ABSTRACT
The E2F family of transcription factors, broadly divided into activator and repressor E2Fs, regulates cell cycle genes. Current models indicate that activator E2Fs are necessary for cell cycle progression and tumorigenesis and are also required to mediate transformation induced by DNA tumor viruses. E2Fs are negatively regulated by the retinoblastoma (RB) family of tumor suppressor proteins, and virus-encoded oncogenes disrupt the RB-E2F repressor complexes. This results in the release of activator E2Fs and induction of E2F-dependent genes. In agreement, expression of large tumor T antigens (TAg) encoded by polyomaviruses in mammalian cells results in increased transcriptional levels of E2F target genes. In addition, tumorigenesis induced by transgenic expression of simian virus 40 (SV40) TAg in choroid plexus or intestinal villi requires at least one activator E2F. In contrast, we show that SV40 TAg-induced transformation in mouse embryonic fibroblasts is independent of activator E2Fs. This work, coupled with recent studies showing that proliferation in stem and progenitor cells is independent of activator E2Fs, suggests the presence of parallel pathways governing cell proliferation and tumorigenesis.
IMPORTANCE The RB-E2F pathway is altered in many cancers and is also targeted by DNA tumor viruses. Viral oncoprotein action on RBs results in the release of activator E2Fs and upregulation of E2F target genes; thus, activator E2Fs are considered essential for normal and tumorigenic cell proliferation. However, we have observed that SV40 large T antigen can induce cell proliferation and transformation in the absence of activator E2Fs. Our results also suggest that TAg action on pRBs regulates both E2F-dependent and -independent pathways that govern proliferation. Thus, specific cell proliferation pathways affected by RB alterations in cancer may be a factor in tumor behavior and response to therapy.
INTRODUCTION
Cell proliferation is a highly regulated process involving four well-coordinated phases of the cell cycle. Each phase is regulated by various pro- and antiproliferation pathways, whose disruption leads to either cell death or uncontrolled cell proliferation and cancer. One of those controllers is the retinoblastoma (RB)/E2F pathway, and mutations in members of this pathway are often found in human cancers, leading to enhanced E2F activity and upregulation of E2F target gene (1). Furthermore, various viral oncoproteins—including the large tumor (T) antigen (TAg) from polyomaviruses, E1A from adenoviruses, and E7 from papillomaviruses—have independently evolved to target this pathway, indicating its central role in cell cycle regulation (2–4).
E2Fs are transcription factors that regulate cell proliferation by controlling the expression of cell cycle genes. In a growth-arrested cell, the E2Fs are bound to and negatively regulated by hypophosphorylated members of the retinoblastoma (RB) protein family (pRb, p107, p130) (1). Upon receiving mitogenic signals, pRBs become hyperphosphorylated and release E2Fs, which then increase the transcription of cell cycle genes. There are nine known mammalian E2Fs, broadly divided into transcriptional activators (E2F1, E2F2, and E2F3a [E2F1-2-3a]) and repressors (E2F3b-4-8) (1). E2F proteins are functionally very similar and often can compensate for each other, both within each subclass and even between the two subclasses, thus complicating their study (1, 5, 6).
Previous reports have suggested an essential role of the activator E2Fs in cell survival and proliferation: genetic ablation of E2F1-2-3 in mice leads to embryonic lethality at 9.5 days, and their depletion from mouse embryonic fibroblasts (MEFs) in cell culture leads to growth arrest and death (5, 7). Similarly, while MEFs derived from E2F single-knockout or double-knockout (DKO) mice are able to survive and proliferate in cell culture, removal of the third activator E2F—the E2F1-2-3 triple-knockout (TKO) MEFs—results in growth arrest and death (5, 7). However, certain murine cell types, both in vivo (the progenitor cells of retina, lens, and small intestinal crypts) and in cell culture (embryonic stem cells), proliferate in the absence of the three activator E2Fs (8–10). Furthermore, several rounds of cell proliferation take place in E2F1-2-3 null embryos until they die by day 9.5. These results suggest that, at least in some instances, mechanisms other than activator E2Fs control cell survival and proliferation.
The large tumor antigen (TAg) of polyomavirus simian virus 40 (SV40) is a multidomain oncoprotein that interacts with and disrupts the function of several host proteins, thereby interfering with specific cellular pathways (11, 12). TAg expression causes transformation of several primary cell types in cell culture and also induces tumors in model organisms. Studies investigating the mechanism of TAg-induced proliferation and transformation have shown that TAg interacts with the Rb/E2F complexes via its LXCXE motif and induces their disruption via its J-domain, thereby upregulating E2F target genes (3, 13, 14). Several studies have led to the current “textbook model” in which release of activator E2Fs by TAg and subsequent upregulation of E2F target genes are required for transformation (11, 15). However, in view of recent studies demonstrating cell proliferation in the absence of activator E2Fs, we investigated whether TAg-induced proliferation and transformation require E2F1-2-3.
MATERIALS AND METHODS
Cell culture.
Mouse embryonic fibroblasts (MEFs) were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Double-knockout (DKO) MEFs (E2F1−/−; E2F2−/−; E2F3floxed/floxed) were obtained from Gustavo Leone. Wild-type (wt) MEFs were generated from embryonic day 13.5 (E13.5) FvB mouse embryos as previously described (7, 16).
Retrovirus and lentivirus production.
Retroviruses containing cre-recombinase were prepared using phoenix-eco packaging cell lines. Particle-containing medium, supplemented with Polybrene (10 μg/ml), was added to the cells being transduced a total of three times during a 24-h interval. After transduction, the cells were selected for cre-expression using puromycin (3 μg/ml) for 5 days. Drug-selected cells were plated at low density (1,000 cells/10-cm-diameter culture dish) to obtain single-cell clones.
Lentiviruses containing E2F3-specific short hairpin RNAs (shRNAs) were prepared using the 293FT packaging cell line following the Invitrogen ViraPower mixture (K4975-00) protocol. Four different E2F3-specific shRNAs were used to prepare separate lentiviruses. Medium containing lentiviruses was collected at 36, 48, and 72 h and was supplemented with Polybrene (10 μg/ml). Cells grown to 80% confluence were then transduced, and drug selection was carried out using blasticidin (3 μg/ml) for 5 days. After drug selection, the cells were allowed to grow and pools of drug-resistant cells were collected and used in subsequent experiments.
