ABSTRACT
The flavivirus NS2A protein is involved in the assembly of infectious particles. To further understand its role in this process, a charged-to-alanine scanning analysis was performed on NS2A encoded by an infectious cDNA clone of yellow fever virus (YFV). Fifteen mutants containing single, double, or triple charged-to-alanine changes were tested. Five of them did not produce infectious particles, whereas efficient RNA replication was detectable for two of the five NS2A mutants (R22A-K23A-R24A and R99A-E100A-R101A mutants). Prolonged cultivation of transfected cells resulted in the recovery of pseudorevertants. Besides suppressor mutants in NS2A, a compensating second-site mutation in NS3 (D343G) arose for the NS2A R22A-K23A-R24A mutant. We found this NS3 mutation previously to be suppressive for the NS2Aα cleavage site Q189S mutant, also deficient in virion assembly. In this study, the subsequently suggested interaction between NS2A and NS3 was proven by coimmunoprecipitation analyses. Using selectively permeabilized cells, we could demonstrate that the regions encompassing R22A-K23A-R24A and Q189S in NS2A are localized to the cytoplasm, where NS3 is also known to reside. However, the defect in particle production observed for the NS2A R22A-K23A-R24A and Q189S mutants was not due to a defect in physical interaction between NS2A and NS3, as the NS2A mutations did not interrupt NS3 interaction. In fact, a region just upstream of R22-K23-R24 was mapped to be critical for NS2A-NS3 interaction. Taken together, these data support a complex interplay between YFV NS2A and NS3 in virion assembly and identify a basic cluster in the NS2A N terminus to be critical in this process.
IMPORTANCE Despite an available vaccine, yellow fever remains endemic in tropical areas of South America and Africa. To control the disease, antiviral drugs are required, and an understanding of the determinants of virion assembly is central to their development. In this study, we identified a basic cluster of amino acids in the N terminus of YFV NS2A which inhibited virion assembly upon mutation. The defect was rescued by a spontaneously occurring mutation in NS3. Our study proves an interaction between NS2A and NS3, which, remarkably, was maintained for the NS2A mutant in the presence and absence of the NS3 mutation. This suggests a role for other viral and/or cellular proteins in virion assembly. Residues important for YFV virion production reported here only partially coincided with those reported for other flaviviruses, suggesting that the determinants for particle production are virus specific. Reconstruction of a YFV encoding tagged NS2A paves the way to identify further NS2A interaction partners.
INTRODUCTION
Yellow fever virus (YFV) belongs to the genus flavivirus within the family Flaviviridae. Clinical disease associated with this prototype flavivirus can result in acute viral hemorrhagic fever (1). Other important human flaviviruses include dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus, and tick-borne encephalitis virus. Members of the Flavivirus genus are typically arthropod borne, and transmission occurs by chronically infected mosquito or tick vectors (2).
Although an effective yellow fever vaccine (YFV17D) has been available for over 70 years, the disease remains endemic in tropical areas of South America and Africa (3, 4). Rare but serious adverse events following immunization with this vaccine have been reported, also demonstrating that YF remains an issue that needs further attention (5–7).
The YFV genome is a single-stranded RNA (ssRNA) of positive polarity which is capped at its 5′ end but lacks a 3′ poly(A) tail. The genome has a length of ∼11 kb, and it encodes a single polyprotein that is cleaved co- and posttranslationally by cellular and viral proteases (8). The first one-third of the genome encodes the structural proteins (capsid [C], premembrane [prM], and envelope [E]), whereas the nonstructural (NS) proteins (NS1-NS2A-NS3-NS4A-2K-NS4B-NS5) are encoded by the remaining two-thirds of the genome (8). For the structural protein region, the anchored C protein is removed from the polyprotein by the viral serine protease, while the N termini of prM, E, and NS1 are generated by cell-derived signalases. The cleavage event responsible for maturation of prM to M is a late event, occurring on egress by Golgi-derived furin. The nonstructural proteins are processed from the polyprotein by a virally encoded serine protease localized within the N-terminal part of NS3 with the exception of the 2K/NS4B junction, which is mediated by a host cell signalase, and the NS1/2A junction, for which the enzyme has yet to be determined. In addition to its serine protease activity, NS3 also contains a helicase and a nucleoside 5′-triphosphatase (NTPase) activity. The viral RNA-dependent RNA polymerase as well as the methyltransferase is localized within NS5.
The nonstructural protein NS2A is a small hydrophobic protein with a size of approximately 22 kDa (9). Kunjin virus (KUNV) NS2A colocalizes with the viral double-stranded RNA species generated during the infectious process and interacts with the 3′ untranslated region of KUNV RNA. During KUNV infections NS2A also interacts with the proposed replicase components NS3 and NS5 in cell lysates (10). Together, these data suggest a role for NS2A in the replication complex, which was supported recently by DENV NS2A mutants defective in viral RNA synthesis (11). An adaptive mutation in KUNV NS2A (A30P) revealed a role for NS2A in the inhibition of beta interferon (IFN-β) promoter-driven transcription (12). Together with reports that the DENV NS2A inhibits interferon signaling (13) and that a single mutation within NS2A of WNV resulted in increased levels of IFN-α/β and attenuation in vivo (14) a role for NS2A in modulating the IFN response is assumed.
In addition to full-length NS2A, a C-terminally truncated version of approximately 20 kDa, NS2Aα, has been identified in YFV-infected cells. It is thought to arise from a cleavage event at the QK↓T cleavage site within NS2A (residues 189 to 191) by the viral serine protease (15). Mutations at this YFV NS2Aα cleavage site selectively blocked the production of infectious particles while subviral particles were still generated (16). For one NS2A mutant (exchange of Q189S at the P2′ position of the NS2Aα cleavage site), the block in infectious particle production could be compensated for by suppressor mutations in the NS3 helicase domain (D343 to A, G, or V) (16). This finding revealed an unexpected role for NS2A and NS3 in the assembly and/or release of infectious flavivirus particles. A Kunjin virus NS2A mutant (I59N mutant) blocked in production of virus particles that could be compensated for by a second site mutation within NS2A (T149P) (17) further suggests a role for NS2A in particle formation. Similarly, the role of DENV NS2A in particle production was recently demonstrated by a set of NS2A mutants, although in this case suppressor mutants have not been described yet (11). A role for NS3 in virion assembly is evident not only from compensating mutants in its helicase domain but also by a mutation in the YFV NS3, which selectively blocked the production of infectious particles (18). This defect could be trans-complemented by NS2B-3 or NS3 alone. Since inactive NS2B-3 still efficiently trans-complemented the mutant, the enzymatic activities of NS3 are not linked to its role in virus assembly (18). An interplay between nonstructural proteins, RNA replication, and virion assembly has also been suggested by studies demonstrating that infectious virus is produced only in the presence of a functionally active replication complex (19).
To further understand the role of YFV NS2A in virus morphogenesis, we performed charged-to-alanine-scanning of this protein. We identified a basic stretch in the N-terminal region of NS2A to be critical for infectious particle production. Further analyses identified a compensatory second-site change in NS3, which was shown before to be compensatory for a previously described NS2Aα cleavage site mutant also deficient in infectious particle production (16). The subsequently hypothesized NS2A-NS3 interaction was proven by coimmunoprecipitation analysis. However, the mutations that interfered with infectious particle production did not interrupt NS2A-NS3 interaction, suggesting that interaction with other proteins might be crucial for particle assembly.
MATERIALS AND METHODS
Cell culture and viruses.
BHK-J cells (kindly provided by Charles M. Rice, Rockefeller University, New York) have been described previously (20). Cells were grown in minimal essential medium (MEM) containing 7.5% fetal calf serum (FCS) and nonessential amino acids.
Establishment, selection, and maintenance of BHK-J cells expressing YFV NS1-2A via a selectable noncytopathic Sindbis virus (SINV) replicon system (SINrep19/NS1-2A) were performed as described previously (16, 20). Wild-type (WT) and mutant YFV were generated via electroporation of infectious cDNA clone-derived in vitro transcripts. Virus was harvested at the desired time points, clarified by centrifugation at 3,000 × g, aliquoted, and frozen at −80°C.
Plasmid constructions.
Restriction enzyme digestion, subcloning, and other standard recombinant DNA techniques were done essentially as previously described (21). All YFV NS2A charged-to-alanine scanning mutants were constructed and analyzed using full-length YFV17D clone pACNR/FLYF (kindly provided by C. M. Rice, Rockefeller University) (22). Amino acid substitutions were introduced by PCR-based site-directed mutagenesis or overlap PCR using standard procedures. Exchanged nucleotides can be seen in Table 2 or are available upon request. To introduce the D343G or D343V exchange in NS3, codon GAC was mutated by site-directed mutagenesis to GGC or GTC. For reconstruction of the second-site mutations K124E, M168K, and M168R in NS2A, fragments encoding the respective mutations were amplified from recovered viruses via reverse transcription-PCR (RT-PCR) and introduced into the original NS2A mutant construct via classical cloning. Further details on cloning strategies are available upon request.
TABLE 2.
