Abstract
Vaccinia virus (VACV) continues to be used in immunotherapy for the prevention of infectious diseases and treatment of cancer since its use for the eradication of smallpox. However, the current method of editing the VACV genome is not efficient. Here, we demonstrate that the CRISPR-Cas9 system can be used to edit the VACV genome rapidly and efficiently. Additionally, a set of 8,964 computationally designed unique guide RNAs (gRNAs) targeting all VACV genes will be valuable for the study of VACV gene functions.
TEXT
Since the eradication of smallpox, vaccinia virus (VACV) has been developed as a vector for vaccines against infectious diseases and immunotherapies for cancer (1–4), including oncolytic virotherapies (5–8). The renewed interest in VACV has driven a number of vaccine and therapeutic candidates to clinical trials, showing especially encouraging results for cancer treatment (5, 7). To improve VACV as a vector for vaccine or cancer therapy, a flexible system is required to delete viral genes or arm the VACV with therapeutic genes. Such a system would also expedite discoveries in cell biology, such as dissection of the signaling pathways used by VACV for its actin-based motility (9). Several strategies have been developed to construct VACV vectors (10–12); the current method for modification of VACV is based on homologous recombination in mammalian cells, but only 1% to 5% of the recombinant plaques contain the inserted DNA (13).
The clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 system is a natural microbial immune mechanism against invading viruses and other genetic elements (14–16). The CRISPR-Cas9 system, consisting of the RNA-guided Cas9 endonuclease (from Streptococcus pyogenes), a single guide RNA (sgRNA), and the trans-activating CRISPR RNA (tracrRNA), has been adapted for genome editing in eukaryotic cells (17, 18). The system has been used successfully for efficient generation of genetically modified cells and animal models (17, 19–21). Recently, the genomes of adenovirus and type I herpes simplex virus were edited using the gRNA-guided Cas9 system (22). We hypothesized that the CRISPR-Cas9 system could specifically generate double-strand breaks (DSBs) in the target DNA sites of VACV, increasing the efficiency of editing VACV genomes and constructing new VACV vectors expressing therapeutic genes.
Given that VACV replicates in the cytoplasm of infected cells, the Cas9 gene without the nuclear localization signal (NLS) was cloned into the pST1374 vector (Fig. 1A). The expression of Cas9 was confirmed (Fig. 1A). Given the important role of the N1L gene in virulence and in regulating the host immune response to VACV (23–25), we chose this as an example to validate the application of CRISPR-Cas9 for editing VACV. Two individual sgRNAs targeting the VACV N1L (LO24) gene were cloned into this guide RNA (gRNA) cloning vector (Fig. 1B) and designated gRNA N1 and N2. To prove that the CRISPR-Cas9 system could improve the efficiency of constructing a VACV vector expressing a therapeutic gene, a shuttle vector (pSh-N1LRFP/TRP2) was constructed to insert red fluorescent protein (RFP) and the tumor-associated antigen TRP2 driven by H5 promoters flanked by homologous sequences targeting both sides of the N1L region (Fig. 1C). The two gRNA plasmids targeting the N1L gene were used separately with Cas9 to generate mutant VACV in the presence of pSh-N1LRFP/TRP2 (donor vector). The success rates of N1L gene deletion in purified RFP-positive plaques (Fig. 1D) were 14.3% (1/7) and 62.5% (5/8) for gRNA N1 and gRNA N2, respectively (Fig. 1E, Table 1), whereas no deletion was detected in the 16 control plaques without prior exposure to CRISPR-Cas9 and the guide RNA (Fig. 1E). Thus, gRNA N2-guided Cas9 significantly increased (about 12 to 60 times) the homologous recombination efficiency in the N1L region. Furthermore, the transgene TRP2 was efficiently expressed in the virus-infected cells (Fig. 1F). For the detailed methods, see the Supplemental Methods.
FIG 1.
gRNA-guided Cas9 induces homologous recombination in the N1L gene. (A) The expression cassette for Cas9 was cloned into the pST1374 vector (pCas9 construct). Cas9 expression was confirmed by reverse transcriptase PCR. (B) Sequences of N1L gRNAs N1 and N2, as well as their alignment on the N1L gene. The PAM is underlined. (C) Schematic of the homologous recombination cassette of the shuttle vector (repair donor vector) and repaired target region in the vaccinia virus genome. X indicates homologous recombination. (D) Image of pure plaques of mutant VACV expressing RFP (magnification, ×400). (E) N1L gene deletion was verified by PCR in pure plaques of mutant VACV obtained from N1L gRNA N2-guided, Cas9-induced homologous recombination. Amplification of the N1L and L026 genes (Target) was carried out by PCR. A52R gene amplification was used as a DNA control. (F) Transgene Trp2 expression in CV1 cells after infection with N1L-deleted VACV was detected by Western blot assay. C, control virus-infected cells; M, mutant virus-infected cells. Actin was used as a loading control.
