Abstract
The cyclin-dependent kinase 5 (CDK-5) activating protein, p35, is important for acute herpes simplex virus 1 (HSV-1) replication in mice. This report shows that HSV-1 increases p35 levels, changes the primary localization of CDK-5 from the nucleus to the cytoplasm, and enhances CDK-5 activity during lytic or acute infection. Infected neurons also stained positive for the DNA damage response (DDR) marker γH2AX. We propose that CDK-5 is activated by the DDR to protect infected neurons from apoptosis.
TEXT
Herpes simplex virus 1 (HSV-1) is a human pathogen that establishes lifelong latent infection in sensory neurons (1, 46). It is not well understood how the virus switches from lytic to latent infections in neurons and the mechanisms involved in reversing this switch under stress stimuli to initiate reactivation. It is vital that the neurons survive an infection by HSV-1 if the virus is to efficiently establish a latent infection or reactivate. Not surprisingly, HSV-1 has developed countermeasures to prevent neuronal apoptosis, in part, with the expression of the latency-associated transcripts (LATs) (2–4). While the contribution of viral gene products that possess antiapoptotic activities has been a major area of interest in HSV-1 research, the role of cellular factors involved in neuronal survival has received limited attention.
One neuronal factor that is highly active in postmitotic neurons and is required for neuronal survival during stress is cyclin-dependent kinase 5 (CDK-5) (5, 6). CDK-5 regulates many neuronal processes (reviewed in reference 7) and has been shown to play important roles in both neuronal survival and death. Inactivation of CDK-5 has been shown to trigger neuronal death (8–11). On the other hand, a survival function for CDK-5 is evident when neurons are stressed (12–16). For its activation, CDK-5 binds to p35 or a related protein, p39, which also modulate CDK-5's subcellular localization (17–20). Notably the activity of CDK-5 directly correlates with the levels of its major activator, p35, and both are expressed predominantly in neurons (21), which likely explains why CDK-5 is mainly active in neurons, although it is constitutively expressed in many cell types (17, 22).
We have previously shown that HSV-1 acute replication is impaired in the eyes and trigeminal ganglia (TG) of p35 knockout mice, reducing the establishment of latency and reactivation (23). Given this previous result, we sought to determine whether HSV-1 altered the expression or subcellular localization of the p35 and CDK-5 proteins during acute infection.
HSV-1 infection induces p35 protein levels.
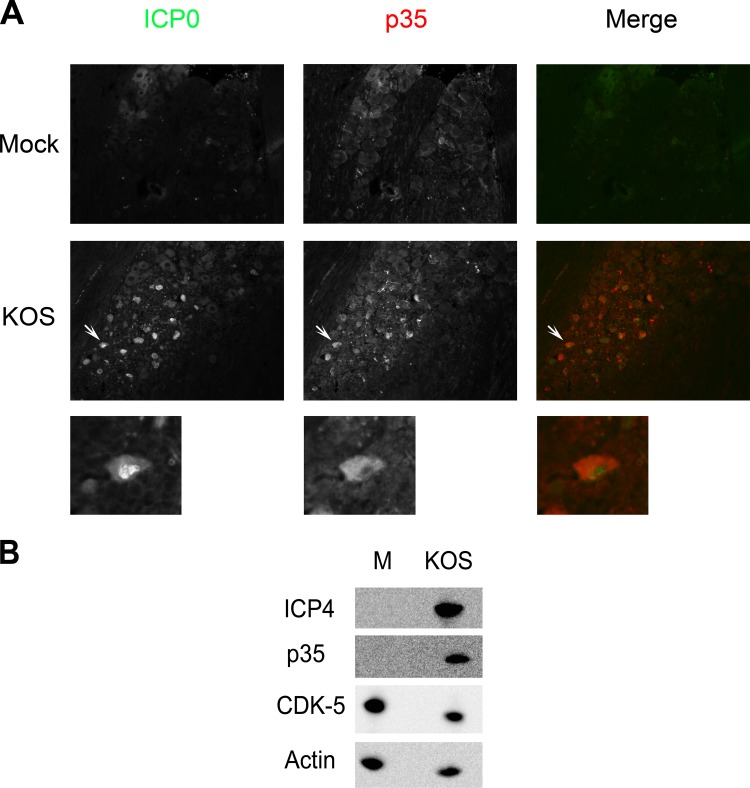
To examine how HSV-1 acute TG infection affects p35, wild-type HSV (KOS)-infected TG were harvested 3 days postinfection, as previously described (24, 25). TG sections were immunostained for both the HSV-1 immediate early protein, ICP0, used as a marker for productively infected neurons, and p35. As shown in Fig. 1A, HSV-1-infected neurons showed an increase in p35 staining compared to mock-infected cells, with a distribution that was primarily cytoplasmic. To confirm this result in cell culture, the human neuronal cell line SK-N-SH was mock infected or infected with KOS. As shown in Fig. 1B, p35 was not detected in mock-infected cells, whereas it was readily detected in KOS-infected cells and reached maximal levels of expression at 24 h postinfection (H. H. Mostafa, J. M. van Loben Sels, and D. J. Davido, unpublished data).
