Abstract
Human cytomegalovirus (HCMV) has emerged as a clinically opportunistic pathogen that targets multiple types of ocular cells and tissues, including the iris region of the uveal tract during anterior uveitis. In this report, we used primary cultures of human iris stroma (HIS) cells derived from human eye donors to investigate HCMV entry. The following lines of evidence suggested the role of 3-O-sulfated heparan sulfate (3-OS HS) during HCMV-mediated entry and cell-to-cell fusion in HIS cells. First, 3-O-sulfotransferase-3 (3-OST-3) expression in HIS cells promoted HCMV internalization, while pretreatment of HIS cells with heparinase enzyme or with anti-3-OS HS (G2) peptide significantly reduced the HCMV-mediated formation of plaques/foci. Second, coculture of the HCMV-infected HIS cells with CHO-K1 cells expressing 3-OS HS significantly enhanced cell fusion. Finally, a similar trend of enhanced fusion was observed with cells expressing HCMV glycoproteins (gB, gO, and gH-gL) cocultured with 3-OS HS cells. Taken together, these results highlight the role of 3-OS HS during HCMV plaque formation and cell-to-cell fusion and identify a novel target for future therapeutic interventions.
TEXT
Human cytomegalovirus (HCMV), a member of the betaherpesvirus family, is the leading cause of congenital neurological complications in neonates as a result of maternal infection (1–4). Among immunocompromised patients—especially those with an organ transplant, chemotherapy, or AIDS—the reactivation of virus causes life-threatening diseases, such as gastroenteritis, encephalitis, pneumonitis, and graft rejection (5–7). In addition, HCMV infection is also implicated in widespread ocular damage and potential vision loss from retinitis, anterior uveitis, and corneal endotheliitis (8–11). Because HCMV infection of the iris is a risk factor during anterior uveitis, understanding the dynamics of viral infection at the molecular level becomes relevant for understanding the viral pathogenesis in general and developing novel strategies to prevent blindness. Therefore, we used primary cultures of human iris stromal (HIS) cells as a model to determine the susceptibility and role of 3-O-sulfated heparan sulfate (3-OS HS) during HCMV uveitis.
Although HCMV entry into host cells is poorly understood (12, 13), it is clearly a multistep process that requires complex interactions between viral envelope glycoproteins and the host cell receptors (12). It has been suggested that HCMV glycoprotein B (gB) binds to heparan sulfate (HS) during viral attachment, resulting in a high virion concentration at the cell surface and further binding to the cellular receptor (14–17). This interaction has been proposed to modulate immune responses (14, 18). To date, three receptors, epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor α (PDGFRα), and cellular integrins (α2β1, α6β1, and αvβ3), have been implicated during HCMV entry (19–24). In addition, recent studies have suggested the potential role of a sulfated form of HS during HCMV entry. For instance, a library of peptides derived from human hemofiltrate and screened for inhibitory effects on HCMV infection implicated the role of 6-O-sulfated HS (6-OS HS) (25) in HCMV entry. Similarly, a peptide generated against 3-OS HS using phage display library screening blocked HCMV entry (26). In addition, several sulfated polysaccharides (dextran sulfate, pentosan polysulfate, and heparin), copolymers of acrylic acid with vinyl alcohol sulfate, and 3-O-sulfated saccharide have proved to be potent inhibitors of HCMV infectivity in vitro (17, 27). In this study, we investigated the susceptibility of HIS cells to HCMV infection and the role of 3-OS HS during this process. Our data demonstrate the significance of 3-OS HS during HCMV entry, and we propose a unique in vitro model for future studies related to 3-OS HS-dependent induction of proinflammatory cytokines and virus tropism.
Susceptibility of primary cultures of HIS cells to HCMV infection.
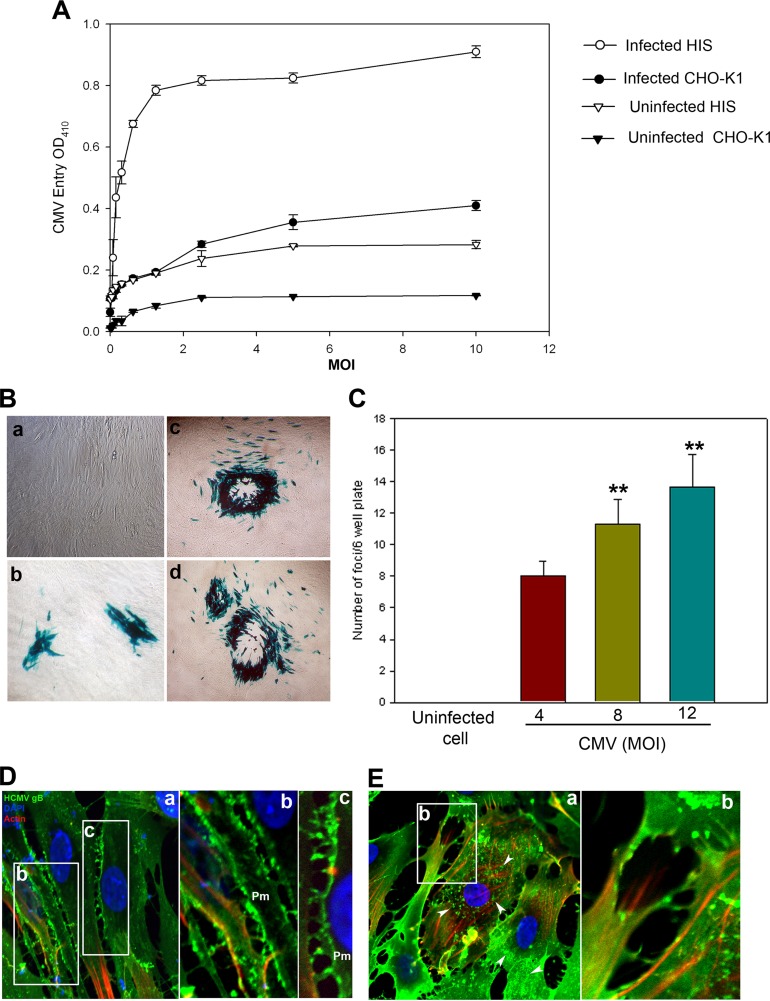