TABLE 1.
Efficiency of gRNA-guided Cas9-induced homologous recombination
| Target site | Sequence (5′–3′)a | % efficiency (no. of plaques with deletion/total no. of plaques) |
|---|---|---|
| N1L gRNA N1 | CGTCTTCTATATTATTAACTGG | 14.3 (1/7) |
| N1L gRNA N2 | GATTCAATCTATCTAGCAATGG | 62.5 (5/8) |
| A46R gRNA A1 | TTCCAGAGGAAACTAATATTGG | 80 (12/15) |
| A46R gRNA A2 | ACAAGTAAGTCATACTAACCGG | 80 (12/15) |
| A46R gRNA A3 | ATGACACCATAGAGATAAGAGG | 85 (6/7) |
Underlining shows the protospacer adjacent motif (PAM) sequence located on the immediate 3′ end of the gRNA recognition sequence.
To test the versatility of gRNA-guided Cas9 in modifying VACV genomes, three gRNAs targeting the VACV A46R gene (Fig. 2A) were cloned into the gRNA cloning vector, as A46R is another important gene that regulates the host immune response and, therefore, the deletion of A46R may further improve the efficacy of VACV therapeutics (26, 27) (see the Supplemental Methods). The three gRNA plasmids were transfected separately with Cas9 to generate mutant VACV in the presence of the A46R homologous recombination shuttle vector (donor vector), using RFP as the plaque purification marker (Fig. 2B). The success rates of A46R gene deletion in RFP-positive plaques were 80% (12/15), 80% (12/15), and 85% (6/7) for gRNA A1, gRNA A2, and gRNA A3, respectively (Fig. 2C; Table 1), whereas there was no deletion detected in the 28 control plaques without prior exposure to CRISPR-Cas9 and the guide RNA (data not shown).
FIG 2.
gRNA-guided, Cas9-induced homologous recombination in the A46R gene. (A) Cloning cassette for gRNA, sequences of A46R gRNAs A1 to A3, and their alignments on the A46R gene. PAM is underlined. (B) Schematics of homologous recombination cassette of shuttle vector (repair donor vector) and repaired target region on VACV genome. X indicates homologous recombination. (C) A46R gene deletion in pure plaques of mutant VACV obtained by using A46R gRNA was verified by PCR. A1-, A2- and A3-guided Cas9, respectively, induced homologous recombination. Amplification of the A46R and A47L genes (Target) was carried out by PCR. A52R gene amplification was used as a DNA control.
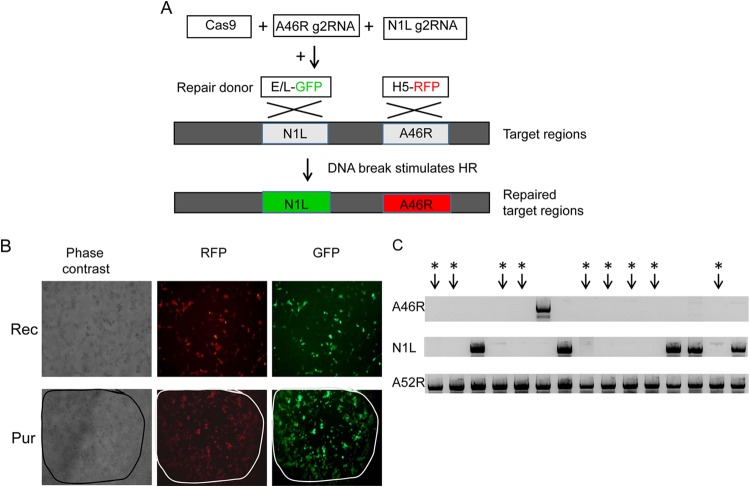
In certain cases, there is a need to modify two or more genes in VACV to generate a mutant virus. Using the current method, more than 4 weeks are required to achieve modification of two genes in the virus. To test whether multiple genes of VACV can be edited simultaneously by gRNA-guided Cas9, the N1L region and the A46R region were targeted. N1L gRNA N2 and A46R gRNA A2 were used to guide Cas9 editing of the VACV genomes (Table 1). The shuttle vector carrying green fluorescent protein (GFP) for the N1L region (Fig. 3A) and donor vector carrying RFP for the A46R region were used for homologous recombination (Fig. 3A). Plaques that were double positive for both GFP and RFP were purified over 3 to 5 rounds (Fig. 3B). N1L and A46R dual deletions were found in 9 of 15 double-positive plaques (60%) (Fig. 3C). This result demonstrates that gRNA-guided Cas9 can simultaneously and rapidly induce homologous recombination across multiple target sites on the VACV genome. For the detailed methods, see the Supplemental Methods.