Similarly, pBABE-puro-H-RasV12 was introduced in shRNA-treated TAg and TKO(cre);TAg MEFs by retrovirus transduction and cell pools were obtained after 5 days of puromycin selection.
DNA constructs.
The SV40 TAg cDNA cloned into the pWZL.BLAST vector was obtained from Ole Gjoerup. The cloning of the early region of SV40—nucleotides 2691 to 5163 of the genome—into pLenti6.3-TOPO vector (Invitrogen) was previously described (17). TE107K mutant was obtained by changing the sequence coding the amino acid Glu at position 107 to encode Lys, using a QuikChange II site-directed mutagenesis kit (Agilent Technologies), following the manufacturer's protocol. The TN136 TAg mutant was TOPO cloned into pLenti6.3 vector (Invitrogen). Depletion of E2F3 was accomplished by retroviral (pBABE–puromycin–cre-recombinase) or lentiviral (pLKO.1-puromycin shRNAs; Sigma-Aldrich) transduction. Cre-recombinase-treated cells were selected with 3 μg/ml puromycin for 5 days and then plated at low density (1,000 cells/10-cm-diameter dish) to obtain single-cell clones. Pools of cells treated with shRNA were collected after selection with 3 μg/ml blasticidin for 5 days.
Western blots.
Protein extracts from whole-cell lysates were prepared as described previously (18), and their concentration was determined using Bradford reagent (Bio-Rad). Total protein extracts were separated using 10% SDS-PAGE gels, transferred onto polyvinylidene difluoride (PVDF) membranes, and incubated with appropriate primary antibodies to detect E2F1 (sc-193; Santa Cruz), E2F2 (sc-9967; Santa Cruz), E2F3 (05-551; Millipore), SV40 TAg (PAb416, PAb419, and PAb901), p107 (sc-318; Santa Cruz), p130 (sc-317x; Santa Cruz), H-Ras (SAB1405964; Sigma-Aldrich), p53 (sc-6243 horseradish peroxidase [HRP]; Santa Cruz), and p21 (sc-471; Santa Cruz). After incubation with appropriate HRP-conjugated secondary antibodies, complexes were detected by chemiluminescence with Luminata Forte (Millipore).
Real-time PCR.
Total RNA was isolated from approximately 1 × 107 cells using a Qiagen RNeasy minikit under the manufacturer's guidance. In-column DNase treatment was performed, and the RNA concentration was determined with NanoDrop (NanoDrop 2000; Thermo Scientific). Reverse transcription from 1 μg of total RNA was done as previously described (9), and samples were incubated with 1.5 U of RNaseH (Promega) at 37°C for 20 min. Real-time PCR was performed using Maxima SYBR green (Thermo Scientific) in a 7300 real-time PCR system (Applied Biosystems). Each reaction was performed in quadruplicate in a final volume of 20 μl. Values of specific transcripts were normalized against the endogenous levels of Rpl5 in the corresponding samples.
Immunofluorescence.
Cells growing on glass coverslips were fixed with 95% ethanol and permeabilized with PBST (phosphate-buffered saline [PBS] buffer; 0.01% Tween 20). Cells were first incubated for 1 h with blocking buffer (PBST; 3% bovine serum albumin [BSA]) and then for 1 to 2 h with hamster anti-TAg antibody diluted (1:1,000) in blocking buffer. After 3 washes with PBST, cells were incubated for 1 h with goat anti-hamster secondary antibody (Alexa Fluor 488; Jackson ImmunoResearch) diluted 1:500 in blocking buffer. The slides were counterstained with DAPI (4′,6-diamidino-2-phenylindole) and mounted for analysis by fluorescence microscopy.
Transformation assays. (i) Soft-agar assay.
About 1.6 × 104 exponentially growing cells were plated in cell culture media containing 0.33% noble agar. Every 3 to 4 days, fresh medium (0.5 ml) was added on top of the agar layer to prevent it from drying. Colonies formed after 3 to 4 weeks of plating were counted by the following method. Each plate was divided into 4 quadrants, and movies of colonies formed in each quadrant were made using the ZEN software package from Ziess Corporation. Each movie captured colonies growing in the different layers of agar, and the size of colonies was calculated by determining the area under the curve. The top 99% of colonies formed by each cell type was used to form box plots, and Wilcox statistical test in R (http://www.r-project.org/) was used to calculate whether variations between populations were significant. All experiments were performed in duplicate.
(ii) Growth in low-level serum.
Cells (5 × 103) were seeded into 12-well plates in media containing 10% FBS. After 24 h, the cells were washed 3 times with PBS and medium containing low-level serum (1% FBS) was added. Cells from two wells per cell type were trypsinized every 2 days and counted using a hemocytometer.
Proliferation assays. (i) Growth curves.
Proliferation curves were generated by plating 2 × 104 cells in 6-well plates, allowing them to proliferate, and then counting the cells from two wells per cell type every 48 h using a hemocytometer.
(ii) Senescence assay (β-Gal staining).
DKO MEFs were infected with lentiviruses containing either control-shRNA- or E2F3-specific shRNAs. Cells were plated in 6-well plates after 5 days of puromycin (3 μg/ml) selection, and staining was performed as previously described (19). Briefly, cells were washed with PBS and fixed using a 2% formaldehyde–0.2% glutaraldehyde solution for 5 min at room temperature. Cells were washed with PBS and incubated overnight at 37°C with fresh β-galactosidase (β-Gal) staining solution (Bluo-Gal [halogenated indoyl-beta-d-galactosidase]; Invitrogen) (pH 6.0). Blue staining was observed with light microscopy, and photographic images were taken at the same magnification.
Genotyping and PCR.
