ABSTRACT
Kaposi's sarcoma-associated herpesvirus (KSHV) encodes ORF57, which enhances the expression of intronless KSHV genes on multiple posttranscriptional levels. However, it remains elusive how ORF57 recognizes viral RNAs. Here, we demonstrate that ORF57 also increases the expression of the multiple intron-containing K15 gene. The nucleotide bias of the K15 cDNA revealed an unusual high AT content. Thus, we optimized the K15 cDNA by raising the frequency of GC nucleotides, yielding an ORF57-independent version. To further prove the importance of the sequence bias of ORF57-dependent RNAs, we grouped KSHV mRNAs according to their AT content and found a correlation between AT-richness and ORF57 dependency. More importantly, latent genes, which have to be expressed in the absence of ORF57, have a low AT content and are indeed ORF57 independent. The nucleotide composition of K15 resembles that of HIV gag, which cannot be expressed unless RNA export is facilitated by the HIV Rev protein. Interestingly, ORF57 can partially rescue HIV Gag expression. Thus, the KSHV target RNAs of ORF57 and HIV gag RNA may share certain motifs based on the nucleotide bias. A bioinformatic comparison between wild-type and sequence-optimized K15 revealed a higher density for hnRNP-binding motifs in the former. We speculate that binding of particular hnRNPs to KSHV lytic transcripts is the prerequisite for ORF57 to enhance their expression.
IMPORTANCE The mostly intronless genes of KSHV are only expressed in the presence of the viral regulator protein ORF57, but how ORF57 recognizes viral RNAs remains elusive. We focused on the multiple intron-containing KSHV gene K15 and revealed that its expression is also increased by ORF57. Moreover, sequences in the K15 cDNA mediate this enhancement. The quest for a target sequence or a response element for ORF57 in the lytic genes was not successful. Instead, we found the nucleotide bias to be the critical determinant of ORF57 dependency. Based on the fact that ORF57 has only a weak affinity for nucleic acids, we speculate that a cellular RNA-binding protein provides the sequence preference for ORF57. This study provides evidence that herpesviral RNA regulator proteins use the sequence bias of lytic genes and the resulting composition of the viral mRNP to distinguish between viral and cellular mRNAs.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV) is a human gammaherpesvirus that was identified in KS tissues from AIDS patients (1). It is also associated with two lymphoproliferative disorders, the primary effusion lymphoma and the plasma cell variant of multicentric Castleman's disease. KSHV-associated malignancies are rare in immunocompetent people but more common in the context of immunosuppression, especially in HIV patients or transplant recipients. The viral life cycle is divided into a latent and a lytic phase. In contrast to other herpesviruses, tumorigenesis is also a consequence of the lytic cycle (2–5).
Herpesviruses replicate in the nucleus, and viral gene expression is dependent on the host mRNA processing and export machinery to translocate viral mRNAs to the cytoplasm for translation. Cellular bulk mRNA export takes place in a stepwise manner: during pre-mRNA splicing the exon-junction complex (EJC) is deposited 20 to 24 nucleotides upstream of every splice event. The EJC then recruits the components of the cellular mRNA export machinery (6, 7). In the present view, the mRNA undergoes a handover mediated by protein interactions from UAP56 to ALY, a TAP-NXT adaptor protein. UAP56 copurifies with the THO/TREX complex, a multimeric complex involved in the coupling of transcription and RNA export (8). Finally, the TAP-NXT dimer is responsible for the interaction with the FG-containing nucleoporins and catalyzes export of the bound RNA (summarized in references 9 to 15).
On average, cellular genes contain eight introns, and only 3% of all human genes are intronless (16), whereas in herpesviruses the majority of genes lack introns. Among the herpesviruses KSHV has the highest number of intron-containing genes (25% [17]). It is believed that splicing leads to a more efficient recruitment of export factors and that intronless genes use transcription-dependent recruitment or auxiliary RNA elements (reviewed in references 18 and 19). Thus, to ensure the expression and stability of their intronless genes, all herpesviruses encode a viral RNA regulator protein that substitutes for the function of the EJC to recruit components of the cellular export machinery. Herpes simplex virus 1 ICP27 is the best-characterized herpesviral RNA regulator (20). In KSHV, ORF57 fulfills this role (21). Although the overall sequence conservation of these regulator proteins is low, they share a highly conserved ICP27 homology region (22). ORF57 has been shown to interact with both ALY and the redundant UAP56-interacting factor to promote export (23, 24). Besides its role in RNA export, ORF57 also has multiple other functions connected with viral RNA biogenesis. Stabilization of transcripts has been described for the long noncoding PAN RNA (polyadenylated nuclear RNA [25, 26]), which accumulates to high levels in the nucleus during the lytic cycle but is not exported to the cytoplasm (27). Furthermore, ORF57 may also regulate splicing (28, 29) and translation of viral RNAs (29–31). A described interaction of ORF57 with the KSHV transcriptional activator RTA also implicates a function in viral transcription (32). Finally, a recent publication demonstrates an involvement of ORF57 in KSHV-induced genome instability by sequestration of the transcription and export complex (TREX) (33; reviewed in reference 34).
These multiple functions of ORF57 demonstrate the importance of this protein for viral lytic gene expression. However, the mechanism for how ORF57 recognizes viral RNA and how it distinguishes between cellular and viral transcripts remains elusive. An ORF57 response element has been identified for PAN and also for viral and human interleukin-6 (35–37). Interestingly, these response elements seem not to be present in other lytic ORF57-dependent genes. This suggests an additional mechanism for how ORF57 recognizes viral RNA. Deletion studies regarding RNA and protein binding of ORF57 yielded conflicting results. Several motifs located at the C and N termini have been implicated in recognition of RNA and protein partners (25, 38). Furthermore, it has been shown that ORF57 lacks RNA binding in the absence of cellular extracts (38). These data clearly indicate that a cellular protein partner is needed for ORF57 to specifically bind viral RNAs (reviewed in references 39 and 40).
Here, we demonstrate that ORF57 also enhances RNA expression of the multiple intron-containing KSHV K15 gene. A decrease in the AT content of K15 mRNA by sequence optimization yielded an ORF57-independent expression. A bioinformatic analysis revealed a drastic decrease of sequence motifs, which bind heterologous nuclear ribonucleoproteins (hnRNPs). This may explain the ORF57-independent expression. A ranking of all KSHV lytic genes according to their AT content revealed a correlation between AT richness and ORF57 dependency. Finally, we explored the HIV gag gene as a reporter for ORF57 activity. We were able to show that ORF57 can partially rescue HIV Gag expression in the absence of the HIV export factor Rev. Moreover, ORF57 can act in a cumulative manner with a constitutive transport element, which is also recruiting Tap/NXT1. We propose that the nucleotide bias of lytic KSHV genes leads to the binding of a distinct set of RNA-binding proteins and represents the basis for ORF57 dependency.
MATERIALS AND METHODS
Plasmids.
For the K15 plasmids, either the cDNA or the genomic sequence was inserted in a pcDNA3.1(+) vector (Invitrogen) using the NheI/XhoI sites. The genomic sequence was amplified from KSHV-BAC36 (41). The synthetic K15 (GeneArt) and the chimeric constructs were cloned into pcDNA3.1 (+) using the BamHI/XhoI site. The chimeric constructs were generated by overlap PCR. The FLAG-tagged ORF57c construct was generated by reverse transcription-PCR (RT-PCR) using RNA from lytic HEK293/BAC36 cells. The PCR product was used for a second PCR with primers containing the tag and appropriate restriction sites. The ORF57 cDNA was then cloned into pcDNA3.1(+) using EcoRI/XhoI sites. The pUL69 plasmid was kindly provided by T. Stamminger, University Erlangen. Hemagglutinin (HA)-tagged ORF47/synthetic ORF47 (GeneArt) and ORF8 were amplified by PCR from KSHV-BAC36. Both were cloned into pcDNA3.1(+) using EcoRI/XhoI sites. The pcDNA3.1-LANA plasmid was described previously (42). The HIV gp-RRE and gp-CTE reporter plasmids were also described previously (43). The HIV gp construct was generated by deletion PCR. For the K15-RRE and sK15-RRE constructs, K15 cDNA and synthetic K15 (sK15) were amplified by using primers with appropriate restriction sites. The HIV gp sequence in the gp-RRE construct was then replaced using BamHI/EcoRI sites. The previously described deoptimized green fluorescent protein (GFP) (44) was kindly provided by R. Wagner (University Regensburg). UL11 plasmid (45) was kindly provided by M. Messerle (Hanover Medical School).
Primers.