Recovery of infectious virus after electroporation of NS2A charged-to-alanine scanning mutants
| Mutationa | Specific infectivityb | Titer atc: |
Compensating mutationd | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| None (WT) | 5 × 105 | 1.3 × 107 | 1.2 × 108 | 8.0 × 107 | None |
| R22A-K23A-R24A | <3.3 × 101 | <5 | 9.3 × 103 | 1.7 × 104 | D343G (NS3) |
| AGG-AAA-AGA | <3.3 × 101 | ND | ND | ND | No virus recovered |
| GCG-GCA-GCA | <3.3 × 101 | ND | ND | ND | No virus recovered |
| D50A-K53A | <3.3 × 101 | <5 | <5 | <5 | No virus recovered |
| GAT-AAA | <3.3 × 101 | ND | ND | ND | No virus recovered |
| GCT-GCA | <3.3 × 101 | ND | ND | ND | No virus recovered |
| D70A | <3.3 × 101 | 1.2 × 103 | 4.0 × 106 | 4.1 × 107 | A70D (NS2A) |
| GAC | <3.3 × 101 | ND | ND | ND | A70D (NS2A) |
| GCC | <3.3 × 101 | ND | ND | ND | A70D (NS2A) |
| R99A-E100A-R101A | <3.3 × 101 | <5 | <5 | 7.5 × 102 | K124E (NS2A) |
| CGG-GAA-CGC | <3.3 × 101 | ND | ND | ND | K124E (NS2A) |
| GCG-GCA-GCC | <3.3 × 101 | ND | ND | ND | K124E (NS2A) |
| E162A-R164A | <3.3 × 101 | <5 | 1.8 × 102 | 1.3 × 104 | M168K (NS2A) |
| GAG-AGA | <3.3 × 101 | ND | ND | ND | M168K (NS2A) |
| GCG-GCA | <3.3 × 101 | ND | ND | ND | M168R (NS2A) |
Amino acids and numbers of residues with regard to the NS2A amino acid sequence mutagenized to alanine are indicated at the top. In the middle, the sequence of the wild type codon(s) is indicated. At the bottom, the codons after mutagenesis are shown, with the mutagenized nucleotide(s) underlined.
Determined by infectious center assay on BHK cells.
Determined by plaque assay on BHK cells. ND, not determined.
Each line represents the result of an independent experiment.
To construct the YFV replicon expressing Renilla luciferase, the Renilla luciferase gene was first amplified with primers BNI339 and BNI340 using phRL-CMV (Promega) as the template (see Table 1). In addition, a fragment encompassing the sequence of the FMDV2A protease fused to the last 22 codons of the YFV envelope protein and the NS1 sequence was amplified from pBK41 (YFV replicon expressing yeast HIS3 [B. M. Kümmerer, unpublished data]) using primers BNI343 and BNI335. The resulting fragment was cut with XbaI and KpnI and fused together with the BamHI-XbaI-restricted Renilla luciferase gene into pBluescript II SK(−) (Stratagene) cut with BamHI and KpnI. A SacII-MluI fragment was released again from the resulting plasmid and cloned together with a NotI-SacII fragment from pBK41 (encompassing the YFV 5′ untranslated region as well as the first 20 codons for the YFV capsid protein fused to an ubiquitin gene downstream of an SP6 promoter) into pACNR/FLYF cut with MluI and NotI. The replicon NS2A mutants were established by exchanging the KpnI-NgoMIV fragment against the respective fragments from the mutant YFV17D full-length clones.
TABLE 1.
Oligonucleotides used in this study
| Name | Orientationa | Sequence (5′ → 3′) |
|---|---|---|
| BNI55 | + | CATGCCATGGTCATGTCCCCTATACTAGGTTATTGG |
| BNI56 | − | TCCCCGCGGAATGCAGGGGCCCCTGGAACAGAAC |
| BNI57 | + | GTTCCGCGGGAGAAATACATGCTGTCCC |
| BNI59 | + | CATGGACTACAAGGACGACGACGACAAGGCCGC |
| BNI60 | − | GGCCTTGTCGTCGTCGTCCTTGTAGTC |
| BNI61 | − | CCGCTCGAGCTACCTCCTACCTTCAGCAAACTTAATA |
| BNI79 | − | CCGCTCGAGTTATCTCCTAGCTCCCCTGACATG |
| BNI99 | + | CATGCCATGGCAAGTGGGGATGTCTTGTGGGATATTC |
| BNI100 | + | TCCCCGCGGCAAGTATCCCAGTGAATGAGGCAC |
| BNI232 | − | GCTCTAGACTACCTTCGCCCAAATATGCGGGTTGC |
| BNI237 | + | CTGTGAGCTTCCTCGAGAGTGATGGGTGTTG |
| BNI239 | + | CTAAGGAAAAGACTCGAGCCAAAGCAAATG |
| BNI241 | + | GTGGGATTGCATCTCGAGGAGATGAACAATG |
| BNI243 | + | CCCTATGGAGCCTCGAGGAACGCCTTGTG |
| BNI245 | + | GCATCAAATACCCTCGAGCCCCTCATGGC |
| BNI247 | + | CTTCACCAGAATCTCGAGGACACCTCCATG |
| BNI249 | + | GCATATTTGGGCTCGAGAGTCTAGACTAC |
| BNI263 | − | CTAGTCTAGACTACTTCTGCATGGAGGTGTCCTTGA |
| BNI264 | − | CTAGTCTAGACTAAGGGCTCCATAGGGTCCTGAGC |
| BNI265 | − | CTAGTCTAGACTACTGTCTTTTCCTTAGGACCACTTCC |
| BNI277 | − | CTAGTCTAGACTACATGCTCACCAAACCAAAAGG |
| BNI335 | − | GAAGATCTAGCTGTAACCCAGGAGCGCAC |
| BNI339 | + | CGGGATCCGCGGTGGCATGGCTTCCAAGGTGTACGACCCCG |
| BNI340 | − | GCTCTAGACTGCTCGTTCTTCAGCACTCGCTC |
| BNI343 | + | GCTCTAGAAACTTTGATTTATTAAAATTAGCAGGAGATGTCGAAT |
| BNI382 | + | TCGAGTACCCGTACGACGTCCCGGACTACGCGC |
| BNI383 | − | TCGAGCGCGTAGTCCGGGACGTCGTACGGGTAC |
| BNI396 | + | CTGTTGACACCTCTCGAGGTCACTATGGCTG |
| BNI470 | − | CGGGATCCCAAGATGGTATTTGATGCTTTC |
| Bo89 | + | TGGACGCAGTCTTTGAATACAACC |
| Bo370 | − | ACCTGATCCGTGGATCTCACCAGCTGTAACCCAGGAGCGCACCAGA |
| Bo371 | + | GGTAGCGGAGGAGAAATACATGCTGTCCCTTTTG |
| Bo372 | + | GCTGGTGAGATCCACGGATCAGGTTACCCATACGATGTCCCAGACTACGCT |
| Bo373 | − | ATGTATTTCTCCTCCGCTACCAGCGTAGTCTGGGACATCGTAACCG |
| Bo508 | − | CTAGTCTAGACTATTTCCTTAGGACCACTTCCATTGC |
| Bo510 | − | CTAGTCTAGACTATAGGACCACTTCCATTGCTATCAT |
| Bo546 | − | CTAGTCTAGACTACATTGCTATCATCATGCTCACCAA |
| Bo547 | − | CTAGTCTAGACTATATCATCATGCTCACCAAACC |
| Bo698 | − | CTAGTCTAGACTACACTTCCATTGCTATCATCATGCTC |
| RU2501 | − | GGAATTCTACCTTCGCCCAAATATGCGGGT |
+, forward orientation; −, reverse orientation. For primers used in site-directed mutagenesis, only forward primers are shown; the corresponding complementary reverse primers were also used.
To generate pFlag-NS2A, an adaptor fragment encoding the Flag tag was annealed using primers BNI59 and BNI60 and was ligated into an NcoI-EcoRI digested pCITE-2a vector along with a SacII-EcoRI cut PCR fragment amplified from pACNR/FL17D using primers BNI57 and RU2501 (Table 1). To establish pFlag-NS2B, a PCR fragment was amplified from pACNR/FL17D using primers BNI100 and BNI79. The fragment was restricted with SacII and XhoI and inserted into pFlag-NS2A cut with the same restriction enzymes. Plasmid pNS3 was generated by ligation of an NcoI-XhoI cut PCR product amplified from pACNR/FL17D using primers BNI99 and BNI61 into pCITE-2a. For pGST-NS2A, a glutathione S-transferase (GST)-encoding amplicon was generated using primers BNI55 and BNI56 cut with NcoI and SacII and inserted into NcoI and XbaI digested pCITE-2a along with a BNI57- and BNI232-amplified NS2A gene restricted with SacII and XbaI. Constructs expressing GST-NS2A mutants were established accordingly, whereas the mutant NS2A genes were amplified from the respective mutant full-length clones. To generate the C-terminally truncated NS2A expression plasmids, truncated NS2A genes were amplified from pACNR/FLYF using BNI57 and BNI263 (pGST-NS2A-K190), BNI57 and BNI264 (pGST-NS2A-P98), BNI57 and BNI265 (pGST-NS2A-Q25), BNI57 and Bo508 (pGST-NS2A-K23), BNI57 and Bo510 (pGST-NS2A-L21), BNI57 and Bo698 (pGST-NS2A-V19), BNI57 and Bo546 (pGST-NS2A-M17), BNI57 and Bo547 (pGST-NS2A-I15), or BNI57 and BNI277 (pGST-NS2A-M13) (Table 1). The resulting PCR fragments were cut with SacII and XbaI and inserted into pGST-NS2A cut with the same restriction enzymes.