Genomic DNA from approximately 1 × 107 cells was extracted using Tail PCR lysis reagent (ViaGen) supplemented with 0.3 μg/ml proteinase K. Briefly, cell pellets were resuspended in 150 μl of tail PCR mix and incubated at 55°C overnight. Proteinase K was inactivated by incubating the samples at 85°C for 1 h. Samples were then centrifuged at 14,000 rpm for 15 min, the supernatant was collected, and the amount of DNA was determined using a NanoDrop instrument. PCRs were carried out with the primers and conditions previously described (10).
RESULTS
SV40 TAg induces proliferation and transformation in MEFs depleted of activator E2Fs.
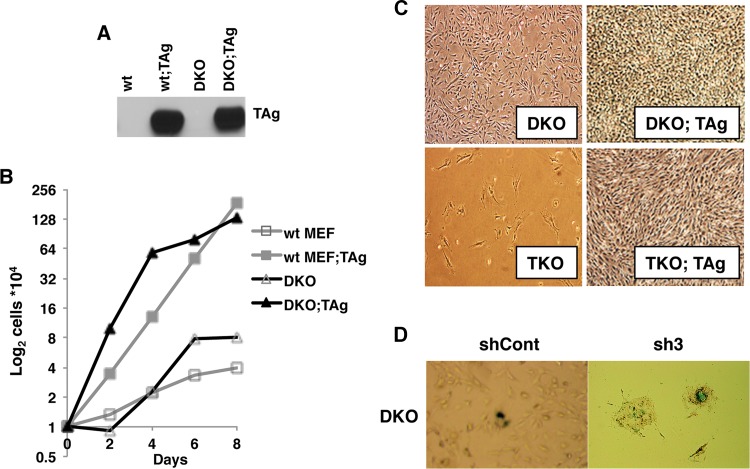
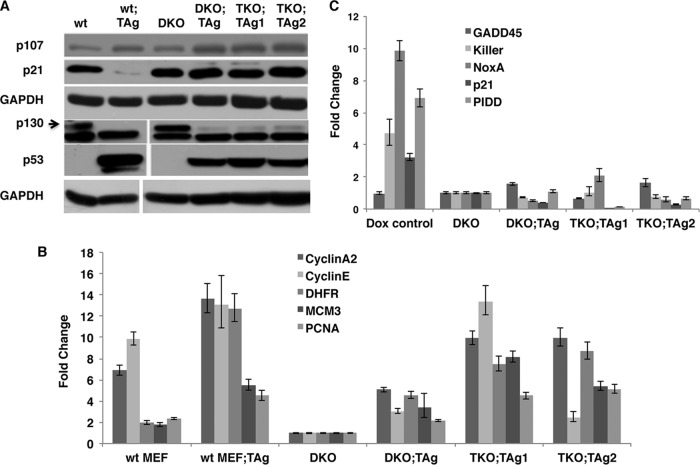
SV40 TAg immortalizes and transforms MEFs, and both events require TAg action on pRb tumor suppressors. In this study, we used a well-characterized cell culture system consisting of E2F1 and E2F2 double-knockout MEFs (DKO MEFs) which carry the E2F3 gene floxed (E2F3f/f) (16, 20). Upon expression of cre-recombinase, these cells become E2F1-2-3 triple-knockout (TKO) MEFs, which then reach growth arrest and die. To test the role of activator E2Fs in TAg-mediated proliferation and transformation, we first generated pools of wt and DKO MEFs expressing TAg. We next confirmed that the absence of E2F1 and E2F2 had no effect on TAg levels (Fig. 1A). DKO and wt MEFs showed similar levels of proliferation under normal culture conditions, and expression of TAg enhanced cell proliferation in both backgrounds (Fig. 1B).
FIG 1.
SV40 TAg does not require activator E2Fs to induce proliferation in MEFs. (A) Western blot analysis showing SV40 TAg expression in wt and DKO backgrounds. (B) Growth curve comparison between wt and DKO MEFs in the presence or absence of TAg. Equal numbers of cells were plated for all cell types, and cells were counted periodically in duplicate to obtain the growth curves (n = 2). (C) Comparison of morphology and cell growth characteristics after removal of E2F3 by cre-recombinase. Images were taken after infection of cells with retroviruses containing either empty vector (DKO and DKO;TAg cells) or cre-recombinase (TKO and TKO;TAg cells). All images were taken at the same time point and with the same magnification. (D) Beta-galactosidase staining to determine senescence in DKO MEFs treated with E2F3-specific or control shRNAs. Four E2F3-specific shRNA treatments were used and yielded the same results. An image representative of one of the shRNA-generated cells is shown. Blue-violet positive staining and enlarged morphology were observed only in E2F3 shRNA-treated DKO MEFs and not in control shRNA-treated cells.
We next depleted E2F3 from DKO and DKO;TAg MEFs by the use of E2F3-specific shRNAs and two different methods: (i) expression of cre-recombinase or (ii) RNA interference (RNAi). Early-passage DKO and DKO:TAg MEFs were transduced with retroviruses containing either the cre-recombinase gene or empty vector, and cell pools and clones were collected upon selection (see Materials and Methods). As an alternative method to deplete E2F3, we used RNAi. Four different E2F3-specific shRNAs (sh1, sh2, sh3, and sh4) plus a control shRNA (shCont) were delivered to DKO and DKO;TAg MEFs by lentivirus transduction. Following infection and selection, pools of cells were collected at early and late passages to test for the depletion of E2F3.