For the amplification of the genomic K15, we used the primers K15g fw (5′-GTCAGCTAGCATGAAGACACTCATATTCTTCTGGAATTTATGGCTTTGGGCCC-3′) and K15g rv (5′-GCTGCTCGAGCTAGTTCCTGGGAAATAAAACCTCCTC-3′). For the ORF57-FLAG construct RT-PCR primers (5′-ATGGTACAAGCAATGATAGACATGGACATTATGAAG-3′ and 5′-AGAAAGTGGATAAAAGAATAAACCCTTGTTAAATTTGGCCG-3′), the ORF57c-FLAG fw (5′-CGCGAATTCATGGTACAAGCAATGATAGACATGGACATTATGAAG-3′) and ORF57c-FLAG rev (5′-GCGCTCGAGTTACTTGTCGTCGTCGTCCTTGTAGTCAGAAAGTGGATAAAAGAATAAACCCTTG) primers were used. For cloning of the ORF47-HA construct, the primers ORF47-HA fw (5′-GTAGAATTCATGGGGATCTTTGCGCTATTTGC-3′) and ORF47-HA rv (5′-GCGCTCGAGTCAAGCGTAATCTGGAACATCGTATGGGTACATGGTTTTTCCCTTTTGACCTGCGTGCGCTCTCCGGC-3′) were used. For cloning of the ORF8 construct, the ORF8 fw (5′-GTAGAATTCATGACTCCCAGGTCTAGATTGGC-3′) and ORF8 rv (5′-GCGCTCGAGTCAAGCGTAATCTGGAACATCGTATGGGTACATGGTCTCCCCCGTTTCCGGACTGATGTCTAGCG-3′) primers were used. For the RRE constructs, the HIV gp fw (5′-GATCCTTAGCACTTATCTGGGACGATC-3′), HIV gp rv (5′-GATCCTTAGCACTTATCTGGGACGATC-3′), K15-RRE fw (5′-CAAGGATCCATGAAGACACTCATATTCTTCTGG-3′), K15-RRE rv (5′-GTTGAATTCCTAGTTCCTGGGAAATAAAACC-3′), sK15-RRE fw (5′-CAAGGATCCATGAAAACCCTGATCTTTTTCTGG-3′), and sK15-RRE rv (5′-GTTGAATTCTCAGTTCCGGGGGAACAG-3′) primers were used. Chimeric constructs were generated by using the primers K15c fw (5′-CAAGGATCCATGAAGACACTCATATTCTTCTGG-3′), sK15 fw (5′-CAAGGATCCATGAAAACCCTGATCTTTTTCTGG-3′), K15c rv (5′-GTTCTCGAGCTAGTTCCTGGGAAATAAAACC-3′), sK15 rv (5′-CGCTCGAGTCAGTTCCGGGGGAACAG-3′), K15c_sK15 500 rv (5′-CAGAAAGGGCAAGCTGAGAGTGAAGCTAAT TGG-3′), K15c_sK15 500 fw (5′-CAGCTTGCCCTTTCTGTACGCCTTCGC-3′), K15c_sK15 1000 rv (5′-GCCTCTGGTTAAACCACTAGGGAGAAGCATTAC-3′), K15c _sK15 1000 fw (5′-GGTTTAACCAGAGGCATTCTGACCATGATC-3′), sK15_K15c 500 rv (5′-AATGGCAAGCTCAGGGTGAAGCTGATGG-3′), sK15_K15c 1000 rv (5′-TCATTGTTAGAATGCCTCTGGTCAGG-3′), sK15_K15c 500 fw (5′-CCCTGAGCTTGCCATTCTTGTATGCATTTGC-3′), and sK15_K15c 1000 fw (5′-GGCATTCTAACAATGATCATCTGCATCAGTAC-3′). For the K15 RT-PCR, the K15 exon 1 fw (5′-GGTGTATCACTCTTGTCTGTGT-3′) and K15 exon 8 rv (5′-CTCATACAGGTCGTCTGTCG-3′) primers were used. For the GAPDH pre-mRNA RT-PCR, a GAPDH intron3 fw primer (5′-CAAGGAGAGCTCAAGGTC-3′) and a GAPDH intron4 rv primer (5′-GGGTGCGGTGGAGATCTG-3′) were used.
Cells and transfection.
HEK293T cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, 1 mM sodium pyruvate, and 1% penicillin-streptomycin. The day before transfection, 5 × 106 293T cells were seeded in a 10-cm plate. Transfections were performed using the calcium phosphate precipitation method with 5 μg of K15 plus 2.5 μg of ORF57, 0.5 μg of enhanced GFP (eGFP) as a transfection control, and 7 μg of plasmid DNA. The medium was not changed before 6 h posttransfection, and RNA and protein were harvested 40 h later. Transfections with the synthetic K15 were harvested at earlier time points (indicated in the figure legends). The HIV reporter plasmids were transfected using 1 μg of the reporter plasmid 400 ng of ORF57 or 100 ng of Rev and 50 ng of eGFP. The day before transfection, 4 × 105 293T cells were seeded and transfected using the Nanofectin transfection reagent (PAA). Protein was harvested at 40 h posttransfection. Transfection efficiency was measured by fluorescence-activated cell sorting analysis (Cytomics FC 500 flow cytometer; Beckman Coulter). For the flow cytometry analysis of the deoptimized GFP-RRE construct, the transfection efficiency was measured by cotransfection of a DsRed plasmid (data not shown), and only double-positive cells were included in the mean fluorescence intensity (MFI) analysis.
RNA preparation and analysis.
RNA methods (preparation of total RNA, gel electrophoresis, blotting, and detection with a radiolabeled probe) were performed as described previously (46). A GAPDH (glyceraldehyde-3-phosphate dehydrogenase)-specific probe was prepared by EcoRI digestion of a GAPDH plasmid (a gift from K. Habers, Heinrich-Pette-Institut, Hamburg). For the detection of K15 mRNA, a specific probe directed against K15 exon 8 was generated from the K15g plasmid by XbaI/BsrGI digestion. The RRE probe was generated by digestion of the HIV-gp plasmid with EcoRI/BamHI. For ORF8, LANA, and ORF47 probes, the ORF8 plasmid was digested with EcoRV/MluI, the LANA plasmid was digested with SalI/BamHI, and the ORF47 plasmid was digested with EcoRI/XhoI. For reverse transcription, 5 μg of total RNA were first DNase digested with Turbo DNase (Ambion) and then purified using an RNeasy minikit (Qiagen). Then, 800 ng of RNA were reverse transcribed with a QuantiTect reverse transcription kit (Qiagen). For the final PCR, a K15 exon 1 fw primer and a K15 exon 8 rv primer were used.
RNA from BJAB cells, which were first selected to harbor a recombinant KSHV genome (47), was subjected to reverse transcription-quantitative PCR (RT-qPCR) as described previously (48). The cells were induced using sodium butyrate and baculovirus coding for KSHV ORF50/RTA or the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA). The value for nonreactivated BJAB rKSHV was set to 1. Relative mRNA expression changes were calculated according to the ΔΔCT method.
RNA fractionation.
For nuclear and cytoplasmic fractionations, HEK293T cells were harvested and washed with phosphate-buffered saline. The cells were lysed in 500 μl of RNA lysis buffer (0.1% NP-40, 0.14 M NaCl, 10 mM Tris [pH 8.4], 1.5 mM MgCl2, 10 mM EDTA [pH 8]), and the nuclei were pelleted by centrifugation at 470 × g for 5 min. The RNA from the cytoplasmic supernatant and the nuclear fraction was extracted using the RNAzol B method as described previously (46). The fractionation was controlled by RT-PCR using GAPDH intron primers to monitor the integrity of the nuclei. As an additional control, an RT-PCR using primers detecting spliced β-actin mRNA was performed.
Western blotting.
For the detection of K15 protein, cell pellets were incubated on ice for 10 min with RIPA-100 buffer (20 mM Tris [pH 7.5], 1 mM EDTA, 100 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) and then centrifuged at 16,000 rpm for 20 min to clear the lysates.
For the detection of all other proteins, the cells were lysed with 1× sodium dodecyl sulfate (SDS) protein lysis buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 10% glycerol, 50 mM dithiothreitol, 0.01% [wt/vol] bromophenol blue) and boiled for 5 min. Lysates were analyzed by immunoblotting. K15 protein was detected with a rabbit polyclonal anti-K15 antibody in a 1:1,000 dilution (49) or rat antibody directed against the cytoplasmic tail of K15 (provided by E. Kremmer, Heimholz Center, Munich). HIV capsid (p24) was detected with a rabbit anti-p24 HIV antibody in a 1:5,000 dilution (kindly provided by H.-G. Kräusslich, University Heidelberg). β-Actin was detected with a mouse monoclonal anti-β-actin antibody in a 1:1,000 dilution (Sigma-Aldrich). HA-tagged proteins were detected with rat monoclonal anti-HA 3F10 in a 1:2,000 dilution (Roche). LANA (ORF73) was detected with a rat monoclonal anti-LANA antibody in a 1:2,000 dilution (ABI). V5-tagged UL11 was detected by a rabbit anti-V5 antibody in a 1:5,000 dilution (Invitrogen). FLAG-tagged ORF57 was detected by a rabbit polyclonal anti-FLAG antibody in a 1:2,000 dilution (Sigma). Detection was carried out by using a standard enhanced chemiluminescence reaction or with the Odyssey infrared imaging system.
Prediction of motifs for RNA-binding proteins.
Motifs were predicted using the RBPmap webserver (http://rbpmap.technion.ac.il/) employing the high-stringency option with no conservation filtering. Motifs for SR (serine-arginine-rich) proteins and hnRNPs were extracted from the RBPmap database, including only validated motifs (50). In order to remove bias due to the repetitive nature of many of the motifs, overlapping predicted motifs were clustered together. Each motif cluster was counted as a single motif and the coordinates of the center of the cluster were considered for further analysis. The RNA-binding proteins CUG-BP and TIA1 were not included due to their high context dependency with respect to intronic or exonic binding sites.
RESULTS
RNA expression of the multiple spliced K15 gene is enhanced by ORF57.
