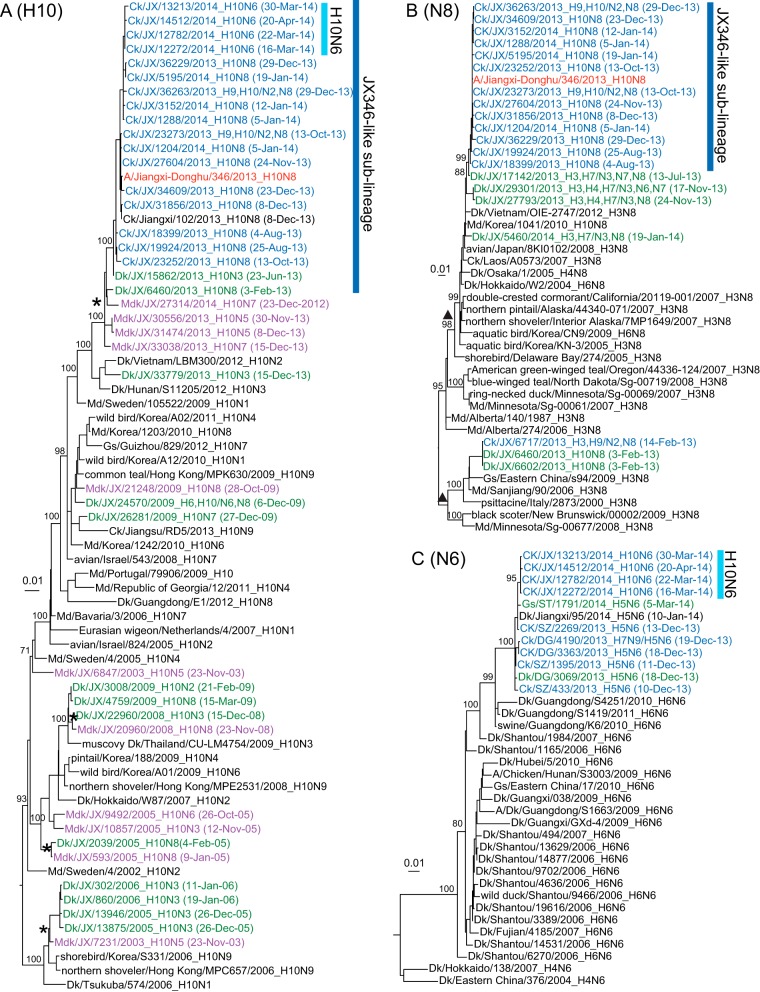
Maximum likelihood phylogenies of the surface genes. Sequences obtained in this study are labeled in blue, green, and purple for isolates from chickens, domestic ducks, and migratory ducks, respectively, in the phylogenetic trees of Eurasian H10 (n = 70) (A), North American N8 (n = 44) (B), and Eurasian N6 (n = 36) (C). The human H10N8 virus is marked in red, and the JX346-like sublineage is indicated by a dark blue bar. Introductions of H10 viruses from migratory to domestic ducks are indicated by asterisks in the H10 phylogeny. Intercontinental introductions of N8 viruses from North America to Eurasia are indicated by triangles in the N8 phylogeny. Bootstrap support values (percentages) from 1,000 pseudoreplicates are shown for selected lineages. Ck, chicken; Dk, duck; Gs, goose; Pg, pigeon; Mdk, migratory duck; Md, mallard; JX, Jiangxi; ST, Shantou; DG, Dongguan; SZ, Shenzhen.
FIG 3.