FIG 3.
Modification of two VACV genes simultaneously using gRNA-guided Cas9. (A) N1L gRNA N2 and A46R gRNA A2 were cotransfected with pCas9 into CV1 cells. A shuttle vector (repair vector) carrying a GFP selection marker was used for N1L region homologous recombination (HR), and an RFP selection marker was used for A46R region homologous recombination. Target regions and repaired regions in the targeted VACV genome region are shown. X indicates homologous recombination. (B) Top, images of cells after N1L gRNA N2 and A46R gRNA A2 were cotransfected with pCas9, followed by VACV infection and transfection of repair vectors with GFP and RFP selection markers. Bottom, images of pure plaques expressing both GFP and RFP markers (magnification, ×200). (C) Deletion of the N1L gene and the A46R gene were verified by PCR in pure plaques of mutant VACV expressing both GFP and RFP. Amplification of the targeted N1L or A46R region was carried out by PCR. A52R gene amplification was used as a DNA control. *, results show double deletion of the N1L gene and the A46R gene in pure GFP-and-RFP dual-positive plaques.
Finally, using a bioinformatics approach for computing all VACV genome targets, we generated a set of 8,964 gene sequences so that gRNA can maximally target specific genes in VACV but minimally target other locations in the genome (see Tables S1 and S2 in the supplemental material). Maximally efficient targeting of Lister clone VACV107 by a gRNA was achieved by identifying 19-nucleotide sequences followed by the nucleotides NGG (protospacer adjacent motif [PAM]), where N can be A, G, C, or T. Identical motif sequences present in multiple genes/locations (usually at either end of the genome) were further excluded, and thus, only unique motif sequences were retained in our final data set (see Table S1 in the supplemental material). The same search and exclusion criteria were also applied to find viral gene sequences for designing gRNA in the reverse complementary strand of coding regions of VACV107. Unique motif sequences are shown in our final data set (see Table S2). Of note, the two N1L gRNAs and all three A46R gRNAs that were functionally validated in this study are covered by the data set (see Tables S1 and S2).
To generate a new mutant of VACV, the current approach can take up to 10 rounds of plaque purification (lasting 4 to 6 weeks) and often results in an unsuccessful outcome in obtaining the desired virus with a purification marker in the target region. In this study, we demonstrate that the gRNA-guided Cas9 system greatly increases the efficiency of generating mutant VACVs without evident off-target effects on the VACV genome. This system can also efficiently engineer two viral genes simultaneously. This approach is likely to have a significant impact by expanding the application of VACV in biomedical research and, potentially, in clinical medicine.
Supplementary Material
ACKNOWLEDGMENTS
This project was funded by the United Kingdom charity Pancreatic Cancer Research Fund and the Ministry of Sciences and Technology, China (grant 2013DFG32080).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00339-15.