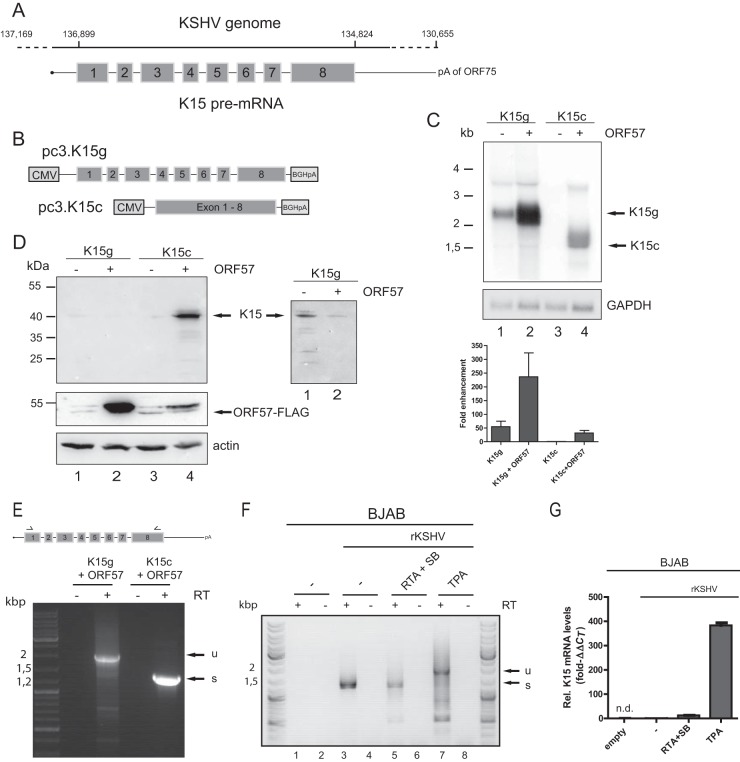
The viral K15 gene mirrors cellular genes in the number of introns. Thus, we set out to determine whether K15 expression requires the viral posttranscriptional activator ORF57. Remarkably, K15 contains seven introns compared to the other mostly intronless or one-intron-containing KHSV genes (Fig. 1A). These introns are characterized by their small size (∼100 nucleotides [nt]) and weak splice sites, on average. Due to the observation that K15 expression vectors only lead to a relatively weak expression (51), we first generated new K15 expression constructs. In these constructs, either the K15 cDNA (pc3.K15c) or the genomic version of K15 (pc3.K15g) containing all seven introns was placed under the control of the cytomegalovirus (CMV) immediate-early promoter and the bovine growth hormone polyadenylation site (Fig. 1B). K15 expression was monitored in cotransfection experiments with ORF57. We observed a strong effect of ORF57 on K15 RNA expression (Fig. 1C). The cotransfection of ORF57 led to a >30-fold enhancement of K15 mRNA expression for the K15 cDNA and an almost 5-fold for the genomic construct, respectively (Fig. 1C). K15 protein is barely detectable in the absence of ORF57 for the cDNA construct (Fig. 1D, lane 3). However, the cotransfection of ORF57 strongly enhanced K15 expression (Fig. 1D, lane 4). Surprisingly, the RNA level of the genomic K15 construct increases, while a weak K15 protein expression could only be detected after longer exposure times (Fig. 1D, right inset). To gain more insights into this expression block, we performed RT-PCRs using total RNA from the cotransfection experiments. The main RNA species for the genomic K15 construct is the unspliced mRNA and only a minor fraction is completely spliced K15 mRNA (Fig. 1E). This mirrors the results from the Northern blot where we also observed poor splicing of the genomic construct (Fig. 1C, lane 2). Also, in the context of B cells harboring a recombinant KSHV (47), induction of the lytic cycle by TPA leads to a mixture of spliced and unspliced K15 variants (Fig. 1F, lane 7). In B cells, K15 is also detectable in the latent state (Fig. 1F, lane 3). However, mRNA quantification by RT-qPCR proved that only reactivation by TPA results in significant levels of K15 RNA (Fig. 1G). In sum, the large amount of unspliced K15 RNA explains the low K15 protein expression from the genomic construct. More importantly, these results show that also the multiply spliced K15 gene is ORF57 dependent. In addition, we could confirm that a target for ORF57 must be present in the cDNA of K15.
FIG 1.
ORF57 promotes K15 expression at the RNA and protein level. HEK293T cells were cotransfected with pc3.K15g or pc3.K15c and ORF57 cDNA. Total RNA and protein were harvested 40 h posttransfection and analyzed by Northern and Western blotting. Transfection efficiency was monitored by cotransfection of an eGFP-encoding plasmid and measured by flow cytometry in all experiments (data not shown). (A) Depiction of the K15 gene in the viral genome. The coordinates in the genome are given according to NCBI accession number AF148805.2. The outer left coordinate represents the start of the terminal repeat. The dashed line indicates the uncharacterized transcriptional start site. K15 uses the poly(A) site of ORF75. The K15 pre-mRNA is drawn with exons (gray boxes; in scale) and introns (not in scale). (B) Scheme of the pcDNA3.1 K15 expression plasmids. For further details, see the text. (C) Northern blot analysis of total RNA from the indicated cotransfection experiments. The 32P-labeled probe is directed against K15 exon 8. As a loading control, the blot was rehybridized with a GAPDH specific probe. On the left, a size marker in kilobases is indicated, and the RNA species are marked on the right. The Northern blots were quantified by phosphorimager analysis. The K15 signal was normalized to the GAPDH loading control. The expression of K15c without ORF57 was set to 1. Quantifications and standard deviations represent three independent experiments. (D) Western blot with protein extracts from the indicated cotransfections. Detection was performed with a K15 antibody raised against epitopes encoded in exon 8. Actin served as a loading control. The lysates were also used to detect ORF57-FLAG with an anti-FLAG antibody. A size marker in kilodaltons is indicated on the left, and the K15- and ORF57-specific band is indicated on the right. The inset on the right shows weak K15 expression after longer exposure times from the genomic constructs. (E) RNA from the indicated cotransfections was analyzed by RT-PCR. The primers bind in K15 exon 1 and exon 8, illustrated by arrows. The addition of the RT enzyme is depicted. A DNA size marker is shown on the left. u, unspliced; s, completely spliced. (F) BJAB cells harboring a recombinant KSHV genome were reactivated as indicated. The RNA was analyzed as in panel E. (G) The RNA from panel F was subjected to RT-qPCR by using a K15-specific probe. The value for nonreactivated BJAB rKSHV was set to 1. n.d., not detected.
K15 sequence optimization leads to an ORF57-independent expression.
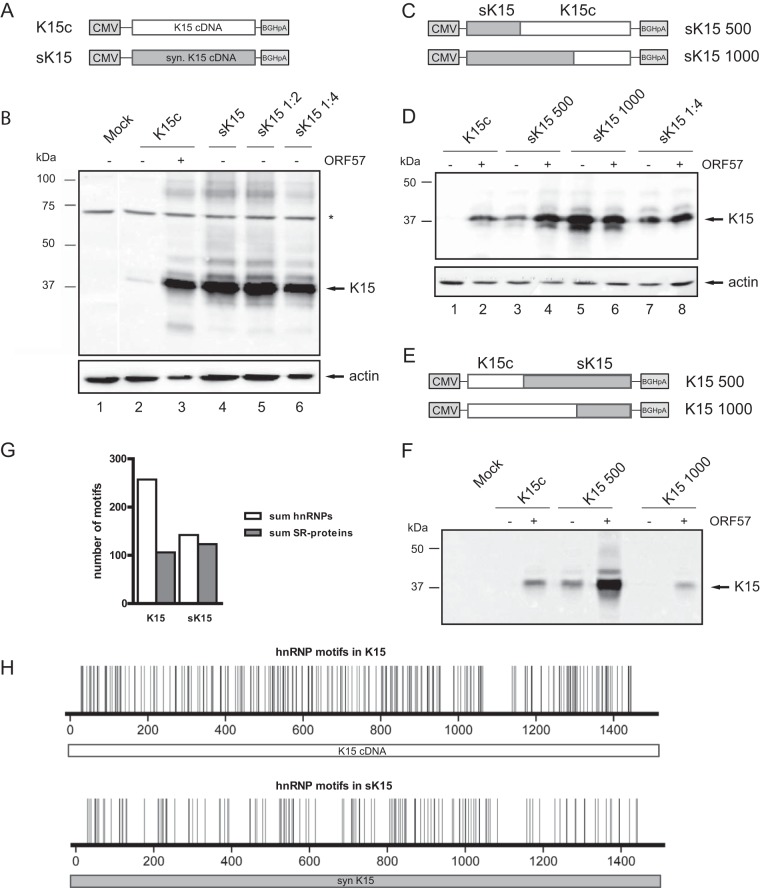
Since the target preference for ORF57 must at least partially be present in the cDNA of K15, we examined the cDNA sequence and noted a high AT content. Therefore, we wondered whether this nucleotide bias might confer ORF57 dependency. To test this hypothesis, we optimized the K15 cDNA sequence (Fig. 2A) by raising the GC content without changing the amino acid sequence (K15 cDNA, 43.1% GC content; sK15, 62.7% GC content). The optimization led to 27% nucleotide changes.
FIG 2.
Synthetic K15 sequence leads to ORF57 independency. HEK293T cells were cotransfected with K15c, sK15, or chimeric constructs and ORF57 cDNA. Protein was harvested at 36 h posttransfection and analyzed by Western blotting. (A) Depiction of K15 and sK15 expression constructs similar to Fig. 1B. The gray shading always represents the synthetic sequence. (B) Western blot with lysates from the indicated transfections. The right part of the blot shows serial dilutions of the sK15 lysate. The asterisk marks an unspecific band. Actin served as a loading control. (C) Scheme of the chimeric constructs with the synthetic part in gray. (D) Western blot with chimeric constructs. K15 was detected with anti-K15 antibody, and actin served as a loading control. (E) Depiction of chimeric constructs with 3′ synthetic K15 sequence (gray). (F) Western blot with lysates from the indicated transfections. K15 was detected with an anti-K15 antibody. (G) Comparison of the total number of predicted hnRNP (white) and SR proteins (gray) motifs between K15 cDNA and the synthetic sequence. (H) Predicted hnRNP motifs in the K15 wild type and the synthetic sequence. The numbering on the x axis corresponds to the nucleotides of the respective sequences. Each line represents the midpoint of cluster of binding motifs for the RNA-binding proteins analyzed (for further details, see Materials and Methods and see also Table S1 in the supplemental material).