The HIS cultures were prepared in accordance with institutional review board-approved protocols and were isolated from anonymously donated human eyes (provided by the Illinois Eye Bank, Chicago, IL) via sterile dissection of the iris and pigmented epithelial layer and subsequent removal with a sterile cotton swab (28). The tissue was then digested with 0.2% type II collagenase (Sigma-Aldrich, St. Louis, MO) in cell culture medium MCDB-131 (Sigma-Aldrich, St. Louis, MO) at 37°C with gentle stirring for 20 to 30 min. Digested tissues were next centrifuged to remove tissue debris, and HIS cells were cultured in MCDB-131 containing 10% fetal bovine serum (FBS) and antibiotics. To establish the HCMV entry model for HIS cells of the iris, both HIS and CHO-K1 cells were infected with serial dilutions of a recombinant derivative of the attenuated Towne strain of HCMV (human herpesvirus 5 [ATCC VR-2356]), which expresses Escherichia coli lacZ as a reporter gene (29). Mock infections with phosphate-buffered saline (PBS) and without virus of both cell lines were used as controls. As shown in Fig. 1A, compared to the mock-infected control cells, the signal intensity during infection of HIS and CHO-K1 cells was significantly higher, demonstrating LacZ expression in a dose-dependent manner. In this assay o-nitrophenyl-β-d-galactopyranoside (ImmunoPure ONPG; Pierce) substrate was used for quantitation of β-galactosidase activity expressed from the input viral genome. The enzymatic activity was measured by spectrophotometer (Molecular Devices) at the optical density at 410 nm (OD410). We further confirmed the above results via X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining in HIS cells infected with HCMV in a dosage-dependent manner. As shown in Fig. 1B, the number of foci or blue plaques/6-well plate increased in correlation with increased multiplicity of infection (MOI) (Fig. 1C). Taken together, our results establish the susceptibility and permissiveness of HIS cells to HCMV infection. Since β-galactosidase activity was determined 9 days postinfection, we decided to investigate the role of 3-O-sulfotransferase-3 (3-OST-3) at an earlier time point by assessing HCMV internalization using immunofluorescence and confocal microscopy. HIS cells were transfected with 3-OST-3 expression plasmid or the empty vector (pDCNA3.1) as a control. Immunofluorescence was detected using a fluorescein isothiocyanate (FITC)-conjugated antibody directed against HCMV envelop glycoprotein B (Virostat, Inc.). As indicated in Fig. 1D, relatively high numbers of virions were sequestered and mostly localized on the plasma membrane (Pm) of HIS cells expressing pCDNA3.1. In contrast, HIS cells expressing 3-OST-3 had larger numbers of virions inside the cell (Fig. 1E, panel a, arrowheads). Interestingly, a significant increase in actin stress fibers was also observed in HIS cells expressing 3-OST-3 (Fig. 1E, panel b).
FIG 1.
(A to C) Susceptibility of primary cultures of human iris stromal (HIS) cells to HCMV infection. (A) Cultured HIS cells along with Chinese hamster ovary (CHO-K1) cells were plated in a 96-well plate and challenged with serial dilutions of a recombinant form of human cytomegalovirus (HCMV) that expresses β-galactosidase at the indicated multiplicity of infection (MOI) or were mock infected with serum-free medium (Optimem) at 37°C at 5% CO2. After 12 h, the cells were washed to remove unbound virus. The media were replenished every other day. After 9 days postinfection, the cells were washed, permeabilized, and incubated with o-nitrophenyl-β-d-galactopyranoside (ImmunoPure ONPG; Pierce) substrate for quantitation of β-galactosidase activity expressed from the input viral genome. The enzymatic activity was measured by spectrophotometer (Molecular Devices) at the optical density at 410 nm (OD410). Values in the figure were plotted as the means from three determinations ± standard deviations (SD). (B) X-Gal staining of HCMV entry. Cultured HIS cells were inoculated with either serum-free Optimem (a) or β-galactosidase-expressing HCMV at MOI of 4 (b), 8 (c), and 12 (d). As indicated in the panels, cytopathic effects (CPE) in the form of blue plaques or foci were noticed in the HCMV-infected HIS cells. Mock-uninfected HIS cells remained colorless. (C) The number of foci per 6-well plate in HIS-infected cells was HCMV concentration dependent. No CPE in the form of foci was noticed in uninfected HIS cells. Asterisks indicate significant difference from the uninfected control (P < 0.05, t test); error bars represent SD (n = 4). (D and E) Immunofluorescence of HCMV-infected (MOI of 20 for 5 h at 37°C at 5% CO2) HIS cells expressing pCDNA3.1 (D) or 3-O-sulfotransferase-3 (3-OST-3) (E). FITC-conjugated antibody against HCMV gB (1:20 dilution) was used to detect the virus particles. Panel D (panels a to c) indicates a higher concentration of HCMV virions on HIS cell plasma membrane (pm). Boxed regions in panel a are highlighted in panels b and c. In panel E, a large number of HCMV virions (arrows in panel a) were seen inside the HCMV-infected HIS cells expressing 3-OST-3. In addition, an increase in actin stress fiber is highlighted in panel b. Confocal microscopy was performed with a 40× oil objective (Nikon Eclipse Ti).