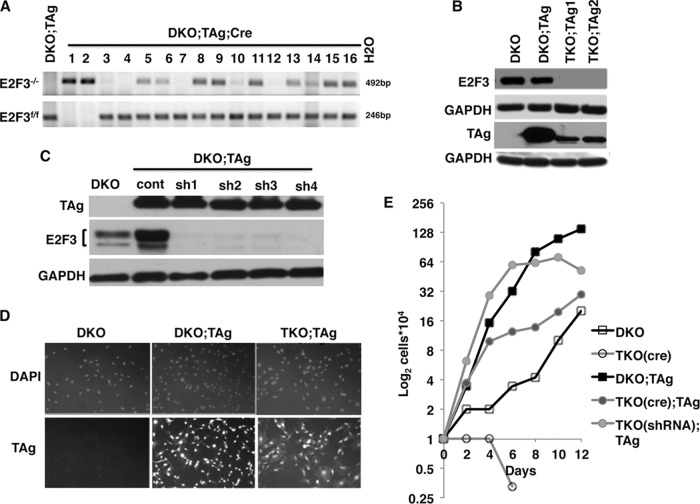
In accordance with previous studies (16, 20), depletion of E2F3 from DKO MEFs, either by the use of cre-recombinase or by the use of RNAi, resulted in senescence and death (Fig. 1C and D). However, upon expression of TAg, we were able to obtain cre-recombinase-induced clones depleted of both E2F3 alleles, with a 19.5% frequency. The status and expression of E2F3 in the resulting clones were tested by PCR-genotyping (Fig. 2A) and Western blot analysis (Fig. 2B). We were also able to generate viable cells by removing E2F3 through the use of RNAi but only in the presence of TAg. We confirmed that depletion of E2F3 in DKO;TAg MEFs via the use of cre-recombinase or via the use of all four E2F3-specific shRNAs reduced the E2F3 protein levels considerably (Fig. 2B and C). We also confirmed by immunofluorescence with a TAg-specific antibody that the oncogene was uniformly expressed in TKO;TAg MEFs and that it was preferentially localized into the nucleus (Fig. 2D). Irrespective of the method of depletion employed, we found that TAg allowed MEFs to proliferate and survive in the absence of all activator E2Fs (Fig. 1D and 2D and E).
FIG 2.
Generation and characterization of cells lacking E2F3 in the presence of TAg. (A) PCR-genotyping analysis to determine the presence or absence of E2F3 in clones of cre-treated DKO;TAg MEFs. DNA from cells containing a successfully deleted E2F3 allele (E2F3−/−) produced an amplified fragment of 492 bp, while those where the floxed allele (E2F3f/f) remained intact produced a 246-bp fragment. DNA from cells where only one copy of E2F3 was removed produced both fragments upon PCR amplification. (B and C) Western blot analysis confirms the absence of E2F3 and presence of TAg protein in the TKO;TAg clones obtained by cre-recombinase (B) or in pools of MEFs expressing E2F3-specific shRNAs (C). Four different E2F3-specific shRNAs (sh1, sh2, sh3, and sh4) were used with a nontargeting control shRNA (cont). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (D) Analysis of TAg expression and localization by immunofluorescence using hamster anti-TAg primary antibody and a fluorophore-attached anti-hamster secondary antibody. DKO MEFs not expressing TAg are shown as negative controls. Negative controls without primary antibody incubation produced no significant signal and are not shown. (E) Representative examples of cell growth curves upon depletion of E2F3 from DKO or DKO;TAg MEFs via the use of either cre-recombinase or RNAi. The data shown represent averages of the results of two separate experiments performed in duplicate. TKO(cre);TAg data represent the averages of the results from the two clones obtained via cre-recombinase, and TKO(shRNA);TAg data represents the averages of the results from two pools of cells generated by two different E2F3-specific shRNAs.
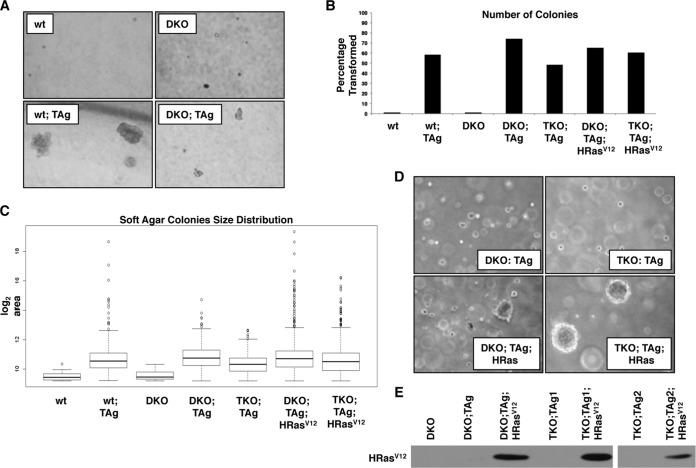
We next examined the ability of TAg to induce transformation of TKO MEFs as measured by anchorage-independent growth. Equal numbers of cells were seeded in soft agar, and the number and size of colonies formed were measured. As expected, both wt and DKO MEFs remained mostly as single cells in soft agar, while TAg expression in these backgrounds induced significant colony formation (Fig. 3A and B). The numbers of colonies generated by expression of TAg in wt or DKO MEFs were similar (Fig. 3B), albeit the size of the colonies was somewhat reduced in the absence of E2F1-2 (Fig. 3A and C). Moreover, TAg was able to induce growth in soft agar in the absence of MEFs lacking all three activator E2Fs, both when TKO MEFs were generated by cre-recombinase and when they were generated by shRNA knockdown (Fig. 3B, C, and D [upper panel] and data not shown).
FIG 3.
SV40 TAg induces growth in soft agar in the absence of E2F1-2-3, and the cooperation between TAg and Hrasv12 does not rely on the presence of E2F activators. To test anchorage independence, equal numbers of cells for all cell types were plated in soft agar and the colonies were analyzed 21 days after plating. Two properties of cells were tested: (i) the percentage of cells able to form colonies within a population and (ii) the size of the colonies formed. Data represent averages of the results from two independent assays (n = 2) performed by using two biological replicates for each cell type. (A) E2F1 and E2F2 are not required for TAg-expressing MEFs to form colonies in soft agar. (B) Bar graph depicting the percentages of cells able to form soft agar colonies within different cell types. The criteria used to identify and count colonies are explained in Materials and Methods. The same results were obtained by independent experiments conducted to evaluate colony formation in cells lacking E2F3 through the use of cre-recombinase (shown) or RNAi (data not shown). (C) Box plots showing the size distributions of colonies for all cell types. Each box plot is divided into four quartiles; the thick line in the middle of the box represents the median for the sample. The median of the sizes of the colonies formed in all cell types expressing TAg (wt, DKO, or TKO MEFs) was significantly larger than those of their respective controls, as determined by the Wilcox test (for wt MEF and wt;TAg, P value < 2.2e-06; for DKO and DKO;TAg, P value = 1.503e-06). (D) Representative images comparing the sizes of soft agar colonies formed by different cell types. Expression of HrasV12 in TAg-expressing cells results in a considerable increase in the sizes of the colonies, particularly in the TKO background. (E) Western blot analysis indicates that HRasV12 is expressed in cells transduced with retroviruses containing HRasV12 cDNA.