Transient transfections now show an ORF57-independent expression of the synthetic K15 construct compared to wild-type K15 (Fig. 2B, lanes 2 and 4). The synthetic version is even expressed at higher levels than wild-type K15 cDNA plus ORF57, as shown by serial dilutions (Fig. 2B, compare lanes 3, 5, and 6). Thus, raising the GC content in the K15 coding sequence leads to ORF57-independent expression, suggesting that the high AT content of K15 cDNA might confer ORF57 dependency.
We also tested different chimeric constructs. In the sK15 500 construct, the first 500 nt (roughly one-third) of the K15 cDNA were replaced with the synthetic sequence (Fig. 2C). This leads to a partial ORF57 independent expression (Fig. 2D, lane 3), which can be still enhanced by cotransfection of ORF57 (Fig. 2D, lane 4). In contrast, exchanging the first 1,000 nt to the synthetic sequence confers complete ORF57-independent expression (Fig. 2D, lanes 7 and 8). Therefore, the greater the segment of synthetic sequence, the more K15 expression becomes ORF57 independent. Interestingly, exchanging the same segments at the 3′ end of K15 cDNA affected ORF57 dependency to a lesser extent (Fig. 2E and F).
We further performed a bioinformatic analysis predicting motifs of RNA-binding proteins in the K15 wild-type and the synthetic sequence (Fig. 2G). The binding motifs for 20 known RNA-binding proteins were predicted using RBPmap (50). As shown in Fig. 2H, the number of motifs for RNA-binding proteins from the hnRNP family was significantly lower in the synthetic K15 sequence compared to the wild type. Notably, the number of predicted hnRNP binding motifs in the synthetic K15 was similar to the number of motifs for SR proteins (Fig. 2G and see Table S1 in the supplemental material). By looking at the motif distribution for individual hnRNPs we observed specific changes and not a general reduction (see Table S1 in the supplemental material). For example, the strongest reduction of hnRNP motifs is observed for hnRNPs A and AB, while others, such as hnRNP C, nearly stays constant. The putative binding sites for hnRNP K even increased (see Table S1 in the supplemental material). These results strengthen the hypothesis that the optimization of the K15 cDNA and the underlying nucleotide substitutions changed the potential for recruiting RNA-binding proteins and as a consequence may alleviate the necessity for ORF57.
ORF57 overcomes the detrimental sequence bias of other KSHV lytic genes.
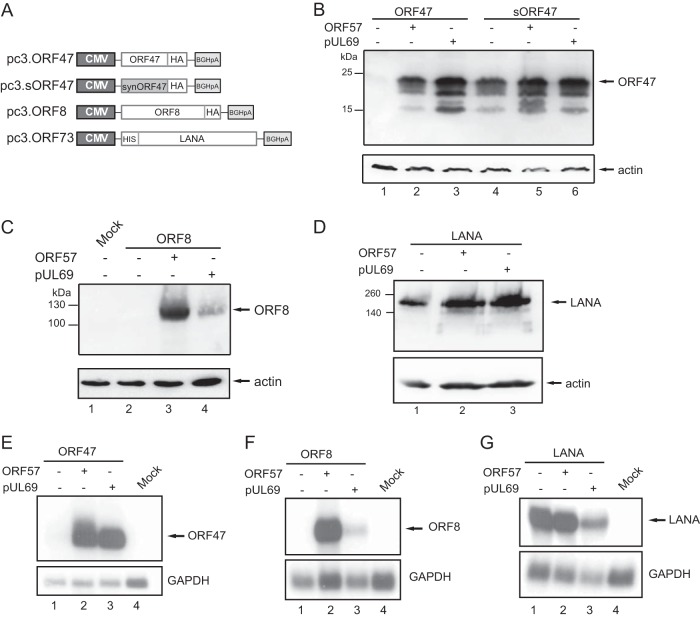
To explore whether the ORF57 dependence of lytic genes is also related to their sequence bias, we grouped the KSHV cDNAs according to their AT content (see Table S2 in the supplemental material). However, several KSHV mRNAs are polycistronic and share polyadenylation sites. We extracted only the protein coding regions from all mRNAs and did not differentiate between the nucleotide bias in the ORFs and the untranslated regions (UTRs). According to this ranking, K15 displays the third highest AT content in the KSHV genome. Based on our hypothesis, we chose another gene exhibiting a high AT content: ORF47. In a manner similar to that for K15, we also optimized the sequence for ORF47 by raising the GC content (pc3.sORF47; Fig. 3A). When we transfected the ORF47 wild-type cDNA, we were unable to detect ORF47 RNA and protein in the absence of ORF57 (Fig. 3B, lane 1, and E) or UL69, the RNA regulator from human cytomegalovirus (HCMV; see also below). However, cotransfection of either RNA regulator rescued ORF47 RNA and protein expression (Fig. 3B, lanes 2 and 3, and 4E). In contrast, the optimized version of ORF47 is ORF57 independent as observed for K15 (Fig. 3B, lane 4). In addition, ORF47, like K15, displays a similar ratio of predicted hnRNP and SR-protein motifs comparing the wild type and the optimized sequences (see Table S1 in the supplemental material). We further tested ORF8, a gene with an average AT content, which was previously described to be ORF57 independent in the context of the closely related rhesus monkey rhadinovirus (RRV) (52). Expression analysis revealed that KSHV ORF8 expression is both ORF57 and pUL69 dependent (Fig. 3C and F) in contrast to RRV ORF8. A sequence comparison reveals a lower AT content for RRV ORF8 (42.6%) than for the KSHV homologue (46.9%). In addition, an RBPmap analysis performed as in Fig. 2 displayed nearly four times more SR protein than hnRNP motifs in RRV ORF8 compared to 1.6-fold more hnRNP binding sites in KSHV ORF8 (data not shown and Table S1 in the supplemental material).
FIG 3.
ORF57 overcomes the detrimental sequence bias of other lytic genes. HEK293T cells were cotransfected with ORF57 or pUL69 and additional KSHV genes with different AT contents. Total RNA and protein were harvested at 40 h posttransfection and analyzed by Northern and Western blotting. (A) Depictions of expression plasmids for the additional KSHV genes analyzed. All proteins were detected by using anti-HA tag antibodies, except for LANA, where a specific antibody was used. (B) Western blot of cotransfections with the wild-type ORF47 and a synthetic version plus ORF57 or pUL69. (C) Western blot of cotransfection with ORF8 and ORF57 or pUL69. Actin served as a loading control. (D) Western blot of cotransfection of ORF57 or pUL69 with LANA (ORF73). Actin served as a loading control. (E) Northern blot analysis from the cotransfection experiment with ORF47 and ORF57 or pUL69. The 32P-labeled probe is directed against ORF47. A GAPDH probe served as loading control. (F) Northern blot analysis of total RNA from the ORF8 cotransfection experiment. The 32P-labeled probe is directed against the ORF8 cDNA. As a loading control, the blot was rehybridized with a GAPDH-specific probe. (G) Northern blot analysis from the cotransfection experiment with ORF57 or pUL69 and LANA. The 32P-labeled probe is directed against LANA. A GAPDH probe served as a loading control.
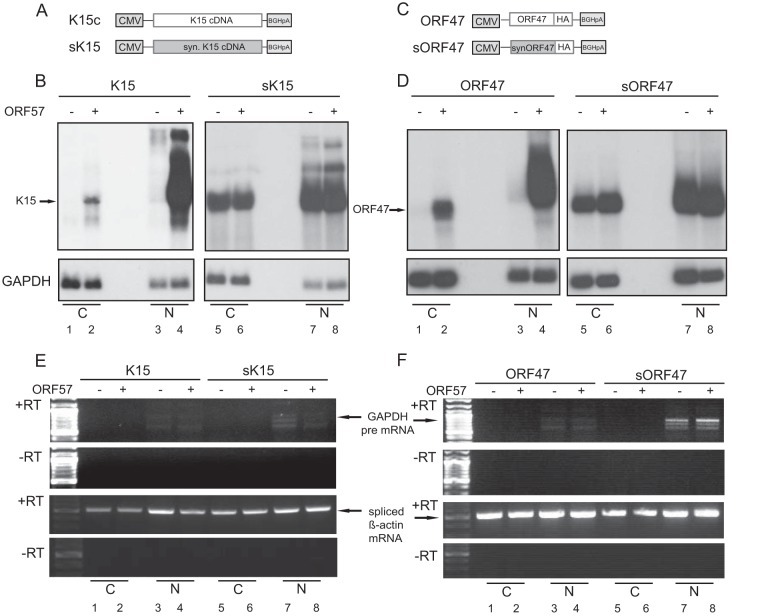
FIG 4.
ORF57 stabilizes KSHV RNAs in the nucleus. For nuclear and cytoplasmic fractionations, HEK293T cells were cotransfected with K15 (A) or ORF47 (C) constructs and ORF57 cDNA. The cells were harvested at 48 h (wild-type genes) or at 24 h (synthetic genes) posttransfection. (B and D) Northern blot analysis of cytoplasmic and nuclear (C and N, respectively) fractions from the indicated cotransfection experiments. For the cytoplasmic fraction, a 4-fold-higher RNA amount compared to the nuclear fraction was loaded. The 32P-labeled probe is directed against K15 exon 8 or GAPDH. (E and F) Semiquantitative RT-PCR controls for GAPDH pre-mRNA and for spliced β-actin mRNA, including the minus RT (reverse transcriptase) controls.
Importantly, LANA (or ORF73) a latent gene with low AT content is indeed ORF57 independent (see Fig. 3D and G). Taken together, these experiments show that ORF57 could overcome the detrimental sequence bias of KSHV lytic genes, whereas latent genes or genes with a low AT content are ORF57 independent.
ORF57 stabilizes KSHV RNAs in the nucleus.