Role for 3-OS HS in plaque formation upon HCMV infection.
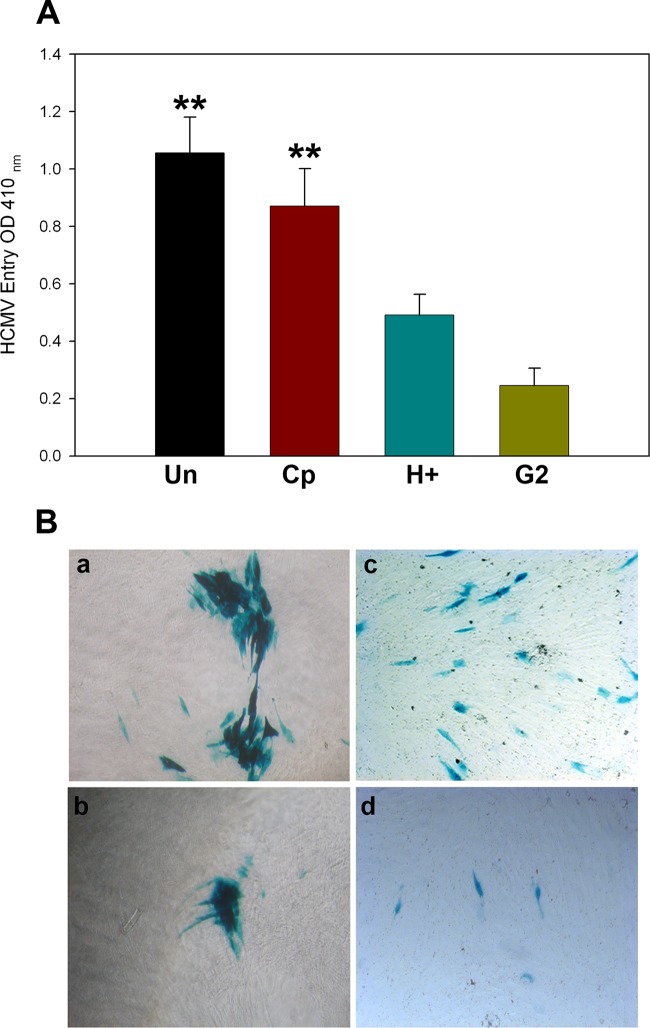
Since HIS cells are already known to express 3-O-sulfotransferase-3 (3-OST-3) (28), an enzyme that generates a modified form of 3-O-sulfated heparan sulfate (3-OS HS) (16, 30, 31), we determined if HS and 3-OS HS play a role during HCMV infection in HIS cells. In this experiment, HIS cells were pretreated with heparinase I, an enzyme that selectively cleaves both heparin and HS chains containing 1→4 linkages between glucosamines and O-sulfated iduronic acid residues (32). In addition, in parallel wells of a 96-well plate HIS cells were pretreated with anti-3-OS HS (G2) peptide, which targets specific regions in 3-OS HS (26). A control peptide (Cp), which is a randomized peptide and does not affect the HS chain, was used a control (26). After 2 h of pretreatment of HIS cells with heparinase and/or with peptides, the cells were challenged with the reporter HCMV for 12 h before being washed to remove the unbound virus. The infected cells were kept at 37°C in 5% CO2. Virus-mediated β-galactosidase activity was assessed 9 days postinfection by colorimetric assays. As indicated in Fig. 2, HCMV LacZ expression was significantly impacted with either heparinase (H+) treatment or G2 peptide, as demonstrated by ONPG (Fig. 2A) and X-Gal (Fig. 2B) assays. The above results suggest the role for 3-OS HS during HCMV infection in HIS cells.
FIG 2.
Interference with cell surface heparan sulfate either by enzymatic removal or by blockage via anti-3-OS HS (G2) peptide negatively affects HCMV infection of HIS cells. (A) Cultured HIS cells were either mock treated or untreated (Un) with serum-free medium or pretreated with 1 mg/ml control peptide (Cp; RVCGSIGKEVLG), anti-3-OS HS (G2; MPRRRRIRRRQK) peptide, and heparinase I (H+) (10 IU) for 2 h followed by addition of the reporter HCMV at an MOI of 4 to individual wells for 12 h before the cells were washed to remove the unbound virus. The cells were kept at 37°C in 5% CO2 for 9 days before the effect of treatment was assessed. As indicated in panel A, HCMV entry was assessed via ONPG substrate (3.0 mg/ml) for quantitation of β-galactosidase activity expressed from the input viral genome. The enzymatic activity was measured at the optical density at 410 nm (OD410). Asterisks indicate significant difference from other treatments (P < 0.05, t test), and error bars represent SD (n = 4). (B) X-Gal staining of the HIS cells for blue plaques or foci in response to following treatments: mock treated (a), Cp treated (b), heparinase treated H+ [c]), and anti-3-OS HS (G2) peptide treated (d).
3-OS HS supports HCMV-mediated cell fusion.