Finally, oncogenic Ras proteins cooperate with TAg to induce transformation. Thus, we tested whether the cooperation between TAg and HRasV12 was affected by the depletion of activator E2Fs. We generated cell pools in which comparable levels of HRasV12 protein were expressed at early passage (not shown) and late passage (Fig. 3E). Not only were MEFs expressing both TAg and HRasV12 able to grow and form similar numbers of colonies in soft agar (Fig. 3B), but the size of the colonies expressing HRasV12 was considerably increased in comparison to those seen with cells expressing TAg alone (Fig. 3C and D).
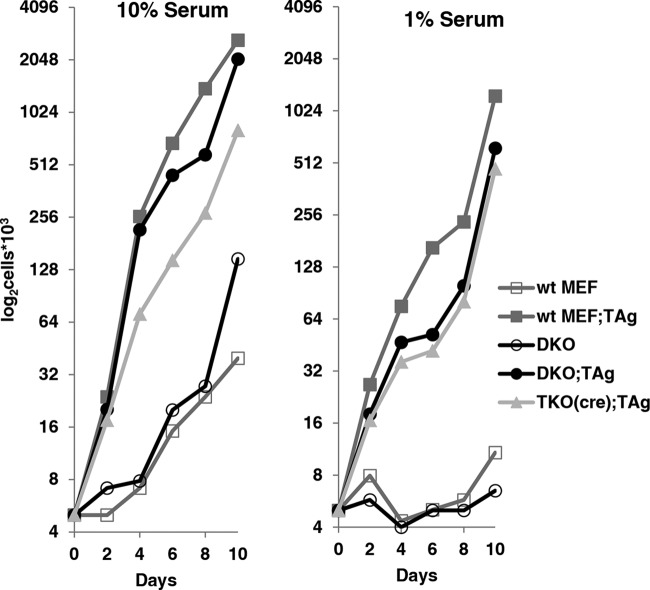
Proliferation of cells in culture media normally decreases under depleted-serum conditions, but TAg is able to circumvent this requirement and induces cell proliferation even in the absence of growth factors. We tested if this property of TAg requires E2F1-2-3. Equal numbers of cells were plated in medium containing a normal (10%) or low (1%) level of serum, and the numbers of cells were assessed at regular intervals (Fig. 4). In regular media (Fig. 4, left panel), all cells survived and proliferated but TAg expression resulted in faster growth. Under depleted-serum conditions (Fig. 4, right panel), all control cells (wt and DKO MEFs) ceased to proliferate and eventually died, while expression of TAg in these backgrounds or in TKO MEFs induced cell proliferation.
FIG 4.
SV40 TAg induces proliferation in MEFs lacking activator E2Fs under low-serum conditions. Equal numbers of cells for each cell type were plated in media containing either 10% (normal) or 1% (low) serum. Cells were counted periodically in duplicate, and growth curves were prepared. Negative (wt) and positive (wt;TAg) controls were included in each experiment. Each data point represents an average of the results from two separate experiments (n = 2), and TKO(cre);TAg represents the average of the results from two biological replicates obtained using cre-recombinase.
TAg disrupts both the pRb and p53 pathways in the absence of activator E2Fs.
We next examined whether the effects of TAg on the RB-E2F and p53 pathways were modified by the presence or absence of activator E2Fs. TAg interacts with all three members of the RB family and induces disruption of the RB/E2F repressor complexes, but it affects different members of the RB family in disparate ways. While TAg expression does not change pRb levels significantly, it induces proteosomal degradation of p130 and results in increased p107 protein levels (15, 21). We found that p107 and p130 respond to TAg in similar ways, in either the TKO or DKO background (Fig. 5A). In addition, the inhibition of RBs by TAg is expected to increase expression levels of E2F target genes, due to the release of activator E2Fs from RB-mediated repression. Real-time PCR analysis showed that cells containing TAg showed increased expression of canonical E2F target genes in the wt, DKO, or TKO background (Fig. 5B and data not shown). We conclude that both TAg-induced cell proliferation and E2F-target gene activation occur in the absence of E2F1-2-3.
FIG 5.
TAg retains the ability to disrupt the pRB and p53 pathways in the absence of activator E2Fs. (A) Western blot analysis of cell cycle regulatory proteins in cells expressing TAg in the presence or absence of activator E2Fs. Both wt and wt;TAg MEFs were used as controls. (B) Real-time PCR analysis of E2F target genes. Cells expressing TAg show increased transcriptional levels of E2F target genes, regardless of the genetic background. wt MEFs and wt;TAg MEFs were used as baseline and positive controls. (C) Real-time PCR analysis of p53 target genes in cells of different backgrounds. In contrast to cells treated with doxorubicin (Dox), which showed a significant increase of p53-target gene expression and were used as a positive control, MEFs expressing TAg failed to activate a p53 response. For both panel B and panel C, real-time PCR was carried out using gene-specific primers and Rpl5 as the cDNA normalization control. Expression of each gene is shown in comparison to its baseline expression in DKO MEFs. Error bars represent standard deviations. TKO;TAg1 and TKO;TAg2 are two clones obtained by cre-recombinase.