As illustrated in Fig. 1 coexpression of ORF57 results in elevated levels of K15 RNA. Since the optimized version of K15 and ORF47 (Fig. 3) are ORF57 independent, we wondered whether increased RNA levels are responsible. In addition, total RNA was used in Fig. 1, which fails to distinguish between enhanced RNA stability or increased RNA export by ORF57. We picked K15 and ORF47 and their respective synthetic counterparts and performed RNA fractionation after transient transfection. Figure 4B reveals that ORF57 leads to a massive accumulation of K15 mRNA in the nucleus (Fig. 4B, lane 4). In the cytoplasmic fraction K15 is only detectable in the presence of ORF57 (Fig. 4B, lane 2). Thus, ORF57 enhances K15 mRNA stability in the nucleus and to a lesser extent induces export of K15 RNA. For the synthetic K15 construct, both fractions show large amounts of K15 mRNA irrespective of ORF57 cotransfection (Fig. 4B, right panel). Thus, the sequence optimization accounts for higher levels of K15 RNA in the nucleus and an efficient RNA export. ORF47 and its synthetic version behaved identical (Fig. 4D). Without ORF57, only traces of ORF47 RNA could be detected in the nucleus. In contrast, the optimized ORF47 is present in both the nuclear and the cytoplasmic fractions in the absence of ORF57 (Fig. 4D, lanes 5 and 7). The quality of the fractionation was controlled by semiquantitative RT-PCR of GAPDH pre-mRNA (Fig. 4E and F, upper panels) and for spliced β-actin mRNA (Fig. 4E and F, lower panels).
K15 expression is enhanced by the betaherpesviral homologue of ORF57.
All members of the herpesvirus family encode a posttranscriptional activator of viral gene expression (53). Since we found no discrete response element within the K15 cDNA as reported for the noncoding PAN RNA, but rather a certain sequence bias illustrated by the high AT content that elicits ORF57 dependency, we wondered whether such a mechanism is conserved among other herpesviral ORF57 homologues. We chose UL69 from HCMV, which belongs to the subfamily of betaherpesviruses. UL69 is able to enhance K15 expression to a greater extent than ORF57 (Fig. 5A, lane 3, and bottom panel). However, this may be due to higher expression levels of UL69 (Fig. 5, middle panel). At the RNA level, both UL69 and ORF57 enhanced K15 expression (data not shown). Since UL69 increases expression of a KSHV gene, we sought to determine whether ORF57 can also boost expression of an HCMV gene. We searched for an AT-rich gene from the otherwise GC-rich HCMV genome and picked UL11. Here, only UL69 leads to detectable UL11 protein expression in contrast to K15 (Fig. 5B). An analysis using RBPmap revealed that the number of hnRNP and SR protein motifs in UL11 is similar to that for K15 (data not shown). This indicates that there are additional features, which lead to UL11 expression in the presence of UL69, but not by cotransfection of ORF57.
FIG 5.
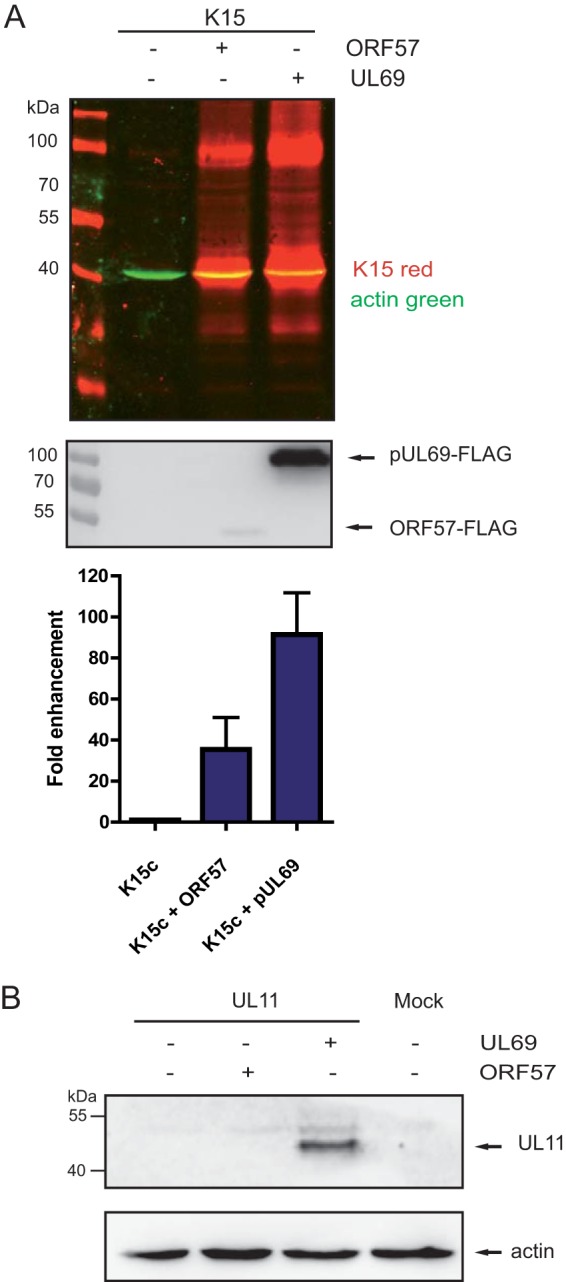
The HCMV homologue of ORF57 also enhances K15 expression. (A) HEK293T cells were cotransfected with K15 cDNA and ORF57 or HCMV pUL69. Protein was harvested at 40 h posttransfection and analyzed by Western blotting. Western blot of cotransfections with K15c and either ORF57 or HCMV pUL69. Detection and quantification were carried out with an Odyssey infrared imaging system. The expression of K15 alone was set to 1. The quantification represents three independent experiments. (B) Activity test of UL69 with UL11 an AT-rich RNA from HCMV. HEK293T cells were cotransfected with HCMV UL11 and pUL69 or KSHV ORF57. Protein was harvested at 40 h posttransfection and analyzed by Western blotting. V5-tagged UL11 was detected by an anti-V5 antibody.
ORF57 partially rescues HIV Gag expression.
Interestingly, HIV gag and KSHV K15 share some striking similarities. They have a comparable lengths (K15 cDNA, 1,470 bp; HIV gag, 1,540 bp) and an almost identical AT content (K15 cDNA, 57%; HIV gag, 56%). For both genes, sequence optimization leads to a comparable reduction in the AT content (sK15, 38%; synHIV gag, 34%) and to expression in the absence of their respective transactivators (54).
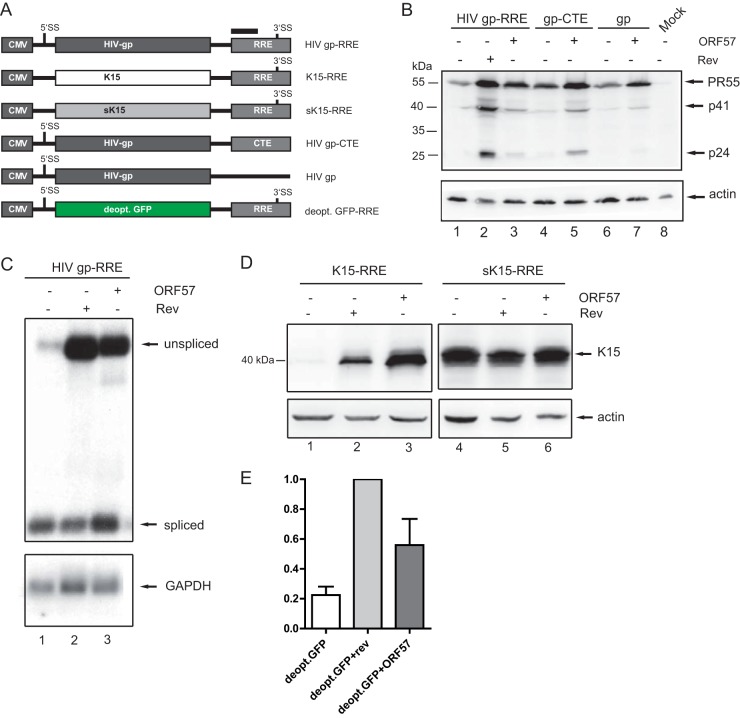
Based on these analogies and previous findings, we decided to test an HIV reporter plasmid (HIV gp-RRE [43]) containing the HIV 5′UTR and the gag/protease gene driven by the CMV promoter. The Rev responsive element (RRE) has been inserted in the 3′UTR (Fig. 6A). In this construct, Gag expression is strictly Rev dependent (Fig. 6B, lanes 1 and 2). Surprisingly, the cotransfection of ORF57 could partially rescue Gag expression (Fig. 6B, compare lanes 1 and 3). One reason for the incomplete rescue of HIV Gag expression could be the usage of a different export pathway by ORF57. Whereas Rev directs the HIV RNA via its nuclear export sequence into the CRM-1 (exportin-1) export pathway, ORF57 facilitates translocation of KSHV RNAs by recruiting the bulk mRNA export factor Tap (23, 55). This resembles the mode of action of the constitutive transport element (CTE) of a simple retrovirus (56). A CTE can also partially rescue Gag expression when incorporated into the Gag reporter plasmid instead of the RRE (Fig. 6A and B, lane 4). When we cotransfected the gp-CTE construct with ORF57, Gag expression is further enhanced arguing for an additive effect of ORF57 and the CTE (Fig. 6B, lane 5). To exclude that the effect of ORF57 on HIV Gag is dependent on the presence of the RRE, we cotransfected ORF57 with an RRE-deleted HIV-gp construct (Fig. 6A) and still observed a partial rescue of HIV Gag expression (Fig. 6B, lanes 6 and 7).
FIG 6.