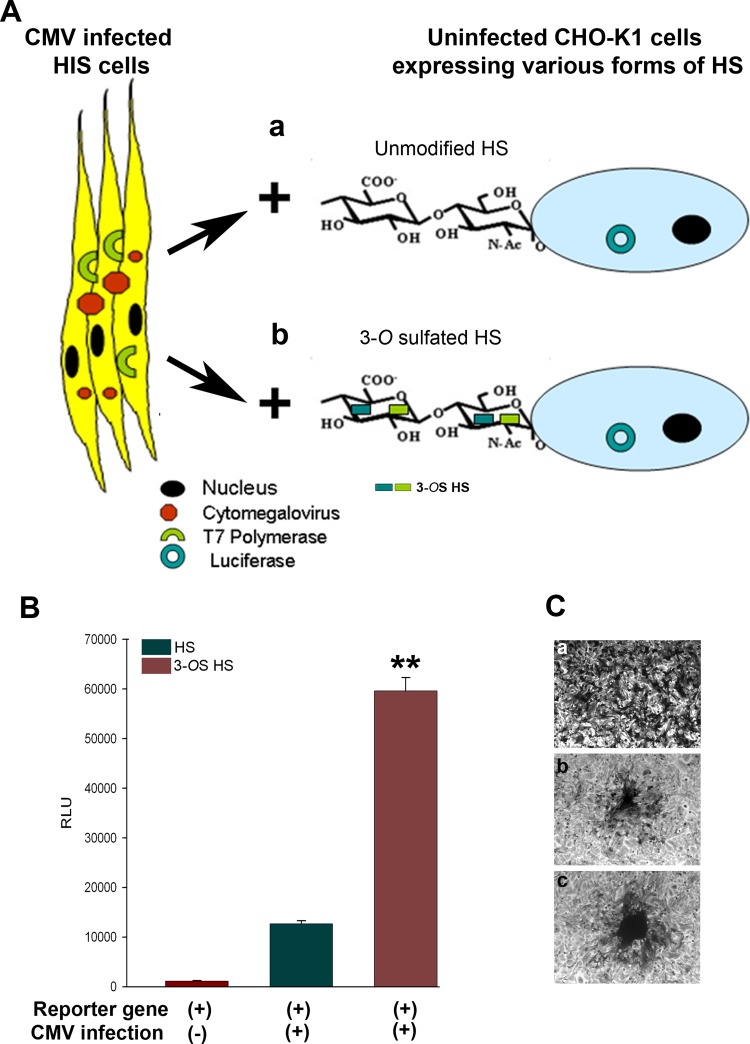
To test the possible significance of 3-OS HS during HCMV-mediated fusion, we used a previously reported luciferase-based reporter gene cell-to-cell fusion assay (33, 34). As demonstrated in Fig. 3A, the HIS cells considered “effector” cells were first infected with HCMV at an MOI of 10 for 7 days followed by transfection with expression plasmid for T7 polymerase. In parallel, CHO-K1 cells which were considered “target” cells were cotransfected with plasmid expressing 3-OST-3 enzymes to generate a modified form of HS or control plasmid pCDNA3.1 to have unmodified HS along with the reporter luciferase gene (Fig. 3A). The DNA concentrations were balanced and kept equal to 2.5 μg in both effector and target cells. Nine days postinfection, HIS cells were mixed in equal proportion (1:1) and cocultured with CHO-K1 cells that express 3-OST-3 enzyme or control plasmid pCDNA3.1 for an additional 24 h before the reporter luciferase assay was performed. As shown in Fig. 3B, a significant cell-to-cell fusion was measured via quantification in terms of relative luciferase units (RLU) in HCMV-infected HIS cells that were cocultured with 3-OST-3-expressing CHO-K1 cells compared to pCDNA3.1-expressing CHO-K1 cells. The uninfected HIS cells were unable to fuse with CHO-K1 cells expressing pCDNA3.1 or 3-OST-3 plasmids. The visual evidence presented in Fig. 3C further indicated that HCMV-infected HIS cells cocultured with 3-OST-3-expressing CHO-K1 cells were able to form a higher number of plaques, and these plaques were comparatively bigger (Fig. 3C, panel c) than CHO-K1 cells that express unmodified HS (Fig. 3C, panel b). Again, the uninfected HIS cells were unable to form plaques with CHO-K1 cells. Therefore, the above results indicate a potential role for 3-OS HS in assisting HCMV fusion.
FIG 3.
Expression of 3-OST-3 enzymes in target CHO-K1 cells facilitates fusion with the HCMV-infected HIS cells. (A) As indicated, HCMV (MOI of 4)-infected HIS cells expressing T7 polymerase at 9 days postinfection (p.i.) were isolated and cocultured independently either with CHO-K1 cells coexpressing HS with luciferase or with CHO-K1 cells expressing 3-O-sulfotransferase-3 (3-OST-3) with luciferase. (B) 3-OST-3-generated 3-OS HS in CHO-K1 cells promotes fusion with HCMV-infected HIS cells. As indicated, no relative luciferase activity (RLU) as a measure of cell-to-cell fusion was recorded when uninfected HIS cells were cultured with CHO-K1 cells (dark red bar (left]), while higher fusion was recorded with HCMV-infected HIS cells when cocultured with CHO-K1 cells expressing plain-type HS (dark green bar [middle]). A significantly higher fusion was obtained with 3-OS HS cells when cocultured with HCMV-infected HIS cells (dark red bar [right]). Relative luciferase units (RLUs) were determined using a Sirius luminometer (Berthold Detection Systems) and are presented as the mean ± SD from three independent experiments. Asterisks indicate significant difference from other treatments (P < 0.05, t test), and error bars represent SD (n = 4). (C) Giemsa stain to visualize multinucleated giant cells is presented for the experiment described above. No giant cell formation was noticed during coculture of uninfected HIS cells with target CHO-K1 cells (a). In contrast, small to massive giant cells were observed during coculture of HCMV (4 MOI)-infected HIS cells with CHO-K1 cells (b) or with 3-OST-3-expressing CHO-K1 cells (c), respectively.