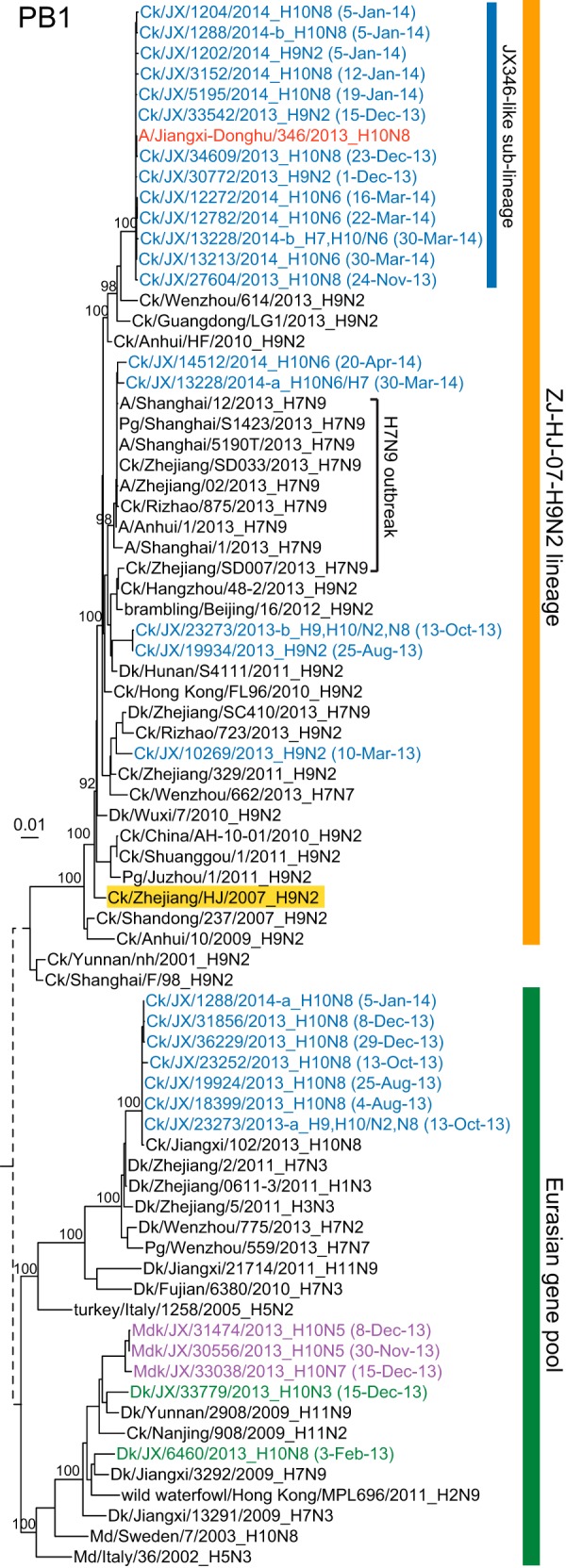
Maximum likelihood phylogenies of the PB1 gene (n = 76). Isolates that contained mixed infections are indicated by “-a” and “-b” after the strain name in the PB1 tree (see Materials and Methods). The ZJ-HJ-07 lineage nominal virus is highlighted in yellow. Abbreviations are described in the legend of Fig. 2.
Molecular dating.
A set of closely related viruses (∼100 sequences for each gene) was chosen for molecular clock estimation. The time-scaled maximum clade credibility phylogenies of the H10 virus segments were inferred by using a relaxed molecular clock model with an uncorrelated log-normal distribution (23) and virus sampling dates accurate to the day of a year. The SRD06 nucleotide substitution model (24) and the coalescent Bayesian skyline model (25) were incorporated into the Bayesian Markov chain Monte Carlo (MCMC) method implemented in BEAST v1.7.5 (26). Multiple independent MCMC runs were performed and assessed for consistency. They were combined to give a total chain length of 2 × 108 to 6 × 108 steps, with sampling at every 1,000 steps. Convergence of relevant parameters was assessed by effective sample sizes of >200 in Tracer v1.5 (http://tree.bio.ed.ac.uk/software/tracer/).
Nucleotide sequence accession numbers.
Sequences generated in this study are available in GenBank under accession numbers KP284874 to KP288026.
RESULTS
Prevalence of H10 AIV in aquatic birds.
From 2002 to 2014, a total of 560 H10 AIVs were isolated from migratory or domestic ducks in six provinces in southern China. Of these, 539 were isolated in Jiangxi from migratory ducks (n = 27) at islets in Poyang Lake, sentinel domestic ducks (n = 407) at farms on Poyang Lake, and domestic ducks (n = 105) at LPMs in Nanchang (40 km southwest of Poyang Lake) (Table 1). In contrast, in the remaining five provinces, only 21 H10 viruses were isolated from domestic ducks, and 3 were isolated from geese. Transmissions of H10 viruses from migratory birds to sentinel domestic ducks in Jiangxi were observed in the winters of 2005 to 2006, 2008 to 2009, and 2012 to 2013, with further transmissions to domestic ducks at LPMs (Table 1). Thus, Poyang Lake, a major aggregative site of migratory birds in Asia, is a major source of H10 viruses in southern China, while sentinel domestic ducks played a key role in spreading the H10 viruses.
Emergence and outbreak of H10 AIV in chickens in Jiangxi.