Effect of ORF57 on HIV gag expression. HEK293T cells were cotransfected with the different RRE and CTE reporter plasmids and with ORF57 or HIV Rev. Total RNA and protein were harvested 40 h posttransfection and analyzed by Northern and Western blotting. The transfection of the sK15-RRE construct (D) was harvested at 24 h posttransfection. (A) All reporter plasmids are based on pcDNA3.1. The gag plasmids containing an RRE or a CTE were described previously (15). The constructs harbor HIV-derived splice sites (SS), as indicated. Cloning of the K15 cDNAs removed the HIV 5′SS. (B) Western blot of cotransfections with HIV-gp-RRE/CTE and ORF57. Gag was detected using an anti–HIV CA p24 antibody, and actin served as a loading control. (C) Northern blot of total RNA hybridized with an RRE probe showing spliced and unspliced RNA, as indicated on the right. As a loading control, the blot was rehybridized with a GAPDH-specific probe. (D) Western blot of transfection with K15-RRE/sK15-RRE constructs. K15 was detected with anti-K15 antibody, and actin served as a loading control. (E) Flow cytometry analysis of the deoptimized GFP-RRE construct. Transfection efficiency was measured by cotransfection of a DsRed plasmid (data not shown), and only double-positive cells were included in the mean fluorescence intensity (MFI) analysis. The MFI of deoptimized GFP when cotransfected with Rev was set to 1. The quantifications and standard deviations represent seven independent experiments.
We also assessed the effect of Rev and ORF57 on HIV gag RNA. The cotransfection of plasmids encoding Rev or ORF57 led to enhanced levels of unspliced gag RNA (Fig. 6C). Interestingly, ORF57 shifts the ratio between unspliced and spliced HIV RNAs toward the spliced RNA (Fig. 6C, compare lanes 2 and 3). Since Rev is recruited cotranscriptionally to the HIV-RNA and may induce rapid export before completion of the splicing reaction (57), we speculate that ORF57 is recruited posttranscriptionally and give the weak HIV 3′splice sites (SS) in our construct more time to splice. We also tried to rescue Gag expression in the context of an integrated HIV provirus. Here, transfection of ORF57 did not lead to a partial rescue of Gag expression (data not shown). A similar effect has been described for the CTE, which has only a weak effect when inserted into an HIV provirus (58).
In addition, we tested homologous constructs wherein HIV gag is replaced by either K15 or the synthetic version of K15 (sK15) (Fig. 6A). In this context, K15 is Rev dependent (Fig. 6D, lane 2). Interestingly, the effect of ORF57 on K15 expression is stronger compared to Rev (Fig. 6D, lanes 2 and 3). The reverse effect was observed for HIV Gag (Fig. 6B). This result confirms that the sequences of HIV gag and K15 share some features but are distinct in others. In addition, we performed a similar motif prediction for RNA-binding proteins (compare to Fig. 2E and F; see also Table S1 in the supplemental material) for HIV gag and its synthetic counterpart (59). Overall, a strong decrease in hnRNP motifs and a moderate increase in SR protein motifs can be observed. However, the numbers of motifs for certain proteins differ between K15 and HIV gag (see Table S1 in the supplemental material). As expected, the sK15-RRE construct is ORF57 and Rev independent due to its optimized sequence (Fig. 6D, lanes 4 to 6).
An adaptation of the humanized eGFP sequence to the HIV codon bias is sufficient to make the GFP expression Rev dependent (44). Based on this study and our hypothesis that the unusual high AT content might confer ORF57 dependency and in conjunction with the RBPmap analysis of both GFP variants (see Table S1 in the supplemental material), we tested a deoptimized GFP placed into the RRE construct (Fig. 6A). The expression of the deoptimized GFP was highly Rev dependent (Fig. 6E) in our flow cytometry analysis. The coexpression of ORF57 also resulted in a significant enhancement of deoptimized GFP expression (Fig. 6E). In summary, ORF57 allows for HIV Gag and deoptimized GFP expression and displayed a cumulative effect with a CTE. This further confirms our hypothesis that a distinct nucleotides bias leads to ORF57 dependency.
DISCUSSION
In this study, we addressed the question of how ORF57 is able to recognize and favor viral RNAs over cellular mRNAs. Interestingly, ORF57 harbors only a weak binding affinity for nucleic acids (38). This argues for one or more cellular cofactors, which at least make partial contact with the viral RNAs (38, 39). However, it remains to be shown how this complex recognizes its targets. Does it occur via a conserved sequence motif or based on other features, which by prediction all viral RNAs should have in common? Data have been published in favor for the first scenario, since an ORF57 response element (ORE) was identified within the long noncoding PAN RNA and viral interleukin-6 (35). These response elements share little sequence similarity, but both are predicted to fold into stem-loop structures containing a core tetranucleotide loop. However, the mechanism for viral interleukin-6 seems to be a special case. Here, ORF57 interacts with a miRNA binding site and thus prevents degradation and translational repression of viral and also human interleukin-6 (35–37). However, the reported elements are not present in other ORF57-dependent lytic genes (data not shown). This suggests an additional mechanism how ORF57 recognizes viral RNAs. We show that the high AT content of KSHV lytic genes is the basis for ORF57 dependency. The observation that cotransfection of ORF57 with a genomic or cDNA construct of the multiply spliced K15 gene enhances its expression demonstrates that the target preference for ORF57 must be present in the cDNA of its target genes (Fig. 1). However, the genomic construct revealed RNA expression, albeit more weakly, in the absence of ORF57 (Fig. 1C, lane1). This finding suggests that the K15 introns might have a stabilizing effect. In addition, K15 RNA from the genomic construct is poorly spliced, but nevertheless stabilized by ORF57, indicating that the effect of ORF57 is splicing independent. In general, K15 expression in the viral life cycle is low and increases after induction of the lytic cycle (Fig. 1F) (51, 60). Our data argue that inefficient splicing accounts for this expression block (Fig. 1E and F), which might be important to maintain latency. Poor splicing of K15 pre-mRNA might be due to the unusually small size of the introns and their weak splice sites. However, splicing does not lead to ORF57-independent expression (Fig. 1D and C). Importantly, it has also been shown in a previous study on ORF57 from rhesus monkey rhadinovirus (RRV) that insertion of a beta-globin intron in ORF57-dependent genes does not rescue their weak expression (61). Thus, the detrimental sequence bias of K15 is a dominant feature and requires ORF57 for efficient expression. This was further confirmed by the construction of an ORF57-independent synthetic K15 cDNA (Fig. 2A and B). A similar sequence optimization was performed for the RRV glycoproteins, which also show a high dependency for RRV ORF57 (52). We also tested chimeric constructs and observed that substitutions of nucleotides from the 5′ end leads to more ORF57 independency compared to synthetic parts in the 3′ end (Fig. 2C, D, and F). The same has been observed in the HIV gag gene, where gradual mutation of instability elements from the 5′ end lead to nuclear RNA export that is independent of the HIV export factor Rev (62). A possible explanation for this polarity is the cotranscriptional formation of stable export competent HIV mRNPs (63). In addition, ORF57 still enhanced K15 expression from the chimeric constructs showing that the effect of ORF57 is cumulative and does not dependent on a single response element. If the assumption is correct that there is no single response element for ORF57 in the majority of KSHV lytic genes, the distinct nucleotide bias may constitute several motifs for RNA-binding proteins. It was previously shown for HIV gag that improvement of expression after sequence optimization is not a consequence of enhanced translation or tRNA availability (64). Thus, we hypothesize that the unbiased increase in the GC content leads to a change in the distribution and density of motifs for RNA-binding proteins along the transcript. Indeed, a bioinformatic comparison between wild-type and synthetic K15 revealed a different frequency of predicted motifs for certain RNA-binding proteins. As expected, based on the higher GC content in the synthetic K15, the number of predicted motifs for hnRNPs is significantly reduced. Although we cannot narrow down a role for individual hnRNPs, the overall density of predicted hnRNP motifs suggests that this is a critical feature conferring ORF57 dependency (Fig. 2H). The same could be observed for ORF47, HIV gag and deoptimized GFP (see Table S1 in the supplemental material). If the sequence bias and the resulting frequency and distribution of RBP/hnRNP motifs are critical determinants of ORF57 dependency, it should be conserved in other lytic genes of KSHV. Indeed, we found a correlation between AT content and ORF57 dependency (Fig. 3). Interestingly, RRV gB (ORF8) is expressed in the absence of ORF57 (52), in contrast to our findings on KSHV ORF8 (Fig. 3C). However, RRV ORF8 displays more SR protein binding motifs than KSHV ORF8, presumably resulting in its ORF57-independent expression. In addition, RRV ORF8 exhibits a low AT content as the KSHV counterpart (data not shown).
Within this line another study observed a potential interaction of ORF57 with the mRNA encoding chloramphenicol transferase (CAT) (21). Interestingly, the CAT mRNA also exhibits an AT-rich nucleotide bias. These observations further support our hypothesis that ORF57 overcomes the detrimental nucleotide bias of KSHV lytic genes.
Additional support in favor for this hypothesis is the partial rescue of HIV Gag expression by ORF57 (Fig. 6B and C). A direct comparison with the effect of the CTE on HIV Gag expression allows us to speculate that the partial rescue is a consequence of the export route. ORF57 and CTE use the Tap/NXT1 export pathway in contrast to Rev which binds to CRM-1. Interestingly, ORF57 and a CTE can display a cumulative effect. This resembles the observation that the presence of multiple CTE copies can completely restore Gag expression (65). Thus, Gag expression may directly correlate with the number of bound Tap/NXT1 complexes. Our finding that ORF57 can partially rescue HIV gag expression does not agree with a previous publication (53). In this study, a chimeric construct consisting of HIV gag and KSHV ORF59 (a reported target of ORF57) displayed no effect of ORF57. However, in our experimental setting we directly compared the effect of ORF57 on HIV gag with the effect of HIV Rev or the CTE (Fig. 6).