FIG 1.
Alterations in p35 expression by HSV-1. (A) p35 staining in response to HSV-1 infection. CD-1 mice were infected with 2 × 105 PFU of HSV-1 (strain KOS) per eye. Three days postinfection, mice were sacrificed, and TG were collected, fixed, paraffin embedded, and processed for immunofluorescence staining of ICP0 and p35. More than 10 sections from two independent experiments were examined, and the image shown is a representative section. Magnification, 200×. Arrows point to the expanded view shown below each panel. (B) p35 and CDK-5 protein levels after HSV-1 infection. SK-N-SH cells were mock infected (M) or infected at a multiplicity of infection (MOI) of 2 with KOS. Twenty-four hours postinfection, cells were harvested, and protein levels from cell extracts were determined by Western blot analysis.
HSV-1 infection changes the localization of CDK-5 and enhances its activity.
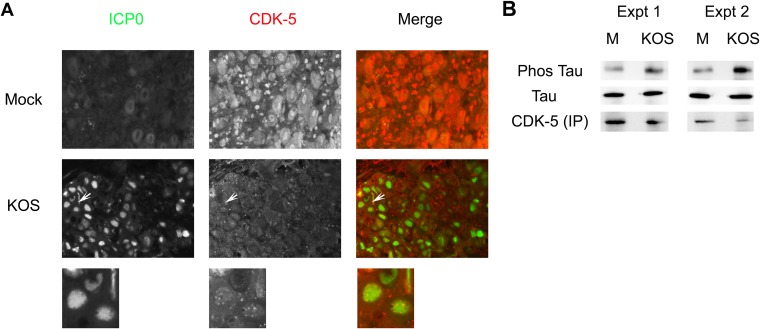
Because p35 function is linked to its binding partner, CDK-5, we next wanted to determine if HSV-1 acute infection affects CDK-5 expression and/or subcellular localization. For these studies, TG sections from 3-day KOS-infected mice were immunostained for ICP0 and CDK-5. As shown in Fig. 2A, in contrast to the mock-infected neurons, where CDK-5 localization was predominantly nuclear with diffuse cytoplasmic staining in most cells, HSV-1-infected neurons had a CDK-5 localization that was primarily punctate and both cytoplasmic and nuclear in ∼70% of cells that stained positive for ICP0. It was difficult to detect CDK-5 localization in SK-N-SH cells, as CDK-5's staining was faint (H. H. Mostafa and D. J. Davido, unpublished data); CDK-5 protein levels, however, were apparent in HSV-1-infected SK-N-SH cells and at comparable levels to those in mock-infected cells (Fig. 1B). Thus, alterations in CDK-5 localization do not appear to correlate with decreased protein levels. In order to test whether HSV-1 infection modulated CDK-5's kinase activity, SK-N-SH cells were mock infected or infected for 24 h with KOS. CDK-5 was immunoprecipitated and incubated with the CDK-5 substrate Tau (26) for 30 min at 37°C. As shown in Fig. 2B, KOS infection increased the amount of phosphorylated Tau by 4- to 5-fold as detected with the monoclonal antibody AT8 (27). This indicates that HSV-1 infection enhances CDK-5 kinase activity in neuronal cells.
FIG 2.