The H10 subtype had never been detected in terrestrial poultry before August 2013 in our surveillance areas. Up to April 2014, a total of 124 H10 viruses were isolated from chickens at LPMs in Nanchang (Table 1 and Fig. 1). Sixty-seven H10N8 viruses were obtained over 13 sampling occasions from August 2013 to January 2014, and 57 H10N6 viruses were isolated on 4 sampling occasions in March and April 2014 (Fig. 1). All chicken H10 viruses were detected from oropharyngeal swabs, suggesting that these chicken H10 viruses mainly replicated in and were shed through the upper respiratory and/or oral tract. Nearly one-half (n = 33) of the chicken H10N8 isolates were detected as mixed infections with H9N2 viruses. Of the H10N6 isolates, six were detected as mixed infections with H7N9 viruses, and one was mixed with an H9N2 virus.
FIG 1.
Isolation of H10 influenza A viruses from migratory and domestic birds in Jiangxi during 2012 to 2014. If two sampling occasions fell within 1 week, this is indicated. LPM, live-poultry market.
Phylogenetic analysis of the H10 viruses.
Whole-genome sequences of H10 viruses isolated in Jiangxi from 2003 to 2014 were obtained. These were H10 chicken isolates, 23 migratory duck isolates, 33 sentinel duck isolates, and 39 viruses from domestic ducks at LPMs (Table 1). Additionally, 68 H9N2 viruses and 56 N8 or N6 viruses isolated from 2013 to 2014 were also sequenced. All the sequences were analyzed along with those of the human H10N8 virus (A/Jiangxi/Donghu-346/2013 [JX346]) and other closely related sequences from publicly available databases.
Phylogenetic analysis of the H10 HA gene showed that there was a recent introduction of H10 viruses from migratory ducks to domestic ducks in Jiangxi from 2012 to 2013 and three earlier introductions in 2003, early 2005, and 2008 (Fig. 2A, asterisks; see also Fig. S1A in the supplemental material, asterisks). Only a single introduction of an H10 virus from domestic ducks to chickens was observed. The HA genes of all the chicken H10N8 viruses isolated at LPMs in Nanchang formed a monophyletic group (the JX346-like sublineage) together with the human H10N8 isolate, consistent with avian-to-human transmission of the virus at the LPM (Fig. 2A). The chicken H10N6 viruses that were isolated subsequently also had HA genes belonging to this sublineage, suggesting that N6 was acquired by a reassortment of the H10N8 viruses.
The N8 gene phylogeny showed that all the NA genes of the chicken H10N8 viruses formed a monophyletic group (the JX346-like sublineage), with >99.5% nucleotide sequence identity to the human isolate. N8 genes from Jiangxi domestic duck isolates with mixed infections involving multiple subtypes were the immediate outlier group of the chicken H10N8 viruses (Fig. 2B; see also Fig. S1B in the supplemental material), suggesting that frequent coinfections and reassortment in ducks may contribute to the assembly of novel HA and NA combinations and may be associated with interspecies transmission to chickens. These viruses were derived from the North American lineage as one of the several intercontinental transmissions of the N8 segment to the Eurasian gene pool that subsequently passed from migratory birds in eastern Asia to domestic ducks in Jiangxi (Fig. 2B, triangles; see also Fig. S1B in the supplemental material, triangle).
The N8 genes of the duck H10N8 viruses isolated at sentinel duck farms on Poyang Lake in February 2013 were genetically distant from those of the human and chicken viruses and originated from a separate intercontinental introduction (Fig. 2B; see also Fig. S1B in the supplemental material). Even though H3N8 viruses have been the most predominant among duck N8 viruses, only one chicken H3 virus (H3N8, isolated in February 2013) was identified in Jiangxi (Fig. 2B; see also Fig. S1B in the supplemental material). This chicken N8 gene was more closely related to those of the duck H10N8 viruses isolated at the sentinel duck farms than to the N8 genes of the JX346-like sublineage that led to the human infection.
The N6 genes of the chicken H10N6 viruses isolated from Jiangxi LPMs were closely related to those of H6N6 viruses established in domestic ducks in southern China (27). They appear to be directly derived from H5N6 viruses isolated from multiple types of poultry from late 2013 to early 2014, which acquired the N6 gene containing an 11-amino-acid stalk deletion from H6N6 viruses of ducks in southern China (Fig. 2C; see also Fig. S1C in the supplemental material) (27). This indicates a recent reassortment, probably between the H10N8 and H5N6 viruses in poultry. Only H10N6 viruses have been detected in chickens at LPMs since March 2014, suggesting that the N8 gene may have been replaced.