Expression of 3-OST enzymes in HIS cells enhances fusion with cells expressing HCMV glycoproteins.
Finally, we tested the possible interaction between 3-OS HS and HCMV glycoproteins in the absence of virus. Again we used a reporter gene-based cell-to-cell fusion assay in the presence of HCMV glycoproteins (33, 34). It has been shown previously that combinations of HCMV glycoproteins (gB, gH-gL, and gO) play a critical role during viral entry and spread in fibroblast cells (12, 35).
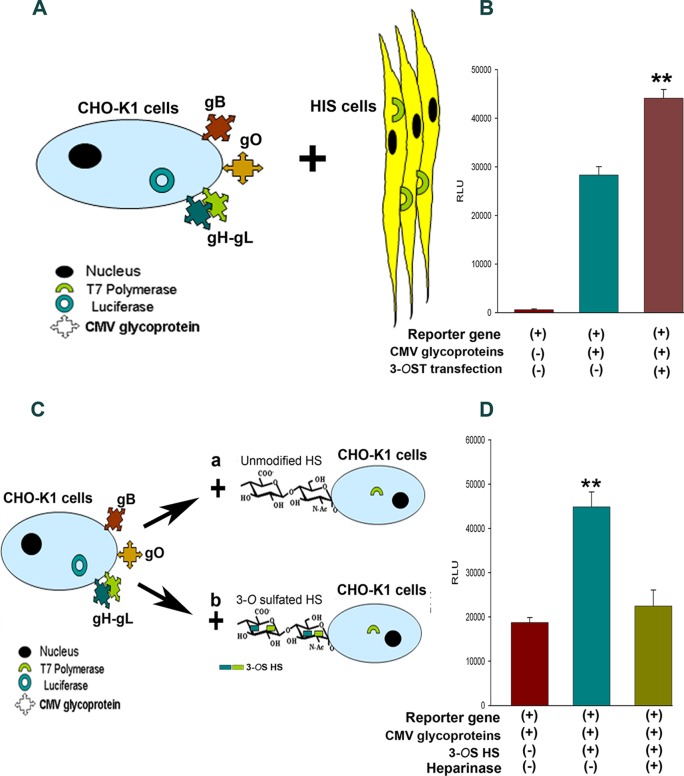
Therefore, effector CHO-K1 cells were cotransfected with the four HCMV glycoproteins described above along with T7 polymerase. In parallel, target HIS cells were cotransfected with 3-OST-3 enzymes with the luciferase gene. A separate group of target HIS cells expressing pCDNA3.1 with the luciferase gene were used as a control. Eighteen hours posttransfection, both effector and target cells were mixed and cocultured for an additional 24 h before cell-to-cell fusion was measured via a reporter luciferase gene-based assay as previously described (33). As indicated in Fig. 4B, expression of 3-OST-3 in HIS cells resulted in higher fusion with HCMV glycoprotein-expressing CHO-K1 cells. In contrast, HIS cells that expressed pCDNA3.1 had lower fusion.
FIG 4.
HCMV glycoprotein-mediated cell-to-cell fusion is enhanced due to overexpression of 3-O-sulfotransferase-3 (3-OST-3) in the target cells. (A) As indicated, effector CHO-K1 cells coexpressing T7 polymerase along with HCMV glycoproteins (gB, gO, and gH-gL) were cocultured with target HIS cells expressing the luciferase gene. (B) Reporter luciferase readings obtained during cell-to-cell fusion assay are presented. No fusion was noticed when effector CHO-K1 cells lacking HCMV glycoprotein were cocultured with HIS cells endogenously expressing 3-OST-3 enzyme (dark red bar [left]), while significant RLU were quantified when effector CHO-K1 cells coexpressing T7 polymerase with HCMV glycoproteins (gB, gO, and gH-gL) were cocultured with target HIS cells expressing the luciferase gene (blue-green bar [middle]). A significantly higher cell fusion was noticed during 3-OST-3 overexpression in HIS cells when cocultured with HCMV glycoprotein-expressing cells (brown bar [right]). Asterisks indicate significant difference from other treatments (P < 0.05, t test), and error bars represent SD (n = 4). (C) CHO-CHO based cell-to-cell fusion assay was further examined to determine the 3-OS HS requirements during HCMV glycoprotein-mediated fusion. (D) Comparison of cell-to-cell fusion between effector CHO-K1 cells coexpressing HCMV glycoproteins (gB, gO, and gH-gL) along with T7 polymerase cocultured with either target CHO-K1 cells (dark red bar [left]) or 3-OST-3-expressing target CHO-K1 cells (blue-green bar [middle]) expressing the luciferase gene. Cell-to-cell fusion activity of 3-OST-3-expressing CHO-K1 cells pretreated with heparinase before coculture with HCMV glycoprotein cells is shown (olive green bar [right]). Asterisks indicate significant difference from other treatments (P < 0.05, t test); error bars represent SD (n = 4).
Characterization of HCMV glycoprotein-mediated cell-to-cell fusion in CHO-K1 cells.