REFERENCES
- 1.Cooney EL, Collier AC, Greenberg PD, Coombs RW, Zarling J, Arditti DE, Hoffman MC, Hu SL, Corey L. 1991. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet 337:567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- 2.DiPaola RS, Chen Y, Bubley GJ, Stein MN, Hahn NM, Carducci MA, Lattime EC, Gulley JL, Arlen PM, Butterfield LH, Wilding G. 18December2014. A national multicenter phase 2 study of prostate-specific antigen (PSA) pox virus vaccine with sequential androgen ablation therapy in patients with PSA progression: ECOG 9802. Eur Urol doi: 10.1016/j.eururo.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo ZS, Bartlett DL. 2004. Vaccinia as a vector for gene delivery. Expert Opin Biol Ther 4:901–917. doi: 10.1517/14712598.4.6.901. [DOI] [PubMed] [Google Scholar]
- 4.Moss B. 2013. Reflections on the early development of poxvirus vectors. Vaccine 31:4220–4222. doi: 10.1016/j.vaccine.2013.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R, Pelusio A, Le Boeuf F, Burns J, Evgin L, De Silva N, Cvancic S, Robertson T, Je JE, Lee YS, Parato K, Diallo JS, Fenster A, Daneshmand M, Bell JC, Kirn DH. 2011. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 6.Chard L, Maniati E, Wang P, Zhang Z, Gao D, Wang J, Cao F, Ahmed J, Khouri ME, Hughes J, Wang S, Li X, Denes B, Fodor I, Hagemann T, Lemoine NR, Wang Y. 2015. A vaccinia virus armed with interleukin-10 is a promising therapeutic agent for treatment of murine pancreatic cancer. Clin Cancer Res 21:405–416. doi: 10.1158/1078-0432.CCR-14-0464. [DOI] [PubMed] [Google Scholar]
- 7.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, Burke J, Lencioni R, Hickman T, Moon A, Lee YS, Kim MK, Daneshmand M, Dubois K, Longpre L, Ngo M, Rooney C, Bell JC, Rhee BG, Patt R, Hwang TH, Kirn DH. 2013. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tysome JR, Briat A, Alusi G, Cao F, Gao D, Yu J, Wang P, Yang S, Dong Z, Wang S, Deng L, Francis J, Timiryasova T, Fodor I, Lemoine NR, Wang Y. 2009. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther 16:1223–1233. doi: 10.1038/gt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisswange I, Newsome TP, Schleich S, Way M. 2009. The rate of N-WASP exchange limits the extent of ARP2/3-complex-dependent actin-based motility. Nature 458:87–91. doi: 10.1038/nature07773. [DOI] [PubMed] [Google Scholar]
- 10.Falkner FG, Moss B. 1990. Transient dominant selection of recombinant vaccinia viruses. J Virol 64:3108–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackett M, Smith GL, Moss B. 1984. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol 49:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domi A, Moss B. 2002. Cloning the vaccinia virus genome as a bacterial artificial chromosome in Escherichia coli and recovery of infectious virus in mammalian cells. Proc Natl Acad Sci U S A 99:12415–12420. doi: 10.1073/pnas.192420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano E, Panicali D, Paoletti E. 1982. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A 79:1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen R, Embden JD, Gaastra W, Schouls LM. 2002. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- 15.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. 2008. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Oost J, Jore MM, Westra ER, Lundgren M, Brouns SJ. 2009. CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem Sci 34:401–407. doi: 10.1016/j.tibs.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, Xiang AP, Zhou J, Guo X, Bi Y, Si C, Hu B, Dong G, Wang H, Zhou Z, Li T, Tan T, Pu X, Wang F, Ji S, Zhou Q, Huang X, Ji W, Sha J. 2014. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156:836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T, Anderson DG. 2014. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol 32:551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. 2013. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi Y, Sun L, Gao D, Ding C, Li Z, Li Y, Cun W, Li Q. 2014. High-efficiency targeted editing of large viral genomes by RNA-guided nucleases. PLoS Pathog 10:e1004090. doi: 10.1371/journal.ppat.1004090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed JY, Yuan M, Li YN, Chu YC, Shi HL, Chard L, Alusi G, Lemoine N, Wang Y. 2014. A novel tumour selective oncolytic vaccinia virus lacking the N1L gene enhances the anti-tumour immune response when used as an anticancer therapeutic. Mol Ther 22:S248–S249. [Google Scholar]
- 24.Bartlett N, Symons JA, Tscharke DC, Smith GL. 2002. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J Gen Virol 83(Pt 8):1965–1976. [DOI] [PubMed] [Google Scholar]
- 25.Billings B, Smith SA, Zhang Z, Lahiri DK, Kotwal GJ. 2004. Lack of N1L gene expression results in a significant decrease of vaccinia virus replication in mouse brain. Ann N Y Acad Sci 1030:297–302. doi: 10.1196/annals.1329.037. [DOI] [PubMed] [Google Scholar]
- 26.Perdiguero B, Gomez CE, Di Pilato M, Sorzano CO, Delaloye J, Roger T, Calandra T, Pantaleo G, Esteban M. 2013. Deletion of the vaccinia virus gene A46R, encoding for an inhibitor of TLR signalling, is an effective approach to enhance the immunogenicity in mice of the HIV/AIDS vaccine candidate NYVAC-C. PLoS One 8:e74831. doi: 10.1371/journal.pone.0074831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stack J, Haga IR, Schroder M, Bartlett NW, Maloney G, Reading PC, Fitzgerald KA, Smith GL, Bowie AG. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med 201:1007–1018. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.