To construct internally hemagglutinin (HA)-tagged NS2A expression constructs, psigNS1-2A (pCITE-2a encoding NS1-2A preceded by the last 23 amino acids [aa] of E) was subjected to site-directed mutagenesis to first introduce XhoI restriction sites at the different insertion sites. For HA tag insertion after NS2A R24, H61, S97, T147, P157, N182, or G222, integration of an XhoI site was performed with primer BNI239, BNI241, BNI243, BNI245, BNI396, BNI247, or BNI249, respectively (Table 1). Insertion of an XhoI site in the NS1-encoding region was done with BNI237. The resulting plasmids were cut with XhoI, and an HA tag adaptor created via annealing of BNI382 and BNI383 was inserted (Table 1).
To generate the infectious YFV17D full-length clone encoding HA-tagged NS2A, a sequence encoding the peptide GEIHGSGYPYDVPDYAGSG (first 4 aa of NS2A, a 3-aa flexible linker [GSG], an HA tag, and a flexible GSG linker) was inserted between NS1 and NS2A. To this end, an HA tag adaptor obtained via overlap PCR of primers Bo372 and Bo373 was N-terminally fused via overlap PCR to a second PCR fragment amplified from the YFV17D genome by Bo89 and Bo370 and C-terminally fused to a YFV17D PCR fragment amplified using Bo371 and BNI470 (Table 1). The resulting fusion product was cut with KpnI and AvrII and ligated together with a YFV17D AvrII-NgoMIV fragment into pACNR/FL17D cut with KpnI and NgoMIV. Further details on cloning are available on request.
In vitro transcription.
WT and mutant pACNR/FLYF clones and YFV replicons were linearized with XhoI, and in vitro transcription was performed using the SP6 mMESSAGE mMACHINE kit (Ambion) according to the manufacturer's instructions. The integrity and amount of the in vitro-transcribed RNA were analyzed by electrophoresis in ethidium bromide agarose gels.
RNA electroporation and recovery of recombinant virus.
In vitro-transcribed RNAs (3 μg) were electroporated into 1 × 106 BHK-J cells resuspended into 100 μl of serum-free OPTI PRO SFM medium (Gibco) using a 2-mm gap electroporation cuvette (VWR). Electroporation was performed with a Gene Pulser Xcell (Bio-Rad) using the Pre-Set BHK21 protocol (square wave, 25 ms, 140 V, 1 pulse). Recombinant viruses were harvested from the supernatant and stored at −80°C.
Identification of second-site mutants.
Total cellular RNA was isolated from infected cells using TRIzol (Invitrogen). Three microliters of RNA was applied to each reverse transcription reaction using a series of YFV reverse primers and SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Subsequent PCR amplification was performed with a set of YFV primers spanning the complete YFV genome. PCR products were purified from preparative agarose gels and subjected to sequencing. PCR fragments containing second-site mutations were cloned into pACNR/FLYF and confirmed by sequencing.
ICA and plaque assay.
Infectious center assays (ICAs) and plaque assays were done as described previously (16). Briefly, for infectious center assays, 10-fold dilutions of the electroporated cells were seeded with nontransfected cells. After 4 to 6 h, the medium of the attached cells was replaced by an agarose overlay. Fixation and staining with crystal violet solution were performed 3 days after infection. Similarly, for plaque assays, BHK-J cells were infected with 10-fold dilutions of cell culture supernatant, overlaid with agarose, and evaluated 3 days after infection by crystal violet staining as described previously (16).
Luciferase assays.
To normalize electroporation efficiencies, 0.5 μg of a firefly luciferase-expressing transcript [firefly luciferase gene under the control of an internal ribosome entry site (IRES) element and terminated with a poly(A) tail] was coelectroporated with the Renilla luciferase-expressing YFV replicon transcripts. Electroporated cells were washed with phosphate-buffered saline (PBS) and subjected to a dual-luciferase assay using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's protocol. Luciferase values were measured using a Junior LB 9509 Luminometer (Berthold). The ratio of Renilla to firefly values was used to compare replication efficiencies of the YFV replicon mutants.
DNA transfection.
For transfection of DNA plasmids, BHK-J cells were seeded in 96-well or 6-well plates at a density of 2 × 104 or 5 × 105 cells per well, respectively. The next day, cells were infected with MVA-T7 vaccinia virus (kindly provided by Gerd Sutter, Helmholtz Center, Munich, Germany) at a multiplicity of infection of 2. After an hour, cells were transfected or cotransfected with a total of 0.2 μg (96-well plates) or 2 to 3 μg (6-well plates) of DNA using Lipofectamin 2000 (Invitrogen) according to the manufacturer's instructions. After 4 h at 37°C, supernatants were replaced with MEM (with 7.5% FCS). Cells were processed for immunofluorescence analysis (96-well plates) or coimmunoprecipitation analysis (6-well plates).
Immunoprecipitation and Western blot analysis.
At 24 h posttransfection, cotransfected cells were washed with PBS and lysed in 200 μl of ice-cold Triton X-100 (0.5% Triton X-100, 50 mM Tris-Cl [pH 7.5], 200 mM NaCl, 1 mM EDTA) lysis buffer containing 1× complete protease inhibitor cocktail (Roche). The lysates were clarified by centrifugation at 15,000 × g for 10 min at 4°C. One hundred microliters of the soluble fraction was mixed with 100 μl of distilled water and 200 μl of 2× TNA buffer (0.5% Triton X-100, 2 mg/ml of bovine serum albumin [BSA], 100 mM Tris-Cl [pH 7.5], 400 mM NaCl, 2 mM EDTA) and incubated overnight at 4°C with goat anti-GST (Amersham), rabbit anti-YFV NS3 (23), or rabbit anti-HA (6908; Sigma) antibody. Preequilibrated Pansorbin cells (Calbiochem) were added and rocked at 4°C for 2 h. Complexes were collected by centrifugation and washed three times with 1× TNA buffer and once with 1× TNE (50 mM Tris-Cl [pH 7.5], 1 mM EDTA, 150 mM NaCl). The washed pellet was resuspended in Laemmli sample buffer (50 mM Tris-Cl [pH 6.8], 10% glycerol, 2% SDS, 0.05% bromophenol blue, 5% 2-mercaptoethanol) and heated for 20 min at 80°C. Precipitated proteins were separated by SDS-PAGE and blotted on a nitrocellulose membrane (Schleicher & Schuell). The membrane was incubated in Roti-Block blocking solution (Roth) for 1 h and then incubated with the respective antibodies in PBS–0.05% Tween for 1 h. Antibodies used were anti-Flag M2-POD (1:5,000; Sigma-Aldrich), rabbit anti-YFV NS3 (1:5,000) followed by goat anti-rabbit IgG-POD (1:10,000; Pierce), or mouse anti-HA (1:1,000, H3663; Sigma) followed by peroxidase-conjugated goat anti-mouse IgG (1:10,000; Jackson ImmunoResearch). After 3 washes in PBS–0.05% Tween, proteins were visualized using ECL Supersignal West Pico chemiluminescent substrate (Thermo Scientific).
Indirect immunofluorescence analyses and selective permeabilization.
BHK-J cells electroporated with pACNR/FLYF transcripts were washed 24 h after electroporation with PBS and fixed with acetone-methanol (1:1) for 20 min at −20°C. Fixed cells were air dried and stored at −20°C until immunofluorescence analyses was performed. Frozen cells were rehydrated in PBS, followed by incubation at 37°C for 1 h with a monoclonal antibody directed against YFV E protein (anti-E6330, kindly provided by M. Niedrig, Robert Koch Institute, Berlin, Germany) diluted at 1:1,000 in PBS. Following three washes with PBS, a fluorescein isothiocyanate (FITC)-labeled secondary anti-mouse antibody (Sifin) was added at a 1:250 dilution for 1 h at 37°C. Washed cells were analyzed using a fluorescence microscope (Leica DMLS).
For detection of internally HA-tagged NS2A proteins, cells were fixed 24 h after transfection with 4% paraformaldehyde for 20 min at room temperature. After three washes with PBS, cells were either permeabilized with 0.1% Triton X-100 (15 min, 4°C) or treated with 2 μg/ml of digitonin (15 min, 4°C) to selectively permeabilize the plasma membrane but not the endoplasmic reticulum (ER) membranes. Cells were then washed with PBS and blocked for 5 min at room temperature with 0.5% bovine serum albumin. As the primary antibody, an anti-HA monoclonal antibody (clone 12CA5; Roche) was used at a 1:100 dilution; as the secondary antibody, goat anti-mouse IgG-FITC (Sifin) was used at a 1:250 dilution.
RESULTS
Phenotypic characterization of charged-to-alanine scanning YFV NS2A mutants.