To further investigate the significance of 3-OST-3 generated 3-OS HS during HCMV cell-to-cell fusion, we utilized a previously characterized CHO-CHO-based cell fusion assay (33). The latter assay has been a very useful tool to identify fusion machinery in several other herpesviruses (34). Since CHO-K1 cells endogenously lack the expression of 3-OST enzymes, which modifies HS, they provide a unique opportunity to study the role of 3-OS HS in HCMV infection (33). As shown in Fig. 4C, CHO-K1 cells were selected as both effector and target cells in this experiment. Using the luciferase-based reporter assay, effector CHO-K1 cells were cotransfected with HCMV glycoproteins (gB, gO, and gH-gL) along with T7 polymerase, while target CHO-K1 cells were cotransfected with 3-OST-3 (to generate 3-OS HS) or pCNDA3.1 (to carry unmodified HS) along with the luciferase gene. The effector cells were mixed and cocultured with 3-OS HS cells or with unmodified HS cells as two separate combinations. The extent of cell-to-cell fusion was measured 24 h postmixing. As shown in Fig. 4D, the presence of 3-OS HS significantly enhanced the cell fusion. To verify that the high extent of cell fusion was due to HS, enzymatic removal of HS and 3-OS HS was carried out using heparinase enzyme pretreatment to target cells expressing 3-OS HS before mixing with effector cells. The heparinase treatment significantly impaired the fusion between HCMV glycoprotein and 3-OS HS-mediated interaction (Fig. 4D).
In summary, we established a novel in vitro model system to study HCMV cell-to-cell fusion by using human eye-derived primary cultures of iris stroma. Our data clearly indicated the cytopathic effects in HIS cells on HCMV infection as demonstrated by reporter virus-based assays (Fig. 1A and B). Previously, a mouse study demonstrated the susceptibility of iris to mouse cytomegalovirus (MCMV) infection by using scanning laser ophthalmoscopy (11). We also observed higher numbers of actin stress fibers early during HCMV internalization in 3-OST-3-expressing HIS cells. Interestingly, similar changes in cytoskeleton have been documented when 3-O-sulfated HS interacts with chemokine CXL-8 during the inflammation process (36). The actin filaments of the cytoskeleton are now widely recognized events by which multiple pathogens, including herpesviruses, highjack the host cell (37, 38).
The primary cultures used in the present study are relevant to further investigate the permissiveness to HCMV since they are derived from human eye donors. Future screening of a library of small molecules or peptide-targeting sulfate moieties on HIS cells will likely advance our current understanding of HCMV cell-specific interactions and the development of novel inhibitors (39, 40). Similarly, understanding the involvement of 3-OS HS for the induction of proinflammatory cytokines is worth investigating. Interestingly, a previous study with MCMV suggested a key role of the HS in the development of a robust immune response as HS expression at the surface of B cells was upregulated during infection via the action of type I IFN (41).
Our data demonstrated the role for 3-OS HS during HCMV-mediated cell fusion. This is the first report of its kind implicating the involvement of 3-OS HS during ocular HCMV infection. Until now, 3-OS HS has been known to mediate herpes simplex virus (HSV) entry in primary cultures of corneal stroma derived from human eye donors (28). Since 3-OS HS facilitates HCMV infection, its further potential in overall disease development, especially in immune modulation or angiogenesis, can be further investigated using corneal endothelial cells (42). Several lines of evidence already suggest that sulfated HS plays a critical role during multiple pathophysiological processes, including inflammation and vascular angiogenesis (43–45). Our study provides a unique example of a naturally susceptible cell type that HCMV targets. It will be useful to address additional key questions, such as which HCMV glycoprotein interact with 3-OS HS during entry and spread using the primary HIS cell cultures. Current evidence indicates the role for HCMV glycoproteins B (gB), M (gM), and N (gN) in binding to cell surface HS (12). Before our study, no evidence existed for the involvement of 3-OS HS during HCMV entry or spread. Furthermore, multiple human tissues are known to express specific isoforms of 3-OST enzymes (16), which may influence HCMV tropism (46). Interestingly, it is known that redistribution of HS greatly impacts HCMV infectivity (47). Further studies are needed to examine potential interaction between HCMV-expressing pentameric complex (gH/gL/UL128) (48) and specific forms of 3-OS HS (30). In addition, human iris cells are known to express HCMV receptors, such as integrins (49), platelet-derived growth factor receptor alpha, and epidermal growth factors (50); however, their expression levels in primary HIS cells together with the receptor preference by HCMV during cell entry and cell-to-cell fusion need to be investigated. The answers to many of the above-mentioned questions will likely rationalize the future therapeutic interventions to develop novel anti-3-OS HS inhibitors targeting virus-cell interaction (51) to prevent blindness and contain other HCMV diseases or complications.
ACKNOWLEDGMENT
This research was supported by an NIH R21 grant (AI105573) to V.T. and D.S.