The internal genes of chicken H10N8 and H10N6 viruses were derived mainly from the locally enzootic chicken H9N2 virus lineage (Ck/Zhejiang/HJ/2007, ZJ-HJ-07 lineage) (Fig. 3; ZJ-HJ-07; see also Fig. S2 in the supplemental material), as were the internal genes of the human H10N8 and the recent H7N9 viruses. For each internal gene, the majority of the H10N8 viruses, including that from the human case, formed a monophyletic clade (JX346-like) (Fig. 3; see also Fig. S2 in the supplemental material). PB1 and PB2 gene segments derived from Eurasian gene pool viruses were commonly identified in the H10N8 viruses, leading to four different genotypes (Fig. 4; see also Tables S1 and S2 in the supplemental material) before January 2014. These segments were then replaced by those from H9N2 viruses (Fig. 3; see also Fig. S2A and S2B and Tables S1 and S2 in the supplemental material), indicating continual reassortments between H10N8 and H9N2 viruses. In the case of a few chicken H10 viruses, the internal genes clustered with those from the first wave of H7N9 viruses (Fig. 3; see also Fig. S2 in the supplemental material), suggesting that H10 viruses might have reassorted in chickens with H7N9 viruses or with H9N2 viruses that carried H7N9-like internal genes.
FIG 4.
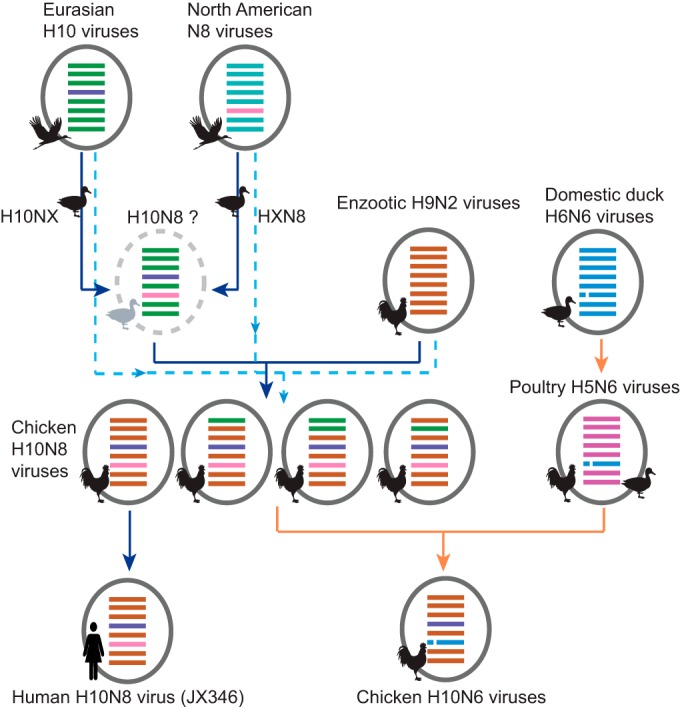
Genesis and evolutionary pathway of H10 viruses in Jiangxi. Either independent H10 and N8 viruses (dotted light blue lines) or an undetected H10N8 precursor in ducks (solid dark blue lines) was introduced to chickens and reassorted with enzootic H9N2 viruses. Reassortment of H10N8 viruses with N6 viruses is indicated by orange lines. Virus particles are indicated by ovals containing bars for the eight gene segments (PB2, PB1, PA, HA, NP, M, and NS, from top to bottom), colored by their source. NA stalk deletions are indicated by a broken line in segment 6.
Dating the emergence of the H10N8 viruses.
The times of the interspecies transmission and reassortment events leading to the emergence of the chicken H10N8 viruses were estimated by molecular dating (see Fig. S3 and S4 in the supplemental material). The H10 gene was transferred from ducks to chickens in April to June 2013, based on estimates for the time of divergence (tDiv) from the duck lineages to the time of the most recent common ancestor (tMRCA) of the chicken viruses. Similarly, the transfer of the N8 segment from ducks to chickens occurred between October 2012 and July 2013 (see Fig. S4 in the supplemental material). For the six internal genes, the tMRCAs of the JX346-like sublineage, containing both H10N8 and H9N2 viruses, were estimated to range from January to September 2013 (see Fig. S4 in the supplemental material). Thus, the generation of the H10N8 viruses in chickens appears to be a recent event that most probably occurred in the middle of 2013.
Molecular characterization of H10N8 and H10N6 viruses.