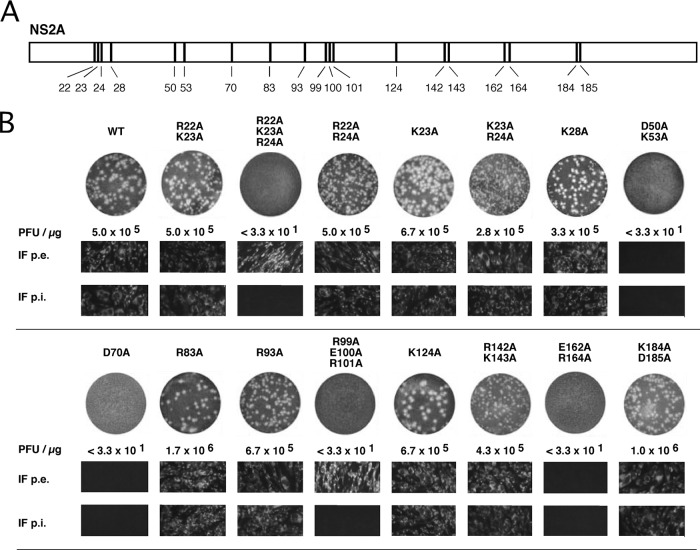
As we reported earlier, mutations at the YFV NS2Aα cleavage site within the C-terminal domain of NS2A selectively blocked production of infectious virus particles despite efficient RNA replication (16). Our data indicated that the sequence of the NS2Aα cleavage site is important to the role of NS2A in virus production rather than for processing by the NS2B-3 serine protease. To further understand the implication of NS2A in virus production, charged-amino-acid-to alanine scanning was used to create a panel of YFV NS2A mutants (Fig. 1). Both single and multiple substitutions were generated, electroporated into BHK cells, and assayed for RNA replication by indirect immunofluorescence at 24 h postelectroporation (p.e.) and for plaque formation by infectious center assay (ICA) at 72 h p.e. In addition, indirect immunofluorescence was performed after inoculation of naive cells with supernatant obtained at 24 h p.e. to analyze for production of infectious noncytopathic virus. Ten of the 15 mutants produced cytopathic virus similar to the wild-type virus (Fig. 1B). These included the NS2A R22A-K23A, R22A-R24A, K23A, K23A-R24A, K28A, R83A, R93A, K124A, R142A-K143A, and K184A-D185A mutants. Three of the 15 tested mutants (D50A-K53A, D70A, and E162A-R164A mutants) did not produce plaques in the infectious center assay, nor was efficient RNA replication detected with these 3 mutants (Fig. 1B). Interestingly, the remaining two mutants, the R22A-K23A-R24A and R99A-E100A-R101A mutants, were clearly positive in the indirect immunofluorescence assay performed after electroporation despite their inability to produce plaques in the infectious center assay (Fig. 1B). The fact that at 24 h p.e. 90 to 100% of the electroporated cells showed positive fluorescence (Fig. 1B) indicated that the signals were due to the replication of the initial RNAs transfected rather than to replication of revertants or second-site mutants. In addition, passage of the supernatant obtained 24 h after electroporation of those two triple mutant RNAs did not result in positive immunofluorescence signals, which rules out the possibility that infectious but noncytopathic virus was generated. The R22A-K23A-R24A and R99A-E100A-R101A mutants may therefore selectively block the production of infectious particles, like the NS2Aα cleavage site NS2A Q189S mutant (16). These data suggest that besides the region around the NS2Aα cleavage site, other regions within NS2A are important for infectious particle production.
FIG 1.
Analysis of YFV NS2A charged-to-alanine scanning mutants. (A) Schematic drawing of NS2A indicating the positions of charged amino acids involved in the charged-to-alanine scanning analysis. (B) Cell culture characterization of NS2A charged-to-alanine scanning mutants. For infectious center assays, BHK cells were electroporated with the WT or mutant transcripts, and serial 10-fold dilutions were seeded on 35-mm-diameter dishes together with untransfected cells. At 4 to 6 h postelectroporation, cells were overlaid with agarose. After 3 days, cells were fixed and stained with crystal violet. The values below the dishes indicate the PFU per microgram of electroporated in vitro-transcribed RNA. In addition, cells were fixed at 24 h p.e. and probed with an E-specific antibody to assess RNA replication (IF p.e.). Supernatant obtained at 24 h p.e. was passaged on naive cells, and indirect immunofluorescence analysis was performed again 24 h postinfection using an E-specific antibody (IF p.i.).
Rescue of infectious particles via trans-complementation.
The defect in infectious particle production of the NS2Aα cleavage site mutant NS2A Q189S could be complemented by providing NS1-2A in trans (16). To determine whether the R22A-K23A-R24A and R99A-E100A-R101A mutants can also be complemented for in trans by NS2A, in vitro transcripts of the respective variants were electroporated into BHK cells expressing YFVNS1-2A via a selectable noncytopathic SINV expression system (16, 24). Electroporated cells were serially diluted and seeded onto the BHK/NS1-2A cells in infectious center assays (Fig. 2A). As described before, in contrast to electroporation into naive BHK cells, the NS2Aα cleavage site mutant (Q189S) formed small plaques on the NS1-2A-expressing BHK cells (16). A similar result was observed for the R22A-K23A-R24A mutant, whereas no plaques were obtained in the infectious center assay for the R99A-E100A-R101A mutant (Fig. 2A). In the latter case, a rather inefficient trans-complementation was indicated only by the production of low levels of infectious virus at 24 h p.e. (1.5 × 101 PFU/ml), as was determined by titration of the supernatant on NS1-2A-expressing cells. For comparison, the other two mutants reached titers in the range of 104 PFU/ml at 24 h p.e. in the trans-complementation assay (data not shown).
FIG 2.
(A) trans-Complementation assay. BHK cells expressing NS1-2A via a Sindbis virus replicon or naive BHK cells were electroporated with WT or mutant YFV full-length in vitro transcripts as indicated and processed for infectious center assays. Plaques were visualized 3 days postelectroporation using crystal violet staining. (B) Replication efficiencies of NS2A mutants analyzed using a Renilla-expressing YFV replicon. BHK cells were electroporated with WT or mutant YFV Renilla replicon transcripts. To control the efficiency of electroporation, a transcript encoding firefly luciferase flanked by an IRES element and a poly(A) tail was cotransfected in each electroporation. Electroporated cells were seeded into several wells of a 24-well plate and used to perform a dual-luciferase assay at the indicated time points. The replication efficiencies are represented as relative light units derived from the ratio of Renilla and firefly values.
Effect of NS2A charged-to-alanine mutations on RNA replication.
To analyze how our mutants effected RNA replication, the mutations R22A-K23A-R24A and R99A-E100A-R101A as well as the previously described NS2A Q189S mutation were introduced into a YFV replicon expressing a Renilla luciferase reporter gene. A replication-deficient replicon in which the polymerase activity was knocked out via mutation (GDD → GAA mutant) served as a negative control. Measurement of the Renilla activity at different times after electroporation demonstrated that the Q189S mutant replicated only slightly less efficiently than the WT (Fig. 2B). RNA replication for the R22A-K23A-R24A mutant was reduced similarly to that for the WT (Fig. 2B). The R99A-E100A-R101A mutant showed a more pronounced delay over the first 30 h (Fig. 2B); however, this delay was not statistically significant (t test, P = 0.2) and the mutant grew to titers comparable to those of the WT at later time points. Still, the data suggest that certain residues within NS2A not only play a role for infectious particle production but also are important for RNA replication.
Rescue of compensating second-site mutants.
To identify mutations that compensate for the defect in infectious particle production, mutants that did not produce plaques in the infectious center assay were monitored for the release of infectious particles by titration of their supernatant at 24, 48, and 72 h postelectroporation (Table 2). Furthermore, electroporated cells were passaged and observed for development of a cytopathic effect (CPE) to indicate the presence of infectious virus. In the case of CPE development, the supernatant was harvested and used for reinfection of naive cells. Total cellular RNA was isolated, and RT-PCR of the NS2A region was performed.
In three independent experiments, no plaque-forming variant could be obtained for the D50A-K53A mutant, even after passaging the cells several times. In contrast, infectious particles could already be restored at low titers after 24 h for mutant D70A (Table 2). Sequencing of the NS2A region of the plaque-forming virus revealed the reversion of A70 to D. This reversion occurred in two further electroporation experiments. Second-site mutations within NS2A were detected for variants that arose from the R99A-E100A-R101A and E162A-R164A mutants. In the case of the R99A-E100A-R101A variant, NS2A amino acid K124 was altered to E. For the E162A-R164A mutant, NS2A residue M168 was changed either to K or R. In the case of the R22A-K23A-R24A mutant, NS2A remained unaltered (Table 2). Further analyses of the triple mutant revealed that in one of three electroporations a second-site mutation in the NS3 region arose, namely, D343G. Interestingly, this second-site mutation was described before to be able to compensate the NS2Aα cleavage site Q189S mutant (16).
Verification of compensatory mutations.
To verify that the second-site exchanges listed in Table 2 are indeed responsible and sufficient for restoration of production of infectious particles, we introduced the second-site mutations into the infectious full-length clone together with the respective original mutations.
Electroporation of the reconstructed variants with second-site mutations in NS2A resulted in the formation of plaques, although the specific infectivities were slightly lower and the plaques were partially smaller than with the wild type (Fig. 3A). Interestingly, the second-site mutation found for the R22A-K23A-R24A mutant was outside NS2A in the NS3 region. This NS3 mutation (D343G) had already been shown to complement the NS2Aα cleavage site mutant (NS2A Q189S) (16). As observed for the second-site mutations within NS2A, reconstruction of the combined R22A-K23A-R24A (NS2A)/D343G (NS3) mutant resulted in formation of plaques with a specific infectivity only slightly lower than that of the wild type (Fig. 3A). The plaque size was reduced but infectious particles could be passaged upon infection of new cells, as demonstrated by immunofluorescence (Fig. 3A).