Binding of p53 by TAg results in p53 inactivation and stabilization; thus, TAg-transformed cells exhibit high levels of p53 (11). Accordingly, we observed an increase in p53 levels in all (wt and DKO) control MEFs expressing TAg (Fig. 5A); furthermore, the presence or absence of activator E2Fs did not impinge on the ability of TAg to increase protein levels of p53 (Fig. 5A). While DKO;TAg and TKO;TAg clones showed different levels of TAg expression (Fig. 2B), all cells contained similar levels of p53 (Fig. 5A), indicating effective stabilization of p53 by TAg in all cases. We next confirmed that, irrespective of the genetic background, p53 was inactive in the presence of TAg. Upon expression of TAg and in response to disruption of the RB pathway, cells normally respond by triggering apoptosis, but TAg prevents this by binding to, stabilizing, and inactivating p53. Thus, TAg-expressing cells fail to show increased levels of p53 target genes. We used doxorubicin (Dox), a DNA-intercalating agent which induces an apoptotic response and a subsequent increase in p53-target gene expression, as a positive control. In contrast to the robust response observed in control cells treated with doxorubicin, p53 failed to induce expression of selected p53 target genes in all TAg-expressing samples (Fig. 5C). These results indicate that TAg fully retains the capacity to inhibit p53 in the absence of activator E2Fs.
The LXCXE motif and p53 binding region in TAg are both required for proliferation and transformation in TKO MEFs.
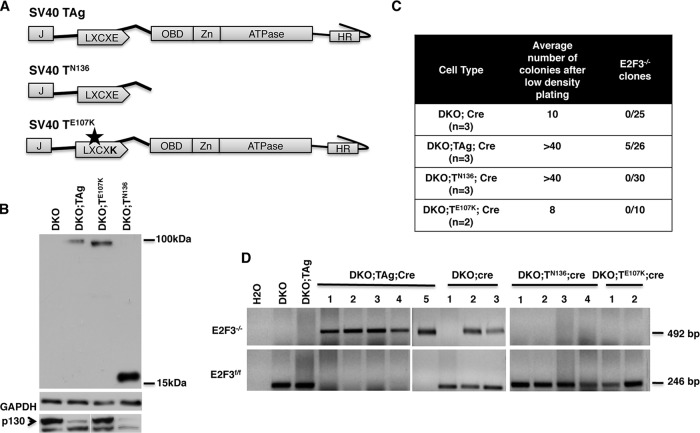
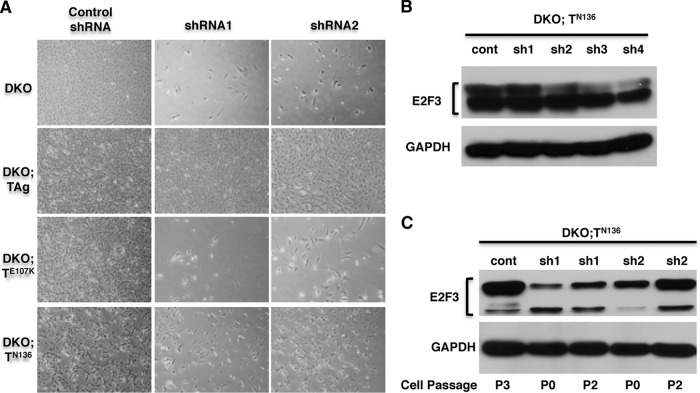
To determine which TAg activities are required to sustain proliferation and survival in TKO MEFs, we expressed TAg mutants unable to interact with RB proteins (TE107K) or p53 (TN136) in DKO MEFs (Fig. 6A). Western blot analysis showed that both mutant proteins were robustly expressed (Fig. 6B). As expected, TN136, but not TE107K, was able to induce p130 degradation in the DKO background (Fig. 6B). To test if the mutant TAg proteins could rescue the proliferation defect observed in TKO MEFs, we used cre-recombinase or RNAi to deplete E2F3. First, DKO cells expressing TAg, TN136, or TE107K were treated with cre-recombinase and plated at low density to obtain single-cell clones. While the cre-treated DKO;TAg and DKO;TN136 strains gave several colonies upon plating at low density, the DKO and DKO;TE107K strains ceased to proliferate and very few colonies were obtained (Fig. 6C). Moreover, further inspection of the colonies arising from TN136- or TE107K-expressing cells revealed that E2F3 had not been successfully removed (Fig. 6C and D). We also depleted E2F3 from DKO MEFs expressing TN136 or TE107K using specific shRNAs. Again, we found that neither TN136-expressing cells nor TE107K-expressing cells were able to grow upon removal of E2F3 (Fig. 7A). In fact, the few TN136 colonies that eventually emerged from these experiments presented evident signs of cell death (Fig. 7A) and did not show significant reduction in the expression of E2F3 (Fig. 7B). This outcome could be explained either if TN136 fails to induce proliferation in E2F3−/− cells and only those cells escaping shRNA-mediated E2F3 depletion survive and grow or if TN136 inhibits RNAi activity. To test this possibility, we transiently expressed E2F3-specific shRNAs in DKO;TN136 MEFs and monitored the depletion of E2F3 by Western blot analyses. As shown in Fig. 7C, depletion of E2F3 was initially successful; however, the cells which were able to grow at later passages presented increased levels of E2F3 protein. Similar experiments monitoring the expression of E2F3 in shRNA-treated DKO;TAg MEFs showed a consistent depletion of E2F3, even at late passages (data not shown). These results suggest that RNAi activity is not inhibited by TAg. We therefore conclude that neither TE107K mutants nor TN136 mutants are able to rescue the proliferation defect observed in TKO MEFs. The most likely hypothesis explaining these results is that TAg actions on both RB proteins and p53 are necessary to allow TKO MEFs to grow and survive.
FIG 6.
TAg requires an intact LXCXE motif and a C-terminal activity to rescue the proliferation defect observed in TKO MEFs. (A) Diagram of the TAg mutants used in this study: wild-type TAg (TAg), an amino-terminal truncation mutant containing the LXCXE RB binding motif (TN136), and a full-length point mutant defective for RB binding (TE107K, indicated by the star). (B) The levels of TAg and p130 were evaluated by Western blot analysis in DKO MEFs expressing different versions of the oncogene. (C) Summary of the numbers of clones obtained from cre-recombinase-treated cells after plating at low density. After treatment with cre-recombinase containing retroviral transduction and drug selection, cells were plated at low density and clonal lines from single cells were obtained. The experiment was performed in duplicate for each cell type and was repeated three times, except in the case of DKO;TE107K, for which the experiment was repeated only twice. The data in the second column indicate the average numbers of colonies per plate observed upon low-density plating. The data in the third column indicate the numbers of clones for which E2F removal was successful versus the number of clones tested. (D) PCR analysis to determine the presence or absence of E2F3 in clones of cre-treated DKO MEFs expressing TAg, TN136, or TE107K, as described in the Fig. 2 legend and Materials and Methods.