HSV-1 affects CDK-5 localization and kinase activity. (A) CDK-5 localization in response to HSV-1 infection. CD-1 mice were infected with 2 × 105 PFU of KOS per eye. Three days postinfection, mice were sacrificed, and TG were collected, fixed, paraffin embedded, and processed for immunofluorescence staining of ICP0 and CDK-5. More than 10 sections from two independent experiments were examined, and the image shown is a representative section. Magnification, 400×. Arrows point to the expanded view shown below each panel. In about 30% of the ICP0-positive neurons, CDK-5 colocalized with ICP0 in the nucleus but showed punctate staining, as evident in the expanded view. (B) In vitro CDK-5 kinase assay. SK-N-SH cells were mock infected or infected with KOS for 24 h. CDK-5 protein was immunoprecipitated (IP) and incubated with bacterially purified Tau protein. Phosphorylated (Phos) versus total Tau was determined by Western blot analysis and quantified by densitometry. Data from two independent experiments are shown.
HSV-1 infection induces DDR in TG.
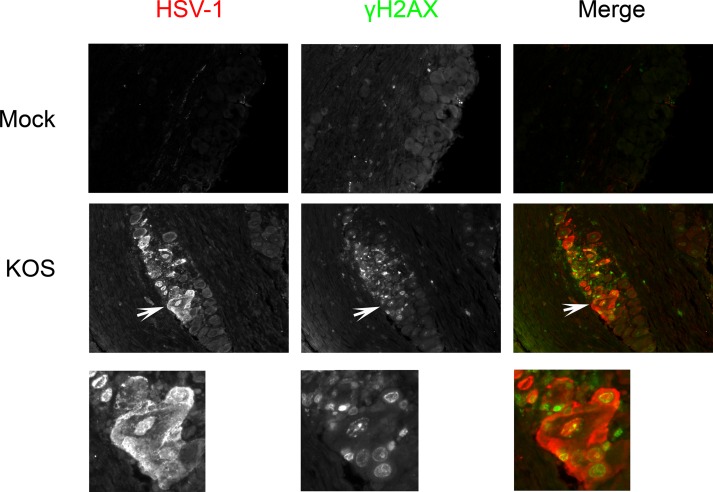
CDK-5 is important for neuronal survival in response to stressful conditions, including the DNA damage response (DDR) (12–16). In this context, HSV-1 infection triggers and counteracts the DDR in nonneuronal cell lines (28–32). However, the induction of the DDR in cultured neurons (28) or in the neurons of HSV-1-infected animals has not been reported. To determine whether HSV-1 infection triggers DDR in infected neurons, KOS-infected TG were isolated 3 days postinfection, sectioned, and immunostained with γH2AX, a histone variant and one of the earliest markers of the DDR (33–35). Interestingly, neurons that stained positive for HSV-1 antigens were positive for γH2AX, whereas mock-infected neurons had no apparent γH2AX staining (Fig. 3). This indicates that acute HSV-1 neuronal infection can trigger early DDR events.
FIG 3.
DNA damage response to HSV-1 infection. CD-1 mice were infected with KOS at 2 × 105 PFU per eye. Three days postinfection, mice were sacrificed, and TG were collected, fixed, paraffin embedded, and processed for immunofluorescence staining of HSV-1 and γH2AX. More than 10 sections from two independent experiments were examined, and the image shown is a representative section. Magnification, 200×. Arrows point to the expanded view shown below each panel.
Model of CDK-5 alteration and p35 induction during HSV-1 infection.