By using a deoptimized GFP construct, we provide further evidence that the nucleotide bias of the respective mRNA is the key feature of ORF57 dependency (Fig. 6E). From a viral perspective, this nucleotide bias is an advantage if the latent state is the rule and not the exception (5, 66). Accidental transcription of lytic genes does not lead to protein expression and presentation of antigens. The same has been proposed also for HIV (67). Background gag RNA expression is confined to the nucleus due to a combination of unused splice sites and an unfavorable nucleotide bias (59, 62, 68; J. Bohne et al., unpublished data).
Based on these experimental data and our bioinformatic analysis, we speculate that ORF57 binds viral RNAs in complex with a cellular hnRNP, which in turn confers the sequence preference due to common nucleotide bias of all lytic genes. Indeed, the interaction of ORF57 with hnRNP E1 and K has been reported previously (69, 70). Unfortunately, a consensus motif for hnRNP E was not available. Interestingly, the number of predicted hnRNP K motifs is slightly increased in all optimized genes analyzed (see Table S1 in the supplemental material). However, this does not exclude hnRNP K as potential interaction partner of ORF57, since four copies of the CTE completely rescued HIV Gag expression (65) and a comparable number of binding motifs could be sufficient for the ORF57 interacting partner. This includes the possibility that ORF57 might also be targeted to the synthetic sequences, but the positive effect might be masked due to the overall favorable mRNP composition. This mRNP signature is also responsible for an ORF57-independent increase in nuclear and cytoplasmic RNA levels for the synthetic versions (Fig. 4B and D). However, nuclear stabilization of the wild-type K15 and ORF47 RNAs is the main phenotype of ORF57, as described for the PAN RNA (25, 26). Interestingly, the protein levels for K15 and sK15 did not differ as much as the RNA levels (Fig. 2D and Fig. 4B). This suggests that ORF57 also influences translation efficiency of KSHV RNAs, as previously described (30). In turn, the data also demonstrate that enhanced expression after sequence optimization is not a consequence of elevated translation due to altered codon usage (Fig. 4B and D). With this strategy, KSHV accepts or even intends that the expression of cellular RNAs with a comparable sequence bias may also be increased upon production of ORF57 during the lytic replication cycle.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Stamminger, M. Messerle, and S. Gramolelli for providing reagents. In addition, we thank A. Grundhoff for critical discussion of the data.
This study was funded by a Niedersachsen-Israeli grant (ZN 2628) to Y.M.-G. and J.B.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03264-14.
REFERENCES
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Damania B. 2004. Oncogenic gammaherpesviruses: comparison of viral proteins involved in tumorigenesis. Nat Rev Microbiol 2:656–668. doi: 10.1038/nrmicro958. [DOI] [PubMed] [Google Scholar]
- 3.Ganem D. 2010. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J Clin Invest 120:939–949. doi: 10.1172/JCI40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganem D. 2006. KSHV infection and the pathogenesis of Kaposi's sarcoma. Annu Rev Pathol 1:273–296. doi: 10.1146/annurev.pathol.1.110304.100133. [DOI] [PubMed] [Google Scholar]
- 5.Schulz TF. 2006. The pleiotropic effects of Kaposi's sarcoma herpesvirus. J Pathol 208:187–198. doi: 10.1002/path.1904. [DOI] [PubMed] [Google Scholar]
- 6.Le Hir H, Gatfield D, Izaurralde E, Moore MJ. 2001. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J 20:4987–4997. doi: 10.1093/emboj/20.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Hir H, Izaurralde E, Maquat LE, Moore MJ. 2000. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon-exon junctions. EMBO J 19:6860–6869. doi: 10.1093/emboj/19.24.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nino CA, Herissant L, Babour A, Dargemont C. 2013. mRNA nuclear export in yeast. Chem Rev 113:8523–8545. doi: 10.1021/cr400002g. [DOI] [PubMed] [Google Scholar]
- 9.Cullen BR. 2003. Nuclear mRNA export: insights from virology. Trends Biochem Sci 28:419–424. doi: 10.1016/S0968-0004(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 10.Gatfield D, Izaurralde E. 2002. REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J Cell Biol 159:579–588. doi: 10.1083/jcb.200207128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatfield D, Le Hir H, Schmitt C, Braun IC, Kocher T, Wilm M, Izaurralde E. 2001. The DExH/D box protein HEL/UAP56 is essential for mRNA nuclear export in Drosophila. Curr Biol 11:1716–1721. doi: 10.1016/S0960-9822(01)00532-2. [DOI] [PubMed] [Google Scholar]
- 12.Herold A, Klymenko T, Izaurralde E. 2001. NXF1/p15 heterodimers are essential for mRNA nuclear export in Drosophila. RNA 7:1768–1780. [PMC free article] [PubMed] [Google Scholar]
- 13.Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, Reed R. 2001. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature 413:644–647. doi: 10.1038/35098106. [DOI] [PubMed] [Google Scholar]
- 14.Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, Bork P, Izaurralde E. 2000. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 6:638–650. doi: 10.1017/S1355838200000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiegand HL, Coburn GA, Zeng Y, Kang Y, Bogerd HP, Cullen BR. 2002. Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes. Mol Cell Biol 22:245–256. doi: 10.1128/MCB.22.1.245-256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grzybowska EA. 2012. Human intronless genes: functional groups, associated diseases, evolution, and mRNA processing in absence of splicing. Biochem Biophys Res Commun 424:1–6. doi: 10.1016/j.bbrc.2012.06.092. [DOI] [PubMed] [Google Scholar]
- 17.Zheng ZM. 2003. Split genes and their expression in Kaposi's sarcoma-associated herpesvirus. Rev Med Virol 13:173–184. doi: 10.1002/rmv.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Hir H, Nott A, Moore MJ. 2003. How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci 28:215–220. doi: 10.1016/S0968-0004(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 19.Reed R, Hurt E. 2002. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell 108:523–531. doi: 10.1016/S0092-8674(02)00627-X. [DOI] [PubMed] [Google Scholar]
- 20.Sandri-Goldin RM. 2008. The many roles of the regulatory protein ICP27 during herpes simplex virus infection. Front Biosci 13:5241–5256. [DOI] [PubMed] [Google Scholar]
- 21.Malik P, Blackbourn DJ, Clements JB. 2004. The evolutionarily conserved Kaposi's sarcoma-associated herpesvirus ORF57 protein interacts with REF protein and acts as an RNA export factor. J Biol Chem 279:33001–33011. doi: 10.1074/jbc.M313008200. [DOI] [PubMed] [Google Scholar]
- 22.Toth Z, Stamminger T. 2008. The human cytomegalovirus regulatory protein UL69 and its effect on mRNA export. Front Biosci 13:2939–2949. doi: 10.2741/2899. [DOI] [PubMed] [Google Scholar]
- 23.Boyne JR, Colgan KJ, Whitehouse A. 2008. Recruitment of the complete hTREX complex is required for Kaposi's sarcoma-associated herpesvirus intronless mRNA nuclear export and virus replication. PLoS Pathog 4:e1000194. doi: 10.1371/journal.ppat.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson BR, Boyne JR, Noerenberg M, Taylor A, Hautbergue GM, Walsh MJ, Wheat R, Blackbourn DJ, Wilson SA, Whitehouse A. 2011. An interaction between KSHV ORF57 and UIF provides mRNA-adaptor redundancy in herpesvirus intronless mRNA export. PLoS Pathog 7:e1002138. doi: 10.1371/journal.ppat.1002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nekorchuk M, Han Z, Hsieh TT, Swaminathan S. 2007. Kaposi's sarcoma-associated herpesvirus ORF57 protein enhances mRNA accumulation independently of effects on nuclear RNA export. J Virol 81:9990–9998. doi: 10.1128/JVI.00896-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stubbs SH, Hunter OV, Hoover A, Conrad NK. 2012. Viral factors reveal a role for REF/Aly in nuclear RNA stability. Mol Cell Biol 32:1260–1270. doi: 10.1128/MCB.06420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun R, Lin SF, Gradoville L, Miller G. 1996. Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A 93:11883–11888. doi: 10.1073/pnas.93.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majerciak V, Lu M, Li X, Zheng ZM. 2014. Attenuation of the suppressive activity of cellular splicing factor SRSF3 by Kaposi sarcoma-associated herpesvirus ORF57 protein is required for RNA splicing. RNA 20:1747–1758. doi: 10.1261/rna.045500.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majerciak V, Yamanegi K, Allemand E, Kruhlak M, Krainer AR, Zheng ZM. 2008. Kaposi's sarcoma-associated herpesvirus ORF57 functions as a viral splicing factor and promotes expression of intron-containing viral lytic genes in spliceosome-mediated RNA splicing. J Virol 82:2792–2801. doi: 10.1128/JVI.01856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyne JR, Jackson BR, Taylor A, Macnab SA, Whitehouse A. 2010. Kaposi's sarcoma-associated herpesvirus ORF57 protein interacts with PYM to enhance translation of viral intronless mRNAs. EMBO J 29:1851–1864. doi: 10.1038/emboj.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diem MD, Chan CC, Younis I, Dreyfuss G. 2007. PYM binds the cytoplasmic exon-junction complex and ribosomes to enhance translation of spliced mRNAs. Nat Struct Mol Biol 14:1173–1179. doi: 10.1038/nsmb1321. [DOI] [PubMed] [Google Scholar]
- 32.Malik P, Blackbourn DJ, Cheng MF, Hayward GS, Clements JB. 2004. Functional co-operation between the Kaposi's sarcoma-associated herpesvirus ORF57 and ORF50 regulatory proteins. J Gen Virol 85:2155–2166. doi: 10.1099/vir.0.79784-0. [DOI] [PubMed] [Google Scholar]