FIG 7.
Proliferation of TKO MEFs expressing TN136 is due to the reemergence of E2F3 expression. (A) Comparison of morphologies and densities of DKO, DKO;TAg, DKO;TN136, and DKO;TE107K cells treated with either E2F3-specific or control shRNAs. All the images were taken at the same time and at the same magnification. (B) Western blot analysis shows considerable amounts of E2F3 in DKO;TN136 MEFs treated with specific shRNAs. (C) E2F3 expression is initially reduced after shRNA treatment but increases upon cell passage as revealed by Western blot analysis. Two E2F3-specific shRNAs (sh1 and sh2) were used to treat DKO;TN136 MEFs. Cells were collected after transient treatment (passage 0 [P0]) and after subsequent passages (passage 2 [P2]).
DISCUSSION
The RB/E2F pathway is a central regulator of cell proliferation, and more than 30% of human cancers bear mutations or deletions in the RB1 gene (1). In addition, DNA tumor viruses also target RB proteins. Activator E2Fs drive proliferation by increasing the transcription of genes required for G1-S phase progression (1), and many pRb mutations result in deregulated activity of activator E2Fs. Consistently, ectopic expression of activator E2Fs increases expression of cell cycle genes and drives cells into S phase (1). Furthermore, depletion of all three activator E2Fs in mice results in embryonic lethality and in growth arrest in MEFs (7). Thus, the current paradigm for spontaneous and viral oncogene-induced tumorigenesis suggests an essential role for the activator E2Fs in tumors containing pRb inactivation. However, many human cancers also bear pRb mutations that do not affect its association with E2Fs, suggesting that some Rb activity other than binding activator E2Fs also contributes to tumorigenesis and that tumorigenic proliferation can occur by mechanisms independent of E2F activators.
Several lines of evidence support this hypothesis. First, reports show that stem and progenitor cells proliferate in vivo and in vitro in the absence of activator E2Fs (8–10). Second, the presence of Rb-dependent but not E2F-dependent pathways contributing to control proliferation in different murine tissues was recently shown through the use of specific Rb mutants (22). Furthermore, we have found that two parallel RB-dependent pathways, one driven by E2F activators and one driven by c-Myc, control normal and tumor-like intestinal proliferation (H. Liu et al., submitted for publication).
In agreement with those results, this report shows that the induction of proliferation and transformation by SV40 TAg in MEFs can take place in the absence of activator E2Fs. However, two previous reports using transgenic models showed that TAg requires at least one activator E2F to induce hyperplasia in intestine or choroid plexus (23, 24). These contrasting results could be due to the use of different model systems, cell culture conditions, cell cycle statuses, and/or variations between in vivo and in vitro systems. For instance, previous studies in transgenic mice expressed TAg in growth-arrested, terminally differentiated cells. In contrast, we have expressed TAg in continuously proliferating, cultured primary cells. Perhaps activator E2Fs are required for the reentry into the cell cycle but are dispensable for continuous cycling cells. Overall, our results suggest that both activator E2F-dependent and -independent pathways can lead to cell proliferation and transformation, perhaps depending on the cell type and environmental context.
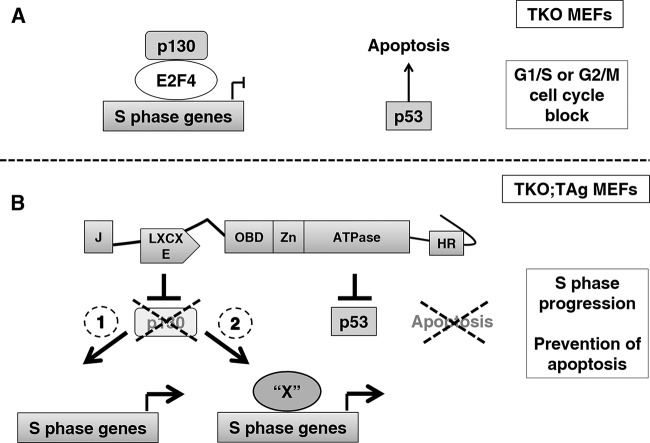
Previous studies in MEFs lacking all three activator E2Fs indicated that growth arrest was due to the transcriptional repression of cell cycle genes by p130/E2F4 complexes and to the activation of the p53 response (16, 20). These results are consistent with our mutant analysis, as TAg proteins unable to disrupt pRB or p53 failed to induce proliferation in the TKO background. We thus propose a model (Fig. 8) to explain TAg-sustained proliferation in the absence of E2F1-2-3. In the absence of E2F1-2-3, p130/E2F4 anchoring the dimerization partner (DP), retinoblastoma (RB)-like, E2F and MuvB (DREAM) complex occupies and represses the promoters of genes required for cell cycle progression, and signaling through p53 results in apoptosis (Fig. 8A). TAg is able both to disrupt p130/E2F4 complexes and to inhibit p53, hence relieving transcriptional repression and preventing apoptosis and thus allowing cell proliferation (Fig. 8B). This model is consistent with previous results showing that deletion of p53 rescues the proliferation defect of TKO MEFs (16, 20). However, it remains to be determined whether the expression of E2F target genes upon TAg expression occurs solely due to the absence of repression or if an unknown activator replaces E2F1-2-3 (Fig. 8B).
FIG 8.