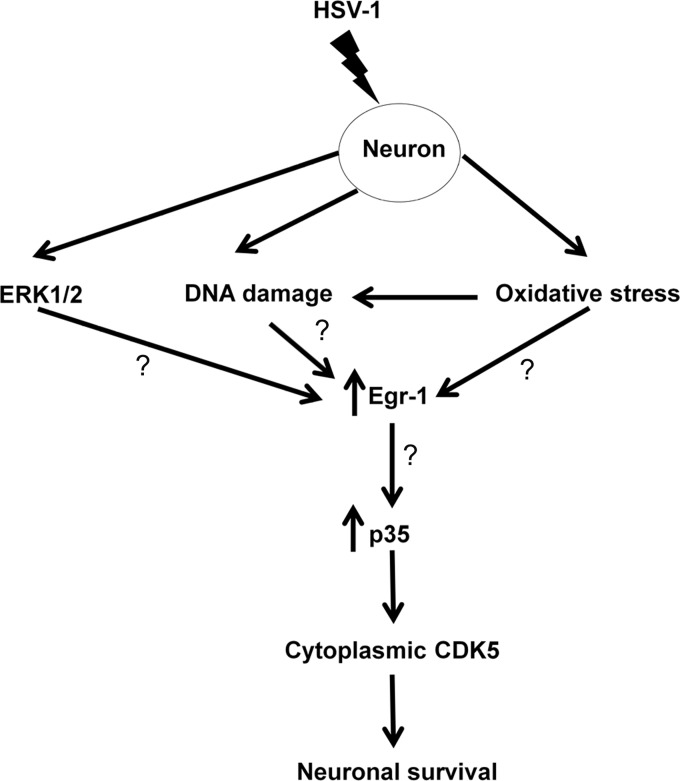
p35 levels are induced secondarily to neurotrophic factors that stimulate extracellular-signal-regulated kinase 1/2 (ERK1/2), activating the transcription factor Egr1 (36–38). Interestingly, a previous report showed that HSV-1 infection activates the ERK/mitogen-activated protein kinase (MAPK) signaling pathway (39). Moreover, it has been shown that HSV-1 lytic infection induces Egr1 in rabbit corneal cells (40). Oxidative stress is capable of initiating the DDR (41) and is reported to enhance the expression of Egr1 (42), and HSV-1 has the ability to induce neuronal oxidative stress (43). With these observations and our data, we propose that oxidative stress and/or activation of DDR during acute HSV-1 infection of neurons stimulates p35 levels via Egr1 (Fig. 4).
FIG 4.
Proposed model of p35 upregulation in response to HSV-1 infection. HSV-1 infection of neurons stimulates the ERK pathway, activates at least one component of the DDR, and triggers the oxidative stress response. These stimuli increase Egr-1 expression, leading to the induction of p35. Elevated p35 protein levels result in the cytoplasmic localization of CDK-5 and enhance its kinase activity, which promotes neuronal survival of the infected neuron.
It has been shown that cytoplasmic CDK-5 inhibits apoptosis by preventing neurons from entering mitosis (15, 44), and its protein levels are stabilized by p35 (45). Our data allow us to propose a model in which HSV-1 induces CDK-5 kinase activity to protect lytically infected neurons from dying (Fig. 4). In support of this possibility, we examined acutely infected TG for signs of apoptosis 3 days postinfection using terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining and did not detect an increase in apoptotic neurons in KOS-infected cells (H. H. Mostafa and D. J. Davido, unpublished data), consistent with other studies (2–4). In the future, we will determine how p35 is upregulated to promote lytic replication.
ACKNOWLEDGMENTS
This work was supported by the Doctoral Student Research Fund and a Hirata Graduate Student Summer Fellowship from the University of Kansas.
We thank Kristi Neufeld, Rick Dobrowsky, Yoshi Azuma, and Chris Gamblin for reagents, comments, and critiques related to this project.