All the H10 chicken viruses and the human isolate had an HA Q220R substitution (residue 210, H3 numbering) relative to the duck viruses. This residue is located in the trimer interface of the HA protein (28). This substitution was shown to increase the pH of virus fusion and viral replication of an H3N2 virus in murine tracheal epithelial cells and lungs of mice (29). Deletions in the stalk region of NA are frequently found in viruses that have become established in terrestrial poultry (30, 31); however, deletions were not seen in N8 of the chicken isolates. The N6 proteins contain a stalk deletion at residues 59 to 69, which was originally identified in H6N6 viruses established in domestic ducks in southern China (27) and the H5N6 viruses identified in 2013 to 2014. None of the H10N8 chicken viruses carry the mammalian adaptation marker substitution E627K or D701N in the PB2 protein. However, PB2 of the human case had a mixture of 627E and 627K, with 627K predominating as the infection progressed (7).
DISCUSSION
The emergence of the H10N8 viruses and the associated human infections highlight the continual threat posed by the influenza ecosystem of China (Fig. 5). Our findings demonstrate how key aspects of the current influenza ecosystem facilitate the emergence and development of novel influenza viruses in the region, as occurred with the highly pathogenic H5N1 and H9N2 viruses and the recently emerged H7N9 viruses (5, 6, 32, 33).
FIG 5.
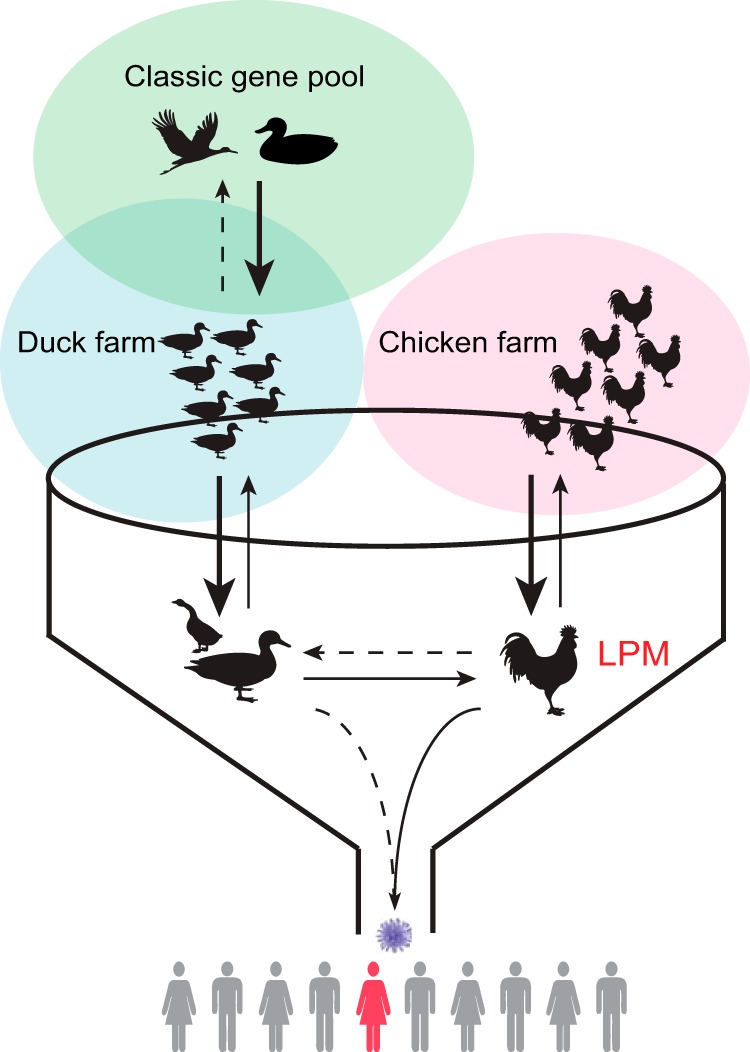
The current ecosystem of avian influenza viruses in China. The diversity of avian influenza viruses is maintained primarily in the classic gene pool viruses of wild waterfowl (green circle). Industrial poultry farming (blue and pink circles) largely separates species, but wild and domestic ducks may interact and transmit viruses. Trading in live-poultry markets (funnel) brings different types of poultry together to facilitate the sharing and mixing of viruses, favoring the emergence of novel AIVs and interspecies transmission of viruses (arrows). The markets are the major contact point between humans and live poultry, and this leads to the transmission of viruses to humans. Improper management may lead to the transfer of viruses from chickens in LPMs to those in farms. The stroke weight of an arrow indicates the frequency of transmissions between different hosts, arrowheads show the direction, and dashed lines indicate sporadic transmissions.