Control of cell proliferation by TAg in the absence of activator E2Fs. (A) Depletion of all three activator E2Fs has a double effect. First, it leads to p53-mediated cell cycle arrest or apoptosis; second, the promoters of the genes driving cell proliferation are occupied by the p130/E2F4 repressive complexes. The overall result is a block in the cell cycle. (B) TAg binds p53 and prevents expression of p53 target genes, thus impeding apoptosis. Simultaneously, TAg disrupts p130/E2F repressor complexes, removing them from the promoters of cell cycle genes. The expression of E2F target genes could then occur solely due to the absence of repression (1) or to the recruitment of an unknown activator “X” to replace E2F1-2-3 (2).
The ability of TAg to upregulate E2F target genes and to induce proliferation and transformation in E2F1-2-3-depleted MEFs contradicts the current model of TAg dependency on activator E2Fs. These results also question the central role of E2F activators as essential drivers of tumorigenesis and suggest that alternative pathways and/or mechanisms are at play.
ACKNOWLEDGMENTS
This work was supported by NIH grant RO1 CA098956 to J.M.P.
We thank Gustavo Leone (Ohio State University) for providing cell lines and reagents and William C. Hahn (Dana Farber) for providing the pBABE-puro-HRasV12 plasmid.
We thank Paul Cantalupo in our laboratory, who kindly did the cloning of plasmids containing the early region of SV40 and/or the TN136 mutant into pL6.3 TOPO vectors.
REFERENCES
- 1.Chen HZ, Tsai SY, Leone G. 2009. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer 9:785–797. doi: 10.1038/nrc2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeCaprio JA, Ludlow JW, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang CM, Livingston DM. 1989. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell 58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- 3.Dyson N, Howley PM, Munger K, Harlow E. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 4.Egan C, Bayley ST, Branton PE. 1989. Binding of the Rb1 protein to E1A products is required for adenovirus transformation. Oncogene 4:383–388. [PubMed] [Google Scholar]
- 5.Tsai SY, Opavsky R, Sharma N, Wu L, Naidu S, Nolan E, Feria-Arias E, Timmers C, Opavska J, de Bruin A, Chong JL, Trikha P, Fernandez SA, Stromberg P, Rosol TJ, Leone G. 2008. Mouse development with a single E2F activator. Nature 454:1137–1141. doi: 10.1038/nature07066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeGregori J, Johnson DG. 2006. Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med 6:739–748. doi: 10.2174/1566524010606070739#sthash.AY7xtHD1.dpuf. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, Greenberg ME, Orkin S, Nevins JR, Robinson ML, Leone G. 2001. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Pacal M, Wenzel P, Knoepfler PS, Leone G, Bremner R. 2009. Division and apoptosis of E2f-deficient retinal progenitors. Nature 462:925–929. doi: 10.1038/nature08544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, Hagan JP, Gomez YM, Sharma N, Chen HZ, Ouseph M, Wang SH, Trikha P, Culp B, Mezache L, Winton DJ, Sansom OJ, Chen D, Bremner R, Cantalupo PG, Robinson ML, Pipas JM, Leone G. 2009. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzel PL, Chong JL, Saenz-Robles MT, Ferrey A, Hagan JP, Gomez YM, Rajmohan R, Sharma N, Chen HZ, Pipas JM, Robinson ML, Leone G. 2011. Cell proliferation in the absence of E2F1-3. Dev Biol 351:35–45. doi: 10.1016/j.ydbio.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An P, Saenz Robles MT, Pipas JM. 2012. Large T antigens of polyomaviruses: amazing molecular machines. Annu Rev Microbiol 66:213–236. doi: 10.1146/annurev-micro-092611-150154. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja D, Saenz-Robles MT, Pipas JM. 2005. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 13.Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, Nevins JR. 1992. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A 89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nevins JR. 1992. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 15.Zalvide J, DeCaprio JA. 1995. Role of pRb-related proteins in simian virus 40 large-T-antigen-mediated transformation. Mol Cell Biol 15:5800–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmers C, Sharma N, Opavsky R, Maiti B, Wu L, Wu J, Orringer D, Trikha P, Saavedra HI, Leone G. 2007. E2f1, E2f2, and E2f3 control E2F target expression and cellular proliferation via a p53-dependent negative feedback loop. Mol Cell Biol 27:65–78. doi: 10.1128/MCB.02147-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seneca NT, Saenz Robles MT, Pipas JM. 2014. Removal of a small C-terminal region of JCV and SV40 large T antigens has differential effects on transformation. Virology 468–470:47–56. doi: 10.1016/j.virol.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verona R, Moberg K, Estes S, Starz M, Vernon JP, Lees JA. 1997. E2F activity is regulated by cell cycle-dependent changes in subcellular localization. Mol Cell Biol 17:7268–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacock M, Campisi J. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma N, Timmers C, Trikha P, Saavedra HI, Obery A, Leone G. 2006. Control of the p53-p21CIP1 Axis by E2f1, E2f2, and E2f3 is essential for G1/S progression and cellular transformation. J Biol Chem 281:36124–36131. doi: 10.1074/jbc.M604152200. [DOI] [PubMed] [Google Scholar]
- 21.Stubdal H, Zalvide J, DeCaprio JA. 1996. Simian virus 40 large T antigen alters the phosphorylation state of the RB-related proteins p130 and p107. J Virol 70:2781–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cecchini MJ, Thwaites MJ, Talluri S, Macdonald JI, Passos DT, Chong JL, Cantalupo P, Stafford PM, Saenz-Robles MT, Francis SM, Pipas JM, Leone G, Welch I, Dick FA. 2014. A retinoblastoma allele that is mutated at its common E2F interaction site inhibits cell proliferation in gene-targeted mice. Mol Cell Biol 34:2029–2045. doi: 10.1128/MCB.01589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sáenz-Robles MT, Markovics JA, Chong JL, Opavsky R, Whitehead RH, Leone G, Pipas JM. 2007. Intestinal hyperplasia induced by simian virus 40 large tumor antigen requires E2F2. J Virol 81:13191–13199. doi: 10.1128/JVI.01658-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan H, Yin C, Dyson NJ, Harlow E, Yamasaki L, Van Dyke T. 1998. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol Cell 2:283–292. doi: 10.1016/S1097-2765(00)80273-7. [DOI] [PubMed] [Google Scholar]