FIG 3.

Phenotypes of YFV charged-to-alanine scanning mutants containing second-site mutations. (A) Infectious center assays for the indicated YFV variants were performed as described in the legend to Fig. 1B. For comparison, an infectious center assay for WT YFV is shown again at the left. The values below the dishes indicate the PFU per microgram of electroporated in vitro-transcribed RNA. In addition, the outcome of the immunofluorescence analysis using a monoclonal antibody against YFV E protein at 24 h postinfection (IF p.e.) or after passaging the cell culture supernatant obtained at 24 h postelectroporation on naive cells (IF p.i.) is shown. (B) Virus release of YFV mutants after electroporation. In vitro transcripts of the indicated WT or mutant YFV genomes were electroporated into BHK cells. Supernatant was harvested at 24, 48, and 72 h postelectroporation, and titers were determined on BHK cells.
In addition, viral titers obtained 24, 48, and 72 h after electroporation of all variants containing the additional second-site mutations were determined (Fig. 3B). Infectious virus was already released at 24 h after electroporation, and titers increased steadily with time. Collectively, the data demonstrate that the identified second-site mutations are compensatory.
Analyses of NS2A variants involving mutations in two different NS2A regions.
Growth defects associated with the NS2A R22A-K23A-R24A and NS2A Q189S mutants were restored by the second-site mutation D343G or D343V within NS3 (Fig. 3A and 4A) (16). In the next step, we wanted to analyze if a variant containing the mutations in both NS2A regions still can be compensated by the NS3 mutation. To this end, the D343V mutation within NS3 was cloned into YFV encoding both the R22A-K23A-R24A and Q189S exchanges. Electroporation of the respective in vitro-transcribed RNA did not result in formation of plaques directly after electroporation, although efficient RNA replication was obtained, as could be seen by immunofluorescence analysis (Fig. 4). Therefore, this NS2A mutant could not be rescued by the second-site mutation within NS3. Nevertheless, trans-complementation analyses using electroporation into the BHK/NS1-2A cells revealed that the NS2A R22A-K23A-R24A+Q189S mutant still could be complemented by NS1-2A, regardless of whether the D343V mutation in NS3 was introduced. This was apparent by the formation of plaques in the ICA as well as by the release of infectious virus after electroporation in NS1-2A-expressing cells (Fig. 4B and C).
FIG 4.
Analysis of NS2A mutants containing exchanges in two different NS2A regions. Electroporation was performed using naive BHK cells (A) or BHK cells expressing NS1-2A (B) via a selectable Sindbis virus replicon for trans-complementation. Infectious center assays were performed as described in legend to Fig. 1B. The values below the dishes indicate the PFU per microgram of electroporated in vitro-transcribed RNA. In addition, the outcome of the immunofluorescence analysis at 24 h postelectroporation (IF p.e.) using an anti E-specific antibody is shown. (C) Virus release after electroporation in NS1-2A expressing cells. Viral titers released into the supernatant at the times indicated were determined on NS1-2A-expressing cells.
Interaction between NS2A and NS3.
Since two of the NS2A variants could be rescued by a compensatory mutation at position D343 within NS3, it was assumed that NS2A and NS3 interact. To confirm this, coprecipitation experiments were performed (Fig. 5). In the absence of an NS2A antibody, NS2A was tagged N-terminally with a Flag sequence (pFlag-NS2A). As NS2B is a cofactor of the viral serine protease and thus interacts with NS3, a plasmid encoding N-terminally Flag-tagged NS2B (pFlag-NS2B) was generated and used as a positive control for interaction. T7 driven expression of pFlag-NS2B and pFlag-NS2A in cells infected with MVA-T7 virus was confirmed by Western blotting with anti-Flag antibodies (Fig. 5A). To analyze for coprecipitation, pFlag-NS2B or pFlag-NS2A was cotransfected with a plasmid expressing NS3 (pNS3) and Flag-tagged protein was precipitated with an anti-Flag antibody. After being subjected to SDS-PAGE, these samples were transferred to membranes. The presence of NS3 was detected using NS3-specific antiserum. NS3 was coprecipitated with both Flag-NS2B and Flag-NS2A (Fig. 5B, lanes 2 and 3). NS3 detected from YFV-infected cells served as a positive control (Fig. 5B, lane 4). In the absence of Flag-NS2A or Flag-NS2B, ectopically expressed NS3 was not precipitated by the anti-Flag antibody (Fig. 5B, lane 5), nor was any protein in the size of NS3 coprecipitated when pFlag-NS2A or pFlag-NS2B was expressed alone (Fig. 5B, lanes 7 and 8), indicating the specificity of the assay. Taken together, the data demonstrate that ectopically expressed NS2A and NS3 interact.
FIG 5.

Interaction of NS2A and NS3. BHK cells were infected with MVA-T7 and subsequently transfected with the indicated plasmids. Untransfected cells (Vacc) served as a control. At 24 h postinfection, cells were lysed and directly analyzed by Western blotting (WB) using anti-Flag antibodies (A) or subjected to immunoprecipitation (IP) under nondenaturing conditions using anti-Flag antibodies (B). Precipitated and coprecipitated proteins were then separated on an SDS gel, and Western blot analysis was performed using rabbit anti-YFV NS3 antiserum. As a control for NS3 detection, cells infected with YFV were also loaded as indicated. Arrows indicate the migration of the relevant proteins. To the left, migration of a size standard (in kilodaltons) is indicated.
Mapping of NS2A-NS3 interaction site.
The additional NS3 protease cleavage site within the C-terminal domain of NS2A (NS2Aα cleavage site) suggests that NS3 interacts, at least transiently, with this region of NS2A. To investigate the significance of amino acids upstream of this cleavage site in interaction between NS2A and NS3, C-terminally truncated NS2A proteins with a GST fusion at the N terminus were tested in NS3 interaction studies. Besides expression of GST-NS2A (R224) and GST-NS2Aα (K190), GST fusion proteins were expressed ending shortly up- or downstream of the basic triple motif R22-K23-R24 or R99-E100-R101, respectively (Fig. 6A). Plasmid pGST expressing only GST and the empty vector (V) served as controls. The plasmids were cotransfected with pNS3-HA expressing C-terminally HA-tagged NS3. Complexes were precipitated with anti-HA antibodies via the HA-tagged NS3, and coimmunoprecipitated NS2A proteins were detected by immunoblotting using anti-GST antibodies (Fig. 6A). Detection of the C-terminally truncated NS2A proteins in the cell lysates by SDS-PAGE and subsequent Western blot analysis using anti-GST antibodies served as the expression control (Fig. 6A). As can be seen in Fig. 6A, C-terminally truncated NS2A proteins coimmunoprecipitated with NS3-HA until truncations up to amino acid Q25. In contrast, GST-NS2A C-terminally truncated up to methionine at position 13 (GST-M13) was not coimmunoprecipitated any longer as was observed for the negative control GST. These data suggest that the region between M13 and Q25 within NS2A is important for NS2A-NS3 interaction. Interestingly, this region includes residues R22-K23-R24 of NS2A, which we have shown in this study are critical for infectious particle production.
FIG 6.

Analysis of interaction between NS3 and C-terminally truncated NS2A deletion mutants. (A) (Top) Schematic presentation of the C-terminally truncated NS2A proteins with N-terminal GST fusion. To the right, results of the interaction studies obtained are indicated. (Bottom) C-terminally truncated GST-NS2A proteins were coexpressed with HA-tagged NS3 and lysed under nondenaturing conditions. Protein complexes were coimmunoprecipitated with anti-HA antibodies. Precipitated proteins were separated by SDS-PAGE, blotted, and detected with anti-HA (expression control) or anti-GST antibodies. As an expression control of C-terminally truncated NS2A proteins, proteins in the cell lysate were analyzed by SDS-PAGE, followed by immunoblotting using anti-GST antibodies. V (empty vector) or GST served as controls. The asterisk indicates a 10-fold-longer exposure to the film than for the other lanes in this blot. (B) Further mapping of the N-terminal region within NS2A involved in NS3 interaction. C-terminal truncations of NS2A were performed up to the amino acids indicated. The C-terminally truncated GST-NS2A proteins were coexpressed with HA-tagged NS3. Sample processing and protein detection were performed as described for panel A.
Cytoplasmic regions within NS2A.