Introductions of influenza viruses from migratory waterfowl (the natural reservoir) through domestic ducks to other poultry were frequently observed in the last decades (34, 35). As >65% of the global population of domestic ducks are bred in China (FAOSTAT [http://faostat.fao.org/]), which forms the largest natural host population for influenza viruses in the world, these birds have become the major source of introductions of influenza viruses to poultry. In this study, >90% of the H10 viruses from ducks in our surveillance were isolated from domestic ducks in Jiangxi, especially from sentinel ducks that share their water body with migratory birds at Poyang Lake (Table 1).
Domestic ducks play a central role in the development of newly introduced viruses in the poultry system by allowing the viruses to spread within their large and highly dense population, by maintaining the local genetic diversity of the viruses through reassortment with other subtypes, and by carrying viruses to LPMs (Fig. 5). After domestic ducks at sentinel duck farms on Poyang Lake acquired H10 viruses similar to those from migratory birds at the lake, related viruses appeared at LPMs in the nearby city of Nanchang. This pattern has occurred previously with H10 viruses during the winter seasons of 2003 to 2004, 2004 to 2005, and 2008 to 2009, although not all introductions persisted or transmitted through the entire chain (Fig. 1 and 2A and Table 1).
In LPMs, the interaction of different types of poultry facilitates interspecies transmissions from domestic ducks to terrestrial poultry. In the emergence of H10N8 viruses, either separate duck H10 and N8 viruses or a precursor H10N8 virus was introduced to chickens, followed by reassortment with H9N2 viruses, leading to the genesis of the H10N8 virus (Fig. 4), which mirrors the scenario observed in the H7N9 outbreak. The subsequent prevalence and outbreak of the novel H10N8 viruses at LPMs in Nanchang became the source of human infections. The existence of distinct genotypes of H10 viruses in chickens, which changed over time, and the replacement of the NA subtype (Fig. 4) suggest that these viruses are subject to ongoing selection and adaptation in this new host species.
Introductions of viruses from aquatic birds to terrestrial poultry occur in most regions of the world (36, 37). However, the current influenza ecosystem of China, associated with the presence of multiple subtypes of influenza viruses enzootic in terrestrial poultry, the huge poultry population size and density, the combination of industrial and mixed-animal backyard farming practices, and the widespread LPM system, provides a unique set of conditions that favor the emergence and genesis of novel viruses. This has been repeatedly demonstrated by the emergence of H5N1, H9N2, and the recent H7N9 and H10N8 viruses in China. All these factors make China a unique influenza epicenter for generating novel viruses with pandemic potential.
This study highlights each of the steps by which the H10N8 virus was generated and evolved (Fig. 4 and 5). Currently, the H10 viruses related to the human infections have been detected only in Jiangxi, reflecting the early phase of an epizootic. If proper measures are implemented at this stage, it will be possible to eradicate these viruses completely. However, the long-term enzootic status of H9N2 and H5N1 viruses and the recent development of H7N9 viruses in China have demonstrated the inefficacy of current disease control systems.
To reduce the emergence of novel viruses and control current enzootic influenza viruses, revolutionary changes to the entire structure of poultry farming practices, LPMs, and the poultry distribution system are needed. Otherwise, H10N8 or H10N6 viruses are likely to disseminate to neighboring regions and follow the trajectory of the H7N9 outbreak. Coupling surveillance with changes in the management of LPMs will provide a higher level of protection against the potential emergence of a pandemic influenza virus from poultry in China.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Li Ka Shing Foundation, the Shenzhen Peacock Plan High-End Talents Program (KQTD201203), the University Grants Committee of the Hong Kong SAR (Area of Excellence Scheme grant AoE/M-12/06), and the National Institute of Allergy and Infectious Diseases (contracts HHSN266200700005C and HHSN272201400006C).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03167-14.