REFERENCES
- 1.Stevens JG. 1987. Defining herpes simplex genes involved in neurovirulence and neuroinvasiveness. Curr Eye Res 6:63–67. doi: 10.3109/02713688709020070. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, BenMohamed L, Fraser NW, Jones C, Wechsler SL. 2011. The herpes simplex virus type 1 latency-associated transcript can protect neuron-derived C1300 and Neuro2A cells from granzyme B-induced apoptosis and CD8 T-cell killing. J Virol 85:2325–2332. doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perng GC, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina SM, Hofman FM, Ghiasi H, Nesburn AB, Wechsler SL. 2000. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science 287:1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RL, Sawtell NM. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J Virol 75:6660–6675. doi: 10.1128/JVI.75.14.6660-6675.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D, Wang JH. 1996. Cyclin-dependent kinase 5 (Cdk5) and neuron-specific Cdk5 activators. Prog Cell Cycle Res 2:205–216. [DOI] [PubMed] [Google Scholar]
- 6.Hisanaga S, Saito T. 2003. The regulation of cyclin-dependent kinase 5 activity through the metabolism of p35 or p39 Cdk5 activator. Neurosignals 12:221–229. doi: 10.1159/000074624. [DOI] [PubMed] [Google Scholar]
- 7.Hisanaga S, Endo R. 2010. Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J Neurochem 115:1309–1321. doi: 10.1111/j.1471-4159.2010.07050.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. 1996. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A 93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka T, Veeranna, Ohshima T, Rajan P, Amin ND, Cho A, Sreenath T, Pant HC, Brady RO, Kulkarni AB. 2001. Neuronal cyclin-dependent kinase 5 activity is critical for survival. J Neurosci 21:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li BS, Zhang L, Takahashi S, Ma W, Jaffe H, Kulkarni AB, Pant HC. 2002. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J 21:324–333. doi: 10.1093/emboj/21.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung ZH, Gong K, Ip NY. 2008. Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2. J Neurosci 28:4872–4877. doi: 10.1523/JNEUROSCI.0689-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li BS, Ma W, Jaffe H, Zheng Y, Takahashi S, Zhang L, Kulkarni AB, Pant HC. 2003. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J Biol Chem 278:35702–35709. doi: 10.1074/jbc.M302004200. [DOI] [PubMed] [Google Scholar]
- 13.Wang CX, Song JH, Song DK, Yong VW, Shuaib A, Hao C. 2006. Cyclin-dependent kinase-5 prevents neuronal apoptosis through ERK-mediated upregulation of Bcl-2. Cell Death Differ 13:1203–1212. doi: 10.1038/sj.cdd.4401804. [DOI] [PubMed] [Google Scholar]
- 14.Vartiainen N, Keksa-Goldsteine V, Goldsteins G, Koistinaho J. 2002. Aspirin provides cyclin-dependent kinase 5-dependent protection against subsequent hypoxia/reoxygenation damage in culture. J Neurochem 82:329–335. doi: 10.1046/j.1471-4159.2002.00959.x. [DOI] [PubMed] [Google Scholar]
- 15.O'Hare MJ, Kushwaha N, Zhang Y, Aleyasin H, Callaghan SM, Slack RS, Albert PR, Vincent I, Park DS. 2005. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci 25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkkoetter PT, Olivier P, Wu JS, Henderson S, Krofft RD, Pippin JW, Hockenbery D, Roberts JM, Shankland SJ. 2009. Cyclin I activates Cdk5 and regulates expression of Bcl-2 and Bcl-XL in postmitotic mouse cells. J Clin Invest 119:3089–3101. doi: 10.1172/JCI37978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. 1994. A brain-specific activator of cyclin-dependent kinase 5. Nature 371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 18.Tsai LH, Delalle I, Caviness VS Jr, Chae T, Harlow E. 1994. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 19.Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. 1995. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem 270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- 20.Humbert S, Dhavan R, Tsai L. 2000. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci 113:975–983. [DOI] [PubMed] [Google Scholar]
- 21.Lee KY, Rosales JL, Tang D, Wang JH. 1996. Interaction of cyclin-dependent kinase 5 (Cdk5) and neuronal Cdk5 activator in bovine brain. J Biol Chem 271:1538–1543. doi: 10.1074/jbc.271.3.1538. [DOI] [PubMed] [Google Scholar]
- 22.Philpott A, Porro EB, Kirschner MW, Tsai LH. 1997. The role of cyclin-dependent kinase 5 and a novel regulatory subunit in regulating muscle differentiation and patterning. Genes Dev 11:1409–1421. doi: 10.1101/gad.11.11.1409. [DOI] [PubMed] [Google Scholar]