During infection, NS3 localizes to the cytoplasm; thus, NS3-interacting domains within other proteins must also locate to the cytoplasm. NS2A is a small, hydrophobic transmembrane protein with an N terminus generated by a cleavage event in the ER lumen (25) and a C terminus generated by the viral serine protease on the cytosolic side of the membrane (23). This suggests a transmembrane topology of NS2A, as was also indicated using TMHMM prediction (Fig. 7B). To support our findings that the area between M13 and Q25 is important for NS3 interaction, we used a selective permeabilization approach to analyze whether this NS2A region faces the cytoplasm, as was also inferred for the NS2Aα cleavage site (26). Unlike Triton X-100, digitonin permeabilizes only the plasma membrane; thus, following digitonin treatment, only cytoplasmic protein regions can be accessed and detected by antibodies. An internal HA tag was introduced after NS2A position R24 and, for comparison, also after positions H61, S97, T147, P157, N182, and G222 into a T7-driven plasmid encoding sigNS1-2A (NS1-2A preceded by the signal sequence of NS1) (Fig. 7A). As a control for ER localization, an HA tag was also introduced in the C-terminal part of NS1 (after NS1 F323), which is known to be localized in the ER. Expression of HA-tagged proteins was confirmed after transfection of these plasmids into MVA-T7-infected BHK cells and permeabilization with Triton X-100 (Fig. 7A). For selective detection of HA tags localized in the cytoplasm, treatment with digitonin was used. Selectivity of the assay was proven by the fact that no fluorescent cells were visible after digitonin treatment for the NS1 F323 HA-tagged protein (Fig. 7A). Similar observations were made with the HA tag introduced downstream of NS2A S97, T147, and P157, suggesting that these regions are in the ER. Since positive fluorescence was obtained for HA tag insertions after positions R24, H61, N182, and G222 of NS2A, these domains are likely to be cytoplasmic. The domains included the region around amino acids R22-K23-R24 of NS2A and the area flanking the NS2Aα cleavage site, which is consistent with these regions of NS2A being involved in interactions with cytoplasmic NS3. Furthermore, these results suggest that NS2A spans the membrane at least three times (Fig. 7B).
FIG 7.
(A) Membrane topology of YFV NS2A and immunofluorescence analysis after selective permeabilization. At the top, a schematic presentation of the NS2A protein with the positions after which HA tags were introduced is shown. “α” indicates the NS2Aα cleavage site. Below the schematic representation are shown results of the immunofluorescence analysis. T7 polymerase-driven plasmids encoding the HA-tagged NS1-2A proteins were transfected into MVA-T7 vaccinia virus-infected BHK cells. The amino acid after which the HA tag is inserted is indicated in each case to the left. Twenty-four hours after transfection, cells were fixed with 4% paraformaldehyde and either treated with digitonin to selectively permeabilize only the outer membrane of the cell without disturbing the ER membrane or treated with Triton X-100 to permeabilize all cellular membranes. Indirect immunofluorescence was performed using a monoclonal anti-HA antibody and a FITC-labeled anti-mouse secondary antibody. A minus or plus sign indicates the negative or positive outcome of the immunofluorescence analysis, respectively. An NS1-2A protein expressing an HA tag in the C-terminal region of NS1 (after F323) served as a control for ER localization; NS1-2A without an HA tag (−HA) served as a negative control. (B) Membrane topology models of YFV NS2A. On top, a topology model based on TMHMM prediction is shown. Black bars represent the transmembrane regions, with the numbers indicating the beginning and end of the transmembrane domains. On the bottom, a rough membrane topology model of YFV NS2A based on the localization of indicated amino acids as determined for panel A is depicted. A minimum of three transmembrane domains is suggested, not ruling out the occurrence of additional transmembrane regions. NS3 colored in gray indicates the NS2A interaction site determined by coimmunoprecipitation studies, whereas NS3 colored in white indicates the NS2A interaction site suggested due to the presence of the NS2Aα cleavage site. ER, ER lumen; CP, cytoplasm.
Studies of interaction between mutant NS2A and NS3 proteins.
To determine whether the mutations in NS2A that selectively block infectious particle production also interfere with its interaction with NS3 and, if so, whether the second-site mutation in NS3 restores this interaction, we generated an infectious YFV cDNA clone expressing NS2A with an N-terminal HA tag in a manner similar to that described for hepatitis C virus (HCV) (27). The first four codons of NS2A were duplicated, and between the duplicated codons an HA tag flanked by G-S-G linkers was inserted. Electroporation of in vitro transcripts derived from the established construct yielded viable virus, although the plaques were smaller than untagged YFV (Fig. 8A). Nevertheless, the NS2A HA-tagged YFV remained stable, as was proven by sequencing analysis of virus that had been passaged five times on BHK cells (data not shown). As for the untagged NS2A Q189S mutant, the introduction of the amino acid substitution Q189S still allowed efficient RNA replication in the absence of infectious particles. Again, the defect could be compensated by the second-site mutation at position D343 within NS3 (data not shown). In contrast, after substitution of amino acids R22-K23-R24 in the HA-tagged NS2A background, RNA replication did not occur. Neither the insertion of different tags nor variation in the number of linker amino acids restored RNA replication. Hence, studies of interaction between NS2A and NS3 proteins based on the HA-NS2A tagged YFV could be performed only for the Q189S variant.
FIG 8.

Interaction studies between different variants of NS2A and NS3. (A) Plaque morphologies of NS2A-HA tagged YFV. BHK cells were electroporated with WT or NS2A-HA tagged YFV transcripts and processed for infectious center assays as described in the legend to Fig. 1B. (B) BHK cells were electroporated with in vitro transcripts derived from untagged or NS2A HA-tagged WT YFV full-length clones or variants containing the indicated mutations in NS2A or NS3 as indicated. Twenty-four hours after electroporation, cells were lysed under nondenaturing conditions and coimmunoprecipitation of protein complexes was performed with anti-HA antibodies. Precipitated proteins and proteins in cytoplasmic lysate (NS3 expression control) were separated by SDS-PAGE, blotted, and detected with anti-HA or anti-NS3 antibodies. (C) Plasmids encoding WT or mutant GST-NS2AQ25 were coexpressed with HA-tagged NS3 and lysed under nondenaturing conditions. Protein complexes were coimmunoprecipitated with anti-HA antibodies. Precipitated proteins were separated by SDS-PAGE, blotted, and detected with anti-HA (expression control) or anti-GST antibodies, respectively. Immunoprecipitation and Western blot analysis using anti-GST antibodies served as expression controls of GST-tagged proteins. pCITE (empty vector) or GST served as controls.
After electroporation of in vitro transcripts derived from the YFV clone encoding wild-type HA-tagged NS2A, immunoprecipitation with anti-HA antibody was performed under nondenaturing conditions. Tagged NS2A was precipitated, the majority of which was of the cleaved NS2Aα form (Fig. 8B). Coimmunoprecipitated NS3 confirmed the interaction between NS2A and NS3. As described before, after introduction of the Q189S exchange, cleavage at the NS2Aα site was prohibited (Fig. 8B) (16). Interestingly, coimmunoprecipitation of NS3 with the HA-tagged NS2A Q189S occurred at levels observed for WT NS2A. Similar levels of NS3 coprecipitation were also observed after introduction of the second-site mutation in NS3 in addition to the NS2A Q189S exchange (Fig. 8B). In contrast, no signal was detected for the untagged YFV despite an even higher NS3 level in the cell lysate, demonstrating the specificity of the coprecipitation.
Since the HA-tagged NS2A R22A-K23A-R24A variant failed to replicate, coimmunoprecipitation studies for this NS2A mutant were performed based on T7-driven expression constructs as described above. The NS2A C-terminally truncated up to residue Q25 was still able to interact with NS3 even when the R22A-K23A-R24A substitutions were introduced into the Q25 background (Fig. 8C).
Taken together, these data suggest that the defect in particle production for the R22A-K23A-R24A and Q189S mutant is not due to a defect in physical interaction between NS2A and NS3.
Further mapping of the N-terminal NS2A region involved in NS3 interaction.
Since interaction with NS3 was still observed in the GST-NS2AQ25/R22A-K23A-R24A background, a site upstream of R22 might be involved in NS2-NS3 interaction. To define residues involved in this interaction, further C-terminal truncations between NS2A R25 and M13 were again generated as GST fusions. After cotransfection of the respective constructs with pNS3-HA and immunoprecipitation with anti-HA antibody, coprecipitated NS2A truncations were detected by immunoblotting using anti-GST antibody (Fig. 6B). C-terminally truncated NS2A proteins coimmunoprecipitated with NS3-HA until the C-terminal truncations reached L21, whereas GST-NS2A V19 or shorter proteins were no longer able to be pulled down by NS3-HA. These data support our findings that the critical region involved in interaction of the NS2A N terminus with NS3 is not within the basic cluster R22-K23-R24 but rather shortly upstream.
DISCUSSION
The first evidence that NS2A contributes to YFV particle assembly was obtained for NS2Aα cleavage site mutants. Thereby it was shown that the sequence of NS2A in this C-terminal NS2A region is important for its role in infectious virus production rather than cleavage by the NS2B-3 serine protease (16). In this study, we used charged-to-alanine scanning mutagenesis to determine further regions within YFV NS2A important for infectious particle production. Using this approach, we identified two YFV NS2A triple mutants (R22A-K23A-R24A and R99A-E100A-R101A mutants) that are defective in infectious particle production despite RNA replication. Our data are consistent with a role for NS2A in virus production of other flaviviruses. KUNV production was affected by an isoleucine-to-asparagine change within NS2A at position 59 (17), as was DENV production by an arginine-to-alanine exchange at position 84 (28). Interestingly, our data demonstrated that exchange of the corresponding YFV residue R83 to alanine did not interfere with particle production, suggesting that the domains important for virus production differ among flaviviruses. On the other hand, comparable data have been obtained with regard to the region encompassing the YFV NS2Aα cleavage site. In this study, mutagenesis of YFV NS2A Q189 and K190 interfered with virion assembly, as was also described for the corresponding amino acids Q187 and K188 of DENV NS2A (11).