REFERENCES
- 1.Butt KM, Smith GJD, Chen H, Zhang LJ, Leung YHC, Xu KM, Lim W, Webster RG, Yuen KY, Peiris JSM. 2005. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol 43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, Norwood M, Senne D, Sims L, Takada A, Webster RG. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen-Van-Tam JS, Nair P, Acheson P, Baker A, Barker M, Bracebridge S, Croft J, Ellis J, Gelletlie R, Gent N, Ibbotson S, Joseph C, Mahgoub H, Monk P, Reghitt TW, Sundkvist T, Sellwood C, Simpson J, Smith J, Watson JM, Zambon M, Lightfoot N, Incident Response Team . 2006. Outbreak of low pathogenicity H7N3 avian influenza in UK, including associated case of human conjunctivitis. Euro Surveill 11(18):pii=2952 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2952. [DOI] [PubMed] [Google Scholar]
- 4.Fouchier RAM, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SAG, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJJ, Koch G, Bosman A, Koopmans M, Osterhaus ADME. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A 101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam TT-Y, Wang J, Shen Y, Zhou B, Duan L, Cheung C-L, Ma C, Lycett SJ, Leung CY-H, Chen X, Li L, Hong W, Chai Y, Zhou L, Liang H, Ou Z, Liu Y, Farooqui A, Kelvin DJ, Poon LLM, Smith DK, Pybus OG, Leung GM, Shu Y, Webster RG, Webby RJ, Peiris JSM, Rambaut A, Zhu H, Guan Y. 2013. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 502:241–244. doi: 10.1038/nature12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li XX, Zou S, Zhang YY, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Yuan H, Gao R, Zhang J, Wang D, Xiong Y, Fan G, Yang F, Li X, Zhou J, Zou S, Yang L, Chen T, Dong L, Bo H, Zhao X, Zhang Y, Lan Y, Bai T, Dong J, Li Q, Wang S, Zhang Y, Li H, Gong T, Shi Y, Ni X, Li J, Zhou J, Fan J, Wu J, Zhou X, Hu M, Wan J, Yang W, Li D, Wu G, Feng Z, Gao GF, Wang Y, Jin Q, Liu M, Shu Y. 2014. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet 383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2014. Influenza at the human-animal interface. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/human_animal_interface/Influenza_Summary_IRA_HA_interface_25Feburary14.pdf?ua=1. [Google Scholar]
- 9.Qi W, Zhou X, Shi W, Huang L, Xia W, Liu D, Li H, Chen S, Lei F, Cao L, Wu J, He F, Song W, Li Q, Li H, Liao M, Liu M. 2014. Genesis of the novel human-infecting influenza A(H10N8) virus and potential genetic diversity of the virus in poultry, China. Euro Surveill 19(25):pii=20841 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20841. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T, Bi Y, Tian H, Li X, Liu D, Wu Y, Jin T, Wang Y, Chen Q, Chen Z, Chang J, Gao GF, Xu B. 2014. Human infection with influenza virus A(H10N8) from live poultry markets, China, 2014. Emerg Infect Dis 20:2076–2079. doi: 10.3201/eid2012.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan American Health Organization. 2004. Avian influenza virus A (H10N7) circulating among humans in Egypt. Pan American Health Organization, Washington, DC: http://new.paho.org/hq/dmdocuments/2010/Avian_Influenza_Egypt_070503.pdf. [Google Scholar]
- 12.Arzey GG, Kirkland PD, Arzey KE, Frost M, Maywood P, Conaty S, Hurt AC, Deng YM, Iannello P, Barr I, Dwyer DE, Ratnamohan M, McPhie K, Selleck P. 2012. Influenza virus A (H10N7) in chickens and poultry abattoir workers, Australia. Emerg Infect Dis 18:814–816. doi: 10.3201/eid1805.111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klingeborn B, Englund L, Rott R, Juntti N, Rockborn G. 1985. An avian influenza A virus killing a mammalian species—the mink. Brief report. Arch Virol 86:347–351. doi: 10.1007/BF01309839. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Zou W, Yang Y, Guo X, Hua Y, Zhang Q, Zhao Z, Jin M. 2012. Complete genome sequence of an H10N5 avian influenza virus isolated from pigs in central China. J Virol 86:13865–13866. doi: 10.1128/JVI.02687-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Smith GJ, Li KS, Wang J, Fan XH, Rayner JM, Vijaykrishna D, Zhang JX, Zhang LJ, Guo CT, Cheung CL, Xu KM, Duan L, Huang K, Qin K, Leung YH, Wu WL, Lu HR, Chen Y, Xia NS, Naipospos TS, Yuen KY, Hassan SS, Bahri S, Nguyen TD, Webster RG, Peiris JS, Guan Y. 2006. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A 103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol 74:9372–9380. doi: 10.1128/JVI.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. 2002. WHO manual on animal influenza diagnosis and surveillance. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/influenza/en/whocdscsrncs20025rev.pdf. [Google Scholar]