- 23.Haenchen SD, Utter JA, Bayless AM, Dobrowsky RT, Davido DJ. 2010. Role of a cdk5-associated protein, p35, in herpes simplex virus type 1 replication in vivo. J Neurovirol 16:405–409. doi: 10.3109/13550284.2010.513030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostafa HH, Thompson TW, Kushnir AS, Haenchen SD, Bayless AM, Hilliard JG, Link MA, Pitcher LA, Loveday E, Schaffer PA, Davido DJ. 2011. Herpes simplex virus 1 ICP0 phosphorylation site mutants are attenuated for viral replication and impaired for explant-induced reactivation. J Virol 85:12631–12637. doi: 10.1128/JVI.05661-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostafa HH, Thompson TW, Davido DJ. 2013. N-terminal phosphorylation sites of herpes simplex virus type 1 ICP0 differentially regulate its activities and enhance viral replication. J Virol 87:2109–2019. doi: 10.1128/JVI.02588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. 2005. Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm 112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 27.Goedert M, Jakes R, Vanmechelen E. 1995. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 189:167–169. doi: 10.1016/0304-3940(95)11484-E. [DOI] [PubMed] [Google Scholar]
- 28.Lilley CE, Carson CT, Muotri AR, Gage FH, Weitzman MD. 2005. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc Natl Acad Sci U S A 102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohni KN, Livingston CM, Cortez D, Weller SK. 2010. ATR and ATRIP are recruited to herpes simplex virus type 1 replication compartments even though ATR signaling is disabled. J Virol 84:12152–12164. doi: 10.1128/JVI.01643-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson DE, Weller SK. 2004. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J Virol 78:4783–4796. doi: 10.1128/JVI.78.9.4783-4796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilley CE, Chaurushiya MS, Boutell C, Everett RD, Weitzman MD. 2011. The intrinsic antiviral defense to incoming HSV-1 genomes includes specific DNA repair proteins and is counteracted by the viral protein ICP0. PLoS Pathog 7:e1002084. doi: 10.1371/journal.ppat.1002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. 2010. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J 29:943–955. doi: 10.1038/emboj.2009.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. 2004. H2AX: the histone guardian of the genome. DNA Repair 3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 35.Bekker-Jensen S, Mailand N. 2010. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair 9:1219–1228. doi: 10.1016/j.dnarep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Harada T, Morooka T, Ogawa S, Nishida E. 2001. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol 3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 37.Tokuoka H, Saito T, Yorifuji H, Wei F, Kishimoto T, Hisanaga S. 2000. Brain-derived neurotrophic factor-induced phosphorylation of neurofilament-H subunit in primary cultures of embryo rat cortical neurons. J Cell Sci 113:1059–1068. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Kim KT. 2004. Induction of cyclin-dependent kinase 5 and its activator p35 through the extracellular-signal-regulated kinase and protein kinase A pathways during retinoic-acid mediated neuronal differentiation in human neuroblastoma SK-N-BE(2)C cells. J Neurochem 91:634–647. doi: 10.1111/j.1471-4159.2004.02770.x. [DOI] [PubMed] [Google Scholar]
- 39.Qin D, Feng N, Fan W, Ma X, Yan Q, Lv Z, Zeng Y, Zhu J, Lu C. 2011. Activation of PI3K/AKT and ERK MAPK signal pathways is required for the induction of lytic cycle replication of Kaposi's sarcoma-associated herpesvirus by herpes simplex virus type 1. BMC Microbiol 11:240. doi: 10.1186/1471-2180-11-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedadala GR, Palem JR, Graham L, Hill JM, McFerrin HE, Hsia SC. 2011. Lytic HSV-1 infection induces the multifunctional transcription factor Early Growth Response-1 (EGR-1) in rabbit corneal cells. Virol J 8:262. doi: 10.1186/1743-422X-8-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canugovi C, Misiak M, Ferrarelli LK, Croteau DL, Bohr VA. 2013. The role of DNA repair in brain related disease pathology. DNA Repair 12:578–587. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohba M, Shibanuma M, Kuroki T, Nose K. 1994. Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol 126:1079–1088. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schachtele SJ, Hu S, Little MR, Lokensgard JR. 2010. Herpes simplex virus induces neural oxidative damage via microglial cell Toll-like receptor-2. J Neuroinflammation 7:35. doi: 10.1186/1742-2094-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Li H, Herrup K. 2010. Cdk5 nuclear localization is p27-dependent in nerve cells: implications for cell cycle suppression and caspase-3 activation. J Biol Chem 285:14052–14061. doi: 10.1074/jbc.M109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Li H, Zhou T, Zhou J, Herrup K. 2012. Cdk5 levels oscillate during the neuronal cell cycle: Cdh1 ubiquitination triggers proteosome-dependent degradation during S-phase. J Biol Chem 287:25985–25994. doi: 10.1074/jbc.M112.343152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roizman R, Knipe DM, Whitley RJ. 2007. Herpes simplex viruses, p 2501–2601 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]