REFERENCES
- 1.Schleiss MR. 2011. Congenital cytomegalovirus infection: molecular mechanisms mediating viral pathogenesis. Infect Disord Drug Targets 11:449–465. doi: 10.2174/187152611797636721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonsenso D, Serranti D, Gargiullo L, Ceccarelli M, Ranno O, Valentini P. 2012. Congenital cytomegalovirus infection: current strategies and future perspectives. Eur Rev Med Pharmacol Sci 16:919–935. [PubMed] [Google Scholar]
- 3.Hamilton ST, van Zuylen W, Shand A, Scott GM, Naing Z, Hall B, Craig ME, Rawlinson WD. 2014. Prevention of congenital cytomegalovirus complications by maternal and neonatal treatments: a systematic review. Rev Med Virol 24:420–433. doi: 10.1002/rmv.1814. [DOI] [PubMed] [Google Scholar]
- 4.Nijman J, Mandemaker FS, Verboon-Maciolek MA, Aitken SC, van Loon AM, de Vries LS, Schuurman R. 2014. Genotype distribution, viral load and clinical characteristics of infants with postnatal or congenital cytomegalovirus infection. PLoS One 9:e108018. doi: 10.1371/journal.pone.0108018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alford CA, Stagno S, Pass RF, Britt WJ. 1990. Congenital and perinatal cytomegalovirus infections. Rev Infect Dis 12(Suppl 7):S745-S753. doi: 10.1093/clinids/12.Supplement_7.S745. [DOI] [PubMed] [Google Scholar]
- 6.Streblow DN, Orloff SL, Nelson JA. 2007. Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol 19:577–582. doi: 10.1016/j.coi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths P, Baraniak I, Reeves M. 2015. The pathogenesis of human cytomegalovirus. J Pathol 235:288–297. doi: 10.1002/path.4437. [DOI] [PubMed] [Google Scholar]
- 8.Yen M, Chen J, Ausayakhun S, Kunavisarut P, Vichitvejpaisal P, Ausayakhun S, Jirawison C, Shantha J, Holland GN, Heiden D, Margolis TP, Keenan JD. 2015. Retinal detachment associated with AIDS-related cytomegalovirus retinitis: risk factors in a resource-limited setting. Am J Ophthalmol 159:185–192. doi: 10.1016/j.ajo.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koizumi N, Inatomi T, Suzuki T, Shiraishi A, Ohashi Y, Kandori M, Miyazaki D, Inoue Y, Soma T, Nishida K, Takase H, Sugita S, Mochizuki M, Kinoshita S, Japan Corneal Endotheliitis Study Group. 2015. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan Corneal Endotheliitis Study. Br J Ophthalmol 99:54–58. doi: 10.1136/bjophthalmol-2013-304625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo JH, Lim WK, Ho SL, Teoh SC. 10September2014. Characteristics of cytomegalovirus uveitis in immunocompetent patients. Ocul Immunol Inflamm 10:1–6. doi: 10.3109/09273948.2014.950384. [DOI] [PubMed] [Google Scholar]
- 11.Zinkernagel MS, Petitjean C, Fleming P, Chinnery HR, Constable IJ, McMenamin PG, Degli-Esposti MA. 2010. In vivo imaging of ocular MCMV infection. Invest Ophthalmol Vis Sci 51:369–374. doi: 10.1167/iovs.09-4083. [DOI] [PubMed] [Google Scholar]
- 12.Vanarsdall AL, Johnson DC. 2012. Human cytomegalovirus entry into cells. Curr Opin Virol 2:37–42. doi: 10.1016/j.coviro.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler B, Sinzger C. 2009. Endothelial cells in human cytomegalovirus infection: one host cell out of many or a crucial target for virus spread? Thromb Haemost 102:1057–1063. doi: 10.1160/TH09-04-0213. [DOI] [PubMed] [Google Scholar]
- 14.Song BH, Lee GC, Moon MS, Cho YH, Lee CH. 2001. Human cytomegalovirus binding to heparan sulfate proteoglycans on the cell surface and/or entry stimulates the expression of human leukocyte antigen class I. J Gen Virol 82:2405–2413. [DOI] [PubMed] [Google Scholar]
- 15.Compton T, Nowlin DM, Cooper NR. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 16.Shukla D, Spear PG. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest 108:503–510. doi: 10.1172/JCI200113799, . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neyts J, Snoeck R, Schols D, Balzarini J, Esko JD, Van Schepdael A, De Clercq E. 1992. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology 189:48–58. doi: 10.1016/0042-6822(92)90680-N. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins JI, Fiander AN, Evans AS, Delchambre M, Gheysen D, Borysiewicz LK. 1996. Cytotoxic T cell immunity to human cytomegalovirus glycoprotein B. J Med Virol 49:124–131. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Huong SM, Chiu ML, Raab-Traub N, Huang ES. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- 20.Chan G, Nogalski MT, Yurochko AD. 2009. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A 106:22369–22374. doi: 10.1073/pnas.0908787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soroceanu L, Akhavan A, Cobbs CS. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391–395. doi: 10.1038/nature07209. [DOI] [PubMed] [Google Scholar]
- 22.Vanarsdall AL, Wisner TW, Lei H, Kazlauskas A, Johnson DC. 2012. PDGF receptor-α does not promote HCMV entry into epithelial and endothelial cells but increased quantities stimulate entry by an abnormal pathway. PLoS Pathog 8:e1002905. doi: 10.1371/journal.ppat.1002905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feire AL, Koss H, Compton T. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A 101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Huang DY, Huong SM, Huang ES. 2005. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat Med 11:515–521. doi: 10.1038/nm1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borst EM, Ständker L, Wagner K, Schulz TF, Forssmann WG, Messerle M. 2013. A peptide inhibitor of cytomegalovirus infection from human hemofiltrate. Antimicrob Agents Chemother 57:4751–4760. doi: 10.1128/AAC.00854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiwari V, Liu J, Valyi-Nagy T, Shukla D. 2011. Anti-heparan sulfate peptides that block herpes simplex virus infection in vivo. J Biol Chem 286:25406–25415. doi: 10.1074/jbc.M110.