A defect in particle production, but not in viral RNA replication, was observed for our first triple mutant, the R22A-K23A-R24A mutant. In contrast, double mutants involving these residues (R22A-K23A and R22A-R24A) exhibited the WT phenotype. This suggests that a complex interplay of residues within the NS2A N terminus or steric conformations at the N terminus of NS2A, or both, are important for particle assembly. As viral RNA replication was delayed for the second triple mutant, the R99A-E100A-R101A mutant, NS2A is also implicated in viral RNA replication. This is consistent with data that show that NS2A residues R84, D125, and G200 of DENV are also critical for viral RNA replication (11, 28). Interestingly, preliminary data indicate that the second-site mutation NS2A:K124E, found to compensate for the defect in particle production of the R99A-E100A-R101A mutant, also mostly restores the delay in RNA replication of the R99A-E100A-R101A mutant. Whether the increase in RNA replication is linked to the ability to produce infectious particles awaits further investigations. In our previous studies, we found that exchange of aspartate 343 of YFV NS3 to either valine, alanine, or glycine was able to restore the defect in infectious virus production observed for the YFV NS2Aα cleavage site Q189S mutant (16). Interestingly, during continual passaging of cells transfected with in vitro-transcribed RNA derived from the YFV R22A-K23A-R24A construct, mutation of NS3 aspartate 343 to glycine also arose. The suppressive nature of this NS3 mutation for the YFV R22A-K23A-R24A mutant was proven using reverse genetics. This result further supported that a genetic interaction between YFV NS2A and NS3 contributes to particle assembly. Interestingly, the combined R22A-K23A-R24A+Q189S mutant could not be restored by the NS3 mutation D343G, suggesting that either of these NS2A sites has to be preserved for a functional interaction network for virion morphogenesis. Hence, the respective NS2A sites seem to be able to compensate for each other. Involvement of YFV NS3 in production of infectious particles was also shown by studies from Patkar and Kuhn, which analyzed the region around the NS3 suppressor mutation in further detail (18). Since D343 lies on a solvent-accessible loop encompassing amino acids E338 to D354 in domain 2 of the helicase, Patkar and Kuhn generated alanine substitution mutations in this region. NS3 substitution W349A was found to halt virus production while having little or no effect on viral RNA replication (18). As aromatic ring structures are involved in nucleic base-stacking interactions, it was suggested that the aromatic nature of the tryptophan at position 349 may be important to interactions between NS3 and viral RNA and/or NS2A. Such interactions might represent a prerequisite for the transport of nascent RNA to the rough ER. For the YFV NS2A mutant reported here, the production of infectious particles was suppressed by the mutation of a cluster of basic residues. Since positively charged amino acids are also involved in RNA interaction, these mutations may also block transport of nascent RNA to the ER or, alternatively, disrupt packaging of viral RNA. Furthermore, encapsidation of genomic flavivirus RNA into progeny virus has been described to involve the modification of ER membrane structures (29). Hence, it would be interesting to analyze whether formation of those ER membrane alterations is disrupted for the NS2A mutants defective in infectious particle production. While colocalization of NS2A with double-stranded RNA has been described (10), interaction with the ssRNA packaged into virions has not been described. Besides charge matching between the positively charged motifs in the capsid protein and the negatively charged phosphate backbone of the RNA, the presence of multiple sites within the ssRNA genomes that contact the capsid proteins during assembly, so-called packaging signals, have been described to be important for efficient and specific packaging of ssRNA genomes (30–32). Whether NS2A has a role in virion assembly at the level of RNA packaging by for example assisting in condensing the RNA remains to be investigated. Alternatively, NS2A may play a role in particle assembly by affecting the electrostatic repulsions that influence the arrangement of capsid proteins.
In this study, we showed that the region encompassing amino acids R22-K23-R24 within the N-terminal region of YFV NS2A localized to the cytoplasm. In contrast, analyses for DENV indicated that in this case the N-terminal 68 amino acids of NS2A are located in the lumen of the ER (28). This discrepancy is similar to data concerning the localization of YFV NS2A residues 189 to 191 in the C-terminal region of NS2A, which encompass the YFV NS2Aα cleavage site. We demonstrate here that the region of the YFV NS2Aα cleavage site is localized in the cytoplasm, which is in accordance with this site being cleaved by the cytoplasmic viral serine protease. In contrast, the data obtained for DENV NS2A locate the corresponding amino acids (DENV 186 to 188) in the ER lumen (28). A different topology between NS2A of YFV and DENV might explain the fact that amino acids critical for infectious particle production partially differ between flaviviruses. For example, as mentioned before, while exchange of DENV NS2A residue R84 to alanine abolished formation of infectious virus (28), mutation of the corresponding YFV residue, R83, to alanine did not interfere with particle production. In any case, NS2A residues determined to localize to the cytoplasm should be accessible for interaction with other cytoplasmic viral or host cell proteins or with viral RNA produced during the replication process in the cytoplasm.
Together, our previous and current studies suggested a genetic interaction between NS2A and NS3. Using coimmunoprecipitation analyses of ectopically expressed NS2A and NS3 proteins, we showed in this study for the first time a direct interaction between these two YFV proteins. After initial experiments involving C-terminal truncations of NS2A, we identified the region encompassing amino acids M14 to Q25 to be involved in interaction. This region contained the basic cluster R22-K23-R24, shown to be important for virus production. For HCV NS2, specific mutations that interfere with particle production also interfered with protein-protein interaction. This prompted us to analyze whether the mutations in YFV NS2A that block production of infectious particles also interfered with interaction of NS3 and, if so, whether the suppressor mutation in NS3 restores the interaction. In the absence of a specific anti-NS2A antibody, an infectious YFV full-length clone with an HA tag insertion in the N-terminal region of NS2A was generated to facilitate NS2A pulldown experiments with infectious virus. Infectious HA-tagged WT-YFV was obtained and efficient RNA replication observed even in the presence of the NS2A Q189S mutation. However, in contrast to the nontagged WT-YFV, RNA replication was abolished for the HA-tagged YFV by the addition of the R22A-K23A-R24A mutations. This supports a role for YFV NS2A in RNA replication in addition to particle production and suggests that a certain conformation of the NS2A N terminus is critical to this role.
Nevertheless, using the NS2A HA-tagged WT-YFV for coimmunoprecipitation analyses, we could confirm the interaction between NS2A and NS3, which we had demonstrated first using ectopically expressed proteins. The fact that the NS2A substitution Q189S, which resulted in a defect in infectious particle production, had little or no effect on the amount of NS3 pulled down by mutant NS2A indicated that there is no direct correlation between YFV NS2A-NS3 interaction and particle production. This contrasts with data for HCV, for which several mutations in NS2 that blocked particle production also disrupted interaction to viral proteins in which suppressor mutations had been found (27). In the same study, the introduction of pseudorevertants that reconstituted virus assembly also restored the protein-protein interactions to various degrees. However, also for HCV, a direct correlation between protein-protein interaction and virus assembly did not seem to be mandatory, as other studies on HCV NS2 mutants and particle assembly showed that second-site suppressors did not restore NS2 interactions; in some cases, they even inhibited them (33, 34). While in our and other studies involving viruses of the genus Flavivirus (16, 17) only suppressor mutations in nonstructural proteins were found, analyses of HCV NS2 mutants revealed pseudorevertants both in the nonstructural proteins and in the structural proteins (27, 33, 34), suggesting that HCV NS2 plays a critical role in HCV assembly by bringing together viral structural and nonstructural proteins. Whether a similar interplay with structural proteins also applies to YFV NS2A needs further investigation.
Since our analyses indicated that the basic residues R22-K23-R24 are not directly involved in NS3 interaction, we tested further C-terminal NS2A truncations and found that the region critical for interaction is directly upstream of the basic cluster encompassing amino acids V20 and/or L21. Preliminary data involving mutation of either V20 or L21 to alanine based on the infectious YFV cDNA clone indicate that NS2A V20A and L21A mutants produced infectious particles, while no plaques were visible in the infectious center assay for the V20A-L21A double mutant despite positive immunofluorescence signals. This suggests that simultaneous mutation of residues V20 and L21 is also critical for particle formation. Interestingly, our first analysis of the double mutant indicated that infectious particles started to arise at day 2 postelectroporation. Preliminary data for the recovered virus indicated that the mutations at positions 20 and 21 remained stable and no other mutation was detectable within NS2A. This suggests the recovery of a second-site mutation outside NS2A, the nature of which still has to be determined.
As mentioned before, amino acids R22-K23-R24 were determined to not be directly involved in NS2A-NS3 interaction. Hence, the basic cluster could remain accessible for interaction with other viral and/or host proteins or viral RNA. Accordingly, mutation of the basic residues might prevent interaction partners from entering the complex necessary for particle formation without affecting the NS2A-NS3 interaction itself or might interfere with RNA binding. For NS2A of DENV and WNV, several host proteins of mosquito origin, like translation factors or proteins involved in the cytoskeleton and cellular trafficking, have been found as binding partners (35). Further studies are necessary to determine whether these and analogous vertebrate proteins are involved in the formation of a complex necessary for assembly and release of infectious flavivirus particles. For HCV, cellular proteins Hsp90 and CIDE-B have been reported to interact with NS2 and were discussed to contribute to HCV particle assembly (34, 36, 37).