- 18.Lam TT, Chong YL, Shi M, Hon CC, Li J, Martin DP, Tang JW, Mok CK, Shih SR, Yip CW, Jiang J, Hui RK, Pybus OG, Holmes EC, Leung FC. 2013. Systematic phylogenetic analysis of influenza A virus reveals many novel mosaic genome segments. Infect Genet Evol 18:367–378. doi: 10.1016/j.meegid.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 21.Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O. 2011. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol 60:685–699. doi: 10.1093/sysbio/syr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 23.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro B, Rambaut A, Drummond AJ. 2006. Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Mol Biol Evol 23:7–9. doi: 10.1093/molbev/msj021. [DOI] [PubMed] [Google Scholar]
- 25.Minin VN, Bloomquist EW, Suchard MA. 2008. Smooth skyride through a rough skyline: Bayesian coalescent-based inference of population dynamics. Mol Biol Evol 25:1459–1471. doi: 10.1093/molbev/msn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang K, Zhu H, Fan X, Wang J, Cheung C-L, Duan L, Hong W, Liu Y, Li L, Smith DK, Chen H, Webster RG, Webby RJ, Peiris M, Guan Y. 2012. Establishment and lineage replacement of H6 influenza viruses in domestic ducks in southern China. J Virol 86:6075–6083. doi: 10.1128/JVI.06389-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vachieri SG, Xiong X, Collins PJ, Walker PA, Martin SR, Haire LF, Zhang Y, McCauley JW, Gamblin SJ, Skehel JJ. 2014. Receptor binding by H10 influenza viruses. Nature 511:475–477. doi: 10.1038/nature13443. [DOI] [PubMed] [Google Scholar]
- 29.Keleta L, Ibricevic A, Bovin NV, Brody SL, Brown EG. 2008. Experimental evolution of human influenza virus H3 hemagglutinin in the mouse lung identifies adaptive regions in HA1 and HA2. J Virol 82:11599–11608. doi: 10.1128/JVI.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banks J, Speidel ES, Moore E, Plowright L, Piccirillo A, Capua I, Cordioli P, Fioretti A, Alexander DJ. 2001. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch Virol 146:963–973. doi: 10.1007/s007050170128. [DOI] [PubMed] [Google Scholar]
- 31.Dlugolenski D, Jones L, Saavedra G, Tompkins SM, Tripp RA, Mundt E. 2011. Passage of low-pathogenic avian influenza (LPAI) viruses mediates rapid genetic adaptation of a wild-bird isolate in poultry. Arch Virol 156:565–576. doi: 10.1007/s00705-010-0891-x. [DOI] [PubMed] [Google Scholar]
- 32.Xu KM, Smith GJD, Bahl J, Duan L, Tai H, Vijaykrishna D, Wang J, Zhang JX, Li KS, Fan XH, Webster RG, Chen H, Peiris JSM, Guan Y. 2007. The genesis and evolution of H9N2 influenza viruses in poultry from southern China, 2000 to 2005. J Virol 81:10389–10401. doi: 10.1128/JVI.00979-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li KS, Guan Y, Wang J, Smith GJD, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie ATS, Chaisingh A, Auewarakul P, Long HT, Hanh NTH, Webby RJ, Poon LLM, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JSM. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 34.Huang K, Bahl J, Fan XH, Vijaykrishna D, Cheung CL, Webby RJ, Webster RG, Chen H, Smith GJD, Peiris JSM, Guan Y. 2010. Establishment of an H6N2 influenza virus lineage in domestic ducks in southern China. J Virol 84:6978–6986. doi: 10.1128/JVI.00256-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan L, Campitelli L, Fan XH, Leung YH, Vijaykrishna D, Zhang JX, Donatelli I, Delogu M, Li KS, Foni E, Chiapponi C, Wu WL, Kai H, Webster RG, Shortridge KF, Peiris JS, Smith GJ, Chen H, Guan Y. 2007. Characterization of low-pathogenic H5 subtype influenza viruses from Eurasia: implications for the origin of highly pathogenic H5N1 viruses. J Virol 81:7529–7539. doi: 10.1128/JVI.00327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campitelli L, Mogavero E, De Marco MA, Delogu M, Puzelli S, Frezza F, Facchini M, Chiapponi C, Foni E, Cordioli P, Webby R, Barigazzi G, Webster RG, Donatelli I. 2004. Interspecies transmission of an H7N3 influenza virus from wild birds to intensively reared domestic poultry in Italy. Virology 323:24–36. doi: 10.1016/j.virol.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Manin TB, Chvala IA, Kolosov SN, Pchelkina IP, Irza VN, Drygin VV. 2010. H5N1 avian influenza outbreak in the Far East of Russia in 2008: new introduction. Avian Dis 54:509–512. doi: 10.1637/8732-032509-Case.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.