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa-Goto K, Arao Y, Ito Y, Ogawa T, Abe T, Kurata T, Irie S, Akanuma H. 1998. Binding of human cytomegalovirus to sulfated glucuronyl glycosphingolipids and their inhibitory effects on the infection. J Gen Virol 79:2533–2541. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin J, Park PJ, Zanotti B, Maus E, Volin MV, Shukla D, Tiwari V. 2013. Susceptibility of human iris stromal cells to herpes simplex virus 1 entry. J Virol 87:4091–4096. doi: 10.1128/JVI.03235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaete RR, Mocarski ES. 1987. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci U S A 84:7213. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esko JD, Lindahl U. 2001. Molecular diversity of heparan sulfate. J Clin Invest 108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13–22. doi: 10.1016/S0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 32.Ernst S, Langer R, Cooney CL, Sasisekharan R. 1995. Enzymatic degradation of glycosaminoglycans. Crit Rev Biochem Mol Biol 30:387–444. doi: 10.3109/10409239509083490. [DOI] [PubMed] [Google Scholar]
- 33.Tiwari V, Clement C, Duncan MB, Chen J, Liu J, Shukla D. 2004. A role for 3-O-sulfated heparan sulfate in cell fusion induced by herpes simplex virus type 1. J Gen Virol 85:805–809. doi: 10.1099/vir.0.19641-0. [DOI] [PubMed] [Google Scholar]
- 34.Pertel PE, Fridberg A, Parish ML, Spear PG. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- 35.Vanarsdall AL, Chase MC, Johnson DC. 2011. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. J Virol 85:11638–11645. doi: 10.1128/JVI.05659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whittall C, Kehoe O, King S, Rot A, Patterson A, Middleton JA. 2013. Chemokine self-presentation mechanism involving formation of endothelial surface microstructures. J Immunol 190:1725–1736. doi: 10.4049/jimmunol.1200867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor MP, Koyuncu OO, Enquist LW. 2011. Subversion of the actin cytoskeleton during viral infection. Nat Rev Microbiol 9:427–439. doi: 10.1038/nrmicro2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Döhner K, Sodeik B. 2005. The role of the cytoskeleton during viral infection. Curr Top Microbiol Immunol 285:67–108. doi: 10.1007/3-540-26764-6_3. [DOI] [PubMed] [Google Scholar]
- 39.Gardner TJ, Cohen TT, Redmann V, Lau Z, Felsenfeld D, Tortorella D. 2015. Development of a high-content screen for the identification of inhibitors directed against the early steps of the cytomegalovirus infectious cycle. Antiviral Res 113:49–61. doi: 10.1016/j.antiviral.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colpitts CC, Schang LM. 2014. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J Virol 88:7806–7817. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarousse N, Trujillo DL, Wilcox-Adelman S, Coscoy L. 2011. Virally-induced upregulation of heparan sulfate on B cells via the action of type I IFN. J Immunol 187:5540–5547. doi: 10.4049/jimmunol.1003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacManiman JD, Meuser A, Botto S, Smith PP, Liu F, Jarvis MA, Nelson JA, Caposio P. 2014. Human cytomegalovirus-encoded pUL7 is a novel CEACAM1-like molecule responsible for promotion of angiogenesis. mBio 5(6):e02035. doi: 10.1128/mBio.02035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferro V. 2013. Heparan sulfate inhibitors and their therapeutic implications in inflammatory illnesses. Expert Opin Ther Targets 17:965–975. doi: 10.1517/14728222.2013.811491. [DOI] [PubMed] [Google Scholar]
- 44.van Wijk XM, van Kuppevelt TH. 2014. Heparan sulfate in angiogenesis: a target for therapy. Angiogenesis 17:443–462. doi: 10.1007/s10456-013-9401-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Song K, Zhou L, Xie Z, Zhou P, Zhao Y, Han Y, Xu X, Li P. 2014. Heparan sulfate d-glucosaminyl 3-O-sulfotransferase-3B1 (HS3ST3B1) promotes angiogenesis and proliferation by induction of VEGF in acute myeloid leukemia cells. J Cell Biochem doi: 10.1002/jcb.25066. [DOI] [PubMed] [Google Scholar]
- 46.Sinzger C, Digel M, Jahn G. 2008. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol 325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 47.Esclatine A, Bellon A, Michelson S, Servin AL, Quéro AM, Géniteau-Legendre M. 2001. Differentiation-dependent redistribution of heparan sulfate in epithelial intestinal Caco-2 cells leads to basolateral entry of cytomegalovirus. Virology 289:23–33. doi: 10.1006/viro.2001.1122. [DOI] [PubMed] [Google Scholar]
- 48.Nogalski MT, Chan GC, Stevenson EV, Collins-McMillen DK, Yurochko AD. 2013. The HCMV gH/gL/UL128-131 complex triggers the specific cellular activation required for efficient viral internalization into target monocytes. PLoS Pathog 9:e1003463. doi: 10.1371/journal.ppat.1003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman MD, Zamora DO, Pan Y, Texeira PV, Planck SR, Rosenbaum JT. 2001. Cell adhesion molecule expression in cultured human iris endothelial cells. Invest Ophthalmol Vis Sci 42:2861–2866. [PubMed] [Google Scholar]
- 50.Kociok N, Heppekausen H, Schraermeyer U, Esser P, Thumann G, Grisanti S, Heimann K. 1998. The mRNA expression of cytokines and their receptors in cultured iris pigment epithelial cells: a comparison with retinal pigment epithelial cells. Exp Eye Res 67:237–250. doi: 10.1006/exer.1998.0517. [DOI] [PubMed] [Google Scholar]
- 51.Tiwari V, Tarbutton MS, Shukla D. 2015. Diversity of heparan sulfate and HSV entry: basic understanding and treatment strategies. Molecules 20:2707–2727. doi: 10.3390/molecules20022707. [DOI] [PMC free article] [PubMed] [Google Scholar]