Overall, the studies substantiate that assembly and release of flavivirus particles constitute a complex process involving an interplay of several components. The NS2A HA-tagged YFV represents a useful tool to identify further NS2A interaction partners relevant for infectious particle production. Better knowledge of those partners and their interaction sites represents a prerequisite for the development of new antivirals that prohibit virus assembly.
ACKNOWLEDGMENTS
We thank Sue Jacobs, Norbert Tautz, Marcel Müller, and Sandra Junglen for helpful comments on the manuscript. We are grateful to Charles M. Rice for providing the infectious YFV cDNA clone and YFV anti-NS3 serum and to Felix Drexler for help with statistical analysis. We thank Stephanie Wurr for excellent technical assistance.
This work was financially supported by the Deutsche Forschungsgemeinschaft (grants KU 1201/3-1 and KU 1201/4-1).
REFERENCES
- 1.Tomori O. 2004. Yellow fever: the recurring plague. Crit Rev Clin Lab Sci 41:391–427. doi: 10.1080/10408360490497474. [DOI] [PubMed] [Google Scholar]
- 2.Gubler DJ, Kuno G, Markoff L. 2007. Flaviviruses, p 1153–1252 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Gardner CL, Ryman KD. 2010. Yellow fever: a reemerging threat. Clin Lab Med 30:237–260. doi: 10.1016/j.cll.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis BR, Barrett AD. 2008. The enigma of yellow fever in East Africa. Rev Med Virol 18:331–346. doi: 10.1002/rmv.584. [DOI] [PubMed] [Google Scholar]
- 5.Breugelmans JG, Lewis RF, Agbenu E, Veit O, Jackson D, Domingo C, Bothe M, Perea W, Niedrig M, Gessner BD, Yactayo S. 2013. Adverse events following yellow fever preventive vaccination campaigns in eight African countries from 2007 to 2010. Vaccine 31:1819–1829. doi: 10.1016/j.vaccine.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 6.Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. 2012. The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: systematic review. Am J Trop Med Hyg 86:359–372. doi: 10.4269/ajtmh.2012.11-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin M, Tsai TF, Cropp B, Chang GJ, Holmes DA, Tseng J, Shieh W, Zaki SR, Al-Sanouri I, Cutrona AF, Ray G, Weld LH, Cetron MS. 2001. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet 358:98–104. doi: 10.1016/S0140-6736(01)05327-2. [DOI] [PubMed] [Google Scholar]
- 8.Lindenbach BD, Thiel H-J, Rice CM. 2007. Flaviviridae: the viruses and their replication, p 1102–1152 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 1 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 9.Chambers TJ, McCourt DW, Rice CM. 1989. Yellow fever virus proteins NS2A, NS2B, and NS4B: identification and partial N-terminal amino acid sequence analysis. Virology 169:100–109. doi: 10.1016/0042-6822(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie JM, Khromykh AA, Jones MK, Westaway EG. 1998. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology 245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 11.Xie X, Zou J, Puttikhunt C, Yuan Z, Shi PY. 12November2014. Two distinct sets of NS2A molecules are responsible for dengue virus RNA synthesis and virion assembly. J Virol doi: 10.1128/JVI.02882-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu WJ, Chen HB, Wang XJ, Huang H, Khromykh AA. 2004. Analysis of adaptive mutations in Kunjin virus replicon RNA reveals a novel role for the flavivirus nonstructural protein NS2A in inhibition of beta interferon promoter-driven transcription. J Virol 78:12225–12235. doi: 10.1128/JVI.78.22.12225-12235.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muñoz-Jordan JL, Sanchez-Burgos GG, Laurent-Rolle M, Garcia-Sastre A. 2003. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci U S A 100:14333–14338. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu WJ, Wang XJ, Clark DC, Lobigs M, Hall RA, Khromykh AA. 2006. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J Virol 80:2396–2404. doi: 10.1128/JVI.80.5.2396-2404.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestorowicz A, Chambers TJ, Rice CM. 1994. Mutagenesis of the yellow fever virus NS2A/2B cleavage site: effects on proteolytic processing, viral replication and evidence for alternative processing of the NS2A protein. Virology 199:114–123. doi: 10.1006/viro.1994.1103. [DOI] [PubMed] [Google Scholar]
- 16.Kümmerer BM, Rice CM. 2002. Mutations in the yellow fever virus nonstructural protein NS2A selectively block production of infectious particles. J Virol 76:4773–4784. doi: 10.1128/JVI.76.10.4773-4784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung JY, Pijlman GP, Kondratieva N, Hyde J, Mackenzie JM, Khromykh AA. 2008. Role of nonstructural protein NS2A in flavivirus assembly. J Virol 82:4731–4741. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patkar CG, Kuhn RJ. 2008. Yellow fever virus NS3 plays an essential role in virus assembly independent of its known enzymatic functions. J Virol 82:3342–3352. doi: 10.1128/JVI.02447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khromykh AA, Varnavski AN, Sedlak PL, Westaway EG. 2001. Coupling between replication and packaging of flavivirus RNA: evidence derived from the use of DNA-based full-length cDNA clones of Kunjin virus. J Virol 75:4633–4640. doi: 10.1128/JVI.75.10.4633-4640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindenbach BD, Rice CM. 1997. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol 71:9608–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 22.Bredenbeek PJ, Kooi EA, Lindenbach B, Huijkman N, Rice CM, Spaan WJ. 2003. A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J Gen Virol 84:1261–1268. doi: 10.1099/vir.0.18860-0. [DOI] [PubMed] [Google Scholar]
- 23.Chambers TJ, McCourt DW, Rice CM. 1990. Production of yellow fever virus proteins in infected cells: identification of discrete polyprotein species and analysis of cleavage kinetics using region-specific polyclonal antisera. Virology 177:159–174. doi: 10.1016/0042-6822(90)90470-C. [DOI] [PubMed] [Google Scholar]
- 24.Agapov EV, Frolov I, Lindenbach BD, Pragai BM, Schlesinger S, Rice CM. 1998. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc Natl Acad Sci U S A 95:12989–12994. doi: 10.1073/pnas.95.22.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falgout B, Markoff L. 1995. Evidence that flavivirus NS1-NS2A cleavage is mediated by a membrane-bound host protease in the endoplasmic reticulum. J Virol 69:7232–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nestorowicz A, Chambers TJ, Rice CM. 1994. Mutagenesis of the yellow fever virus NS2A/2B cleavage site: effects on proteolytic processing, viral replication, and evidence for alternative processing of the NS2A protein. Virology 199:114–123. doi: 10.1006/viro.1994.1103. [DOI] [PubMed] [Google Scholar]
- 27.Jirasko V, Montserret R, Lee JY, Gouttenoire J, Moradpour D, Penin F, Bartenschlager R. 2010. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog 6:e1001233. doi: 10.1371/journal.ppat.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie X, Gayen S, Kang C, Yuan Z, Shi PY. 2013. Membrane topology and function of dengue virus NS2A protein. J Virol 87:4609–4622. doi: 10.1128/JVI.02424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. 2009. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garmann RF, Comas-Garcia M, Koay MS, Cornelissen JJ, Knobler CM, Gelbart WM. 2014. Role of electrostatics in the assembly pathway of a single-stranded RNA virus. J Virol 88:10472–10479. doi: 10.1128/JVI.01044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dykeman EC, Stockley PG, Twarock R. 2013. Packaging signals in two single-stranded RNA viruses imply a conserved assembly mechanism and geometry of the packaged genome. J Mol Biol 425:3235–3249. doi: 10.1016/j.jmb.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Stockley PG, Twarock R, Bakker SE, Barker AM, Borodavka A, Dykeman E, Ford RJ, Pearson AR, Phillips SE, Ranson NA, Tuma R. 2013. Packaging signals in single-stranded RNA viruses: nature's alternative to a purely electrostatic assembly mechanism. J Biol Phys 39:277–287. doi: 10.1007/s10867-013-9313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stapleford KA, Lindenbach BD. 2011. Hepatitis C virus NS2 coordinates virus particle assembly through physical interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J Virol 85:1706–1717. doi: 10.1128/JVI.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan T, Beran RK, Peters C, Lorenz IC, Lindenbach BD. 2009. Hepatitis C virus NS2 protein contributes to virus particle assembly via opposing epistatic interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J Virol 83:8379–8395. doi: 10.1128/JVI.00891-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colpitts TM, Cox J, Nguyen A, Feitosa F, Krishnan MN, Fikrig E. 2011. Use of a tandem affinity purification assay to detect interactions between West Nile and dengue viral proteins and proteins of the mosquito vector. Virology 417:179–187. doi: 10.1016/j.virol.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erdtmann L, Franck N, Lerat H, Le Seyec J, Gilot D, Cannie I, Gripon P, Hibner U, Guguen-Guillouzo C. 2003. The hepatitis C virus NS2 protein is an inhibitor of CIDE-B-induced apoptosis. J Biol Chem 278:18256–18264. doi: 10.1074/jbc.M209732200. [DOI] [PubMed] [Google Scholar]
- 37.Waxman L, Whitney M, Pollok BA, Kuo LC, Darke PL. 2001. Host cell factor requirement for hepatitis C virus enzyme maturation. Proc Natl Acad Sci U S A 98:13931–13935. doi: 10.1073/pnas.241510898. [DOI] [PMC free article] [PubMed] [Google Scholar]