- 33.Jackson BR, Noerenberg M, Whitehouse A. 2014. A novel mechanism inducing genome instability in Kaposi's sarcoma-associated herpesvirus-infected cells. PLoS Pathog 10:e1004098. doi: 10.1371/journal.ppat.1004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumann S, Jackson BR, Baquero-Perez B, Whitehouse A. 2013. Kaposi's sarcoma-associated herpesvirus ORF57 protein: exploiting all stages of viral mRNA processing. Viruses 5:1901–1923. doi: 10.3390/v5081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JG, Pripuzova N, Majerciak V, Kruhlak M, Le SY, Zheng ZM. 2011. Kaposi's sarcoma-associated herpesvirus ORF57 promotes escape of viral and human interleukin-6 from microRNA-mediated suppression. J Virol 85:2620–2630. doi: 10.1128/JVI.02144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massimelli MJ, Kang JG, Majerciak V, Le SY, Liewehr DJ, Steinberg SM, Zheng ZM. 2011. Stability of a long noncoding viral RNA depends on a 9-nt core element at the RNA 5′ end to interact with viral ORF57 and cellular PABPC1. Int J Biol Sci 7:1145–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sei E, Conrad NK. 2011. Delineation of a core RNA element required for Kaposi's sarcoma-associated herpesvirus ORF57 binding and activity. Virology 419:107–116. doi: 10.1016/j.virol.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majerciak V, Yamanegi K, Nie SH, Zheng ZM. 2006. Structural and functional analyses of Kaposi sarcoma-associated herpesvirus ORF57 nuclear localization signals in living cells. J Biol Chem 281:28365–28378. doi: 10.1074/jbc.M603095200. [DOI] [PubMed] [Google Scholar]
- 39.Majerciak V, Zheng ZM. 2009. Kaposi's sarcoma-associated herpesvirus ORF57 in viral RNA processing. Front Biosci (Landmark ed) 14:1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson BR, Noerenberg M, Whitehouse A. 2012. The Kaposi's sarcoma-associated herpesvirus ORF57 protein and its multiple roles in mRNA biogenesis. Front Microbiol 3:59. doi: 10.3389/fmicb.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou FC, Zhang YJ, Deng JH, Wang XP, Pan HY, Hettler E, Gao SJ. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J Virol 76:6185–6196. doi: 10.1128/JVI.76.12.6185-6196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ottinger M, Christalla T, Nathan K, Brinkmann MM, Viejo-Borbolla A, Schulz TF. 2006. Kaposi's sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J Virol 80:10772–10786. doi: 10.1128/JVI.00804-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wodrich H, Bohne J, Gumz E, Welker R, Krausslich HG. 2001. A new RNA element located in the coding region of a murine endogenous retrovirus can functionally replace the Rev/Rev-responsive element system in human immunodeficiency virus type 1 Gag expression. J Virol 75:10670–10682. doi: 10.1128/JVI.75.22.10670-10682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graf M, Ludwig C, Kehlenbeck S, Jungert K, Wagner R. 2006. A quasi-lentiviral green fluorescent protein reporter exhibits nuclear export features of late human immunodeficiency virus type 1 transcripts. Virology 352:295–305. doi: 10.1016/j.virol.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Gabaev I, Steinbruck L, Pokoyski C, Pich A, Stanton RJ, Schwinzer R, Schulz TF, Jacobs R, Messerle M, Kay-Fedorov PC. 2011. The human cytomegalovirus UL11 protein interacts with the receptor tyrosine phosphatase CD45, resulting in functional paralysis of T cells. PLoS Pathog 7:e1002432. doi: 10.1371/journal.ppat.1002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zychlinski D, Erkelenz S, Melhorn V, Baum C, Schaal H, Bohne J. 2009. Limited complementarity between U1 snRNA and a retroviral 5′ splice site permits its attenuation via RNA secondary structure. Nucleic Acids Res 37:7429–7440. doi: 10.1093/nar/gkp694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kati S, Tsao EH, Gunther T, Weidner-Glunde M, Rothamel T, Grundhoff A, Kellam P, Schulz TF. 2013. Activation of the B cell antigen receptor triggers reactivation of latent Kaposi's sarcoma-associated herpesvirus in B cells. J Virol 87:8004–8016. doi: 10.1128/JVI.00506-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas DA, Bala K, Busche G, Weidner-Glunde M, Santag S, Kati S, Gramolelli S, Damas M, Dittrich-Breiholz O, Kracht M, Ruckert J, Varga Z, Keri G, Schulz TF. 2013. The inflammatory kinase MAP4K4 promotes reactivation of Kaposi's sarcoma herpesvirus and enhances the invasiveness of infected endothelial cells. PLoS Pathog 9:e1003737. doi: 10.1371/journal.ppat.1003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brinkmann MM, Glenn M, Rainbow L, Kieser A, Henke-Gendo C, Schulz TF. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J Virol 77:9346–9358. doi: 10.1128/JVI.77.17.9346-9358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paz I, Kosti I, Ares M Jr, Cline M, Mandel-Gutfreund Y. 2014. RBPmap: a web server for mapping binding sites of RNA-binding proteins. Nucleic Acids Res 42:W361–W367. doi: 10.1093/nar/gku406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brinkmann MM, Pietrek M, Dittrich-Breiholz O, Kracht M, Schulz TF. 2007. Modulation of host gene expression by the K15 protein of Kaposi's sarcoma-associated herpesvirus. J Virol 81:42–58. doi: 10.1128/JVI.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bilello JP, Morgan JS, Desrosiers RC. 2008. Extreme dependence of gH and gL expression on ORF57 and association with highly unusual codon usage in rhesus monkey rhadinovirus. J Virol 82:7231–7237. doi: 10.1128/JVI.00564-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pilkington GR, Majerciak V, Bear J, Uranishi H, Zheng ZM, Felber BK. 2012. Kaposi's sarcoma-associated herpesvirus ORF57 is not a bona fide export factor. J Virol 86:13089–13094. doi: 10.1128/JVI.00606-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner R, Graf M, Bieler K, Wolf H, Grunwald T, Foley P, Uberla K. 2000. Rev-independent expression of synthetic gag-pol genes of human immunodeficiency virus type 1 and simian immunodeficiency virus: implications for the safety of lentiviral vectors. Hum Gene Ther 11:2403–2413. doi: 10.1089/104303400750038507. [DOI] [PubMed] [Google Scholar]
- 55.Fornerod M, Ohno M, Yoshida M, Mattaj IW. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051–1060. doi: 10.1016/S0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 56.Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber BK, Izaurralde E. 1998. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell 1:649–659. doi: 10.1016/S1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 57.Iacampo S, Cochrane A. 1996. Human immunodeficiency virus type 1 Rev function requires continued synthesis of its target mRNA. J Virol 70:8332–8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zolotukhin AS, Valentin A, Pavlakis GN, Felber BK. 1994. Continuous propagation of RRE(−) and Rev(−) RRE(−) human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J Virol 68:7944–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graf M, Bojak A, Deml L, Bieler K, Wolf H, Wagner R. 2000. Concerted action of multiple cis-acting sequences is required for Rev dependence of late human immunodeficiency virus type 1 gene expression. J Virol 74:10822–10826. doi: 10.1128/JVI.74.22.10822-10826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bala K, Bosco R, Gramolelli S, Haas DA, Kati S, Pietrek M, Havemeier A, Yakushko Y, Singh VV, Dittrich-Breiholz O, Kracht M, Schulz TF. 2012. Kaposi's sarcoma herpesvirus K15 protein contributes to virus-induced angiogenesis by recruiting PLCγ1 and activating NFAT1-dependent RCAN1 expression. PLoS Pathog 8:e1002927. doi: 10.1371/journal.ppat.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin YC, Desrosiers RC. 2011. Rhesus monkey rhadinovirus ORF57 induces gH and gL glycoprotein expression through posttranscriptional accumulation of target mRNAs. J Virol 85:7810–7817. doi: 10.1128/JVI.00493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol 71:4892–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nawroth I, Mueller F, Basyuk E, Beerens N, Rahbek UL, Darzacq X, Bertrand E, Kjems J, Schmidt U. 2014. Stable assembly of HIV-1 export complexes occurs cotranscriptionally. RNA 20:1–8. doi: 10.1261/rna.038182.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kofman A, Graf M, Deml L, Wolf H, Wagner R. 2003. Codon usage-mediated inhibition of HIV-1 gag expression in mammalian cells occurs independently of translation. Tsitologiia 45:94–100. [PubMed] [Google Scholar]
- 65.Wodrich H, Schambach A, Krausslich HG. 2000. Multiple copies of the Mason-Pfizer monkey virus constitutive RNA transport element lead to enhanced HIV-1 Gag expression in a context-dependent manner. Nucleic Acids Res 28:901–910. doi: 10.1093/nar/28.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santag S, Jager W, Karsten CB, Kati S, Pietrek M, Steinemann D, Sarek G, Ojala PM, Schulz TF. 2013. Recruitment of the tumour suppressor protein p73 by Kaposi's sarcoma herpesvirus latent nuclear antigen contributes to the survival of primary effusion lymphoma cells. Oncogene 32:3676–3685. doi: 10.1038/onc.2012.385. [DOI] [PubMed] [Google Scholar]
- 67.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, Siliciano RF. 2006. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog 2:e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bohne J, Wodrich H, Krausslich HG. 2005. Splicing of human immunodeficiency virus RNA is position-dependent suggesting sequential removal of introns from the 5′ end. Nucleic Acids Res 33:825–837. doi: 10.1093/nar/gki185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malik P, Clements JB. 2004. Protein kinase CK2 phosphorylation regulates the interaction of Kaposi's sarcoma-associated herpesvirus regulatory protein ORF57 with its multifunctional partner hnRNP K. Nucleic Acids Res 32:5553–5569. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Nishimura K, Ueda K, Guwanan E, Sakakibara S, Do E, Osaki E, Yada K, Okuno T, Yamanishi K. 2004. A posttranscriptional regulator of Kaposi's sarcoma-associated herpesvirus interacts with RNA-binding protein PCBP1 and controls gene expression through the IRES. Virology 325:364–378. doi: 10.1016/j.virol.2004.04.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.