Abstract
Background
The association between plasminogen activator inhibitor-1 (PAI-1) 4G/5G polymorphism and recurrent pregnancy loss (RPL) risk is still contradictory. We thus performed a meta-analysis.
Material/Methods
Relevant studies were searched for in PubMed, Web of Science, Embase, and Cochrane Library. An odds ratio (OR) with a 95% confidence interval (CI) was used to assess the association between PAI-1 4G/5G polymorphism and RPL risk.
Results
A total of 22 studies with 4306 cases and 3076 controls were included in this meta-analysis. We found that PAI-1 4G/5G polymorphism was significantly associated with an increased RPL risk (OR=1.89; 95% CI 1.34–2.67; P=0.0003). In the subgroup analysis by race, PAI-1 4G/5G polymorphism was significantly associated with an increased RPL risk in Caucasians (OR=2.23; 95% CI 1.44–3.46; P=0.0003). However, no significant association was observed in Asians (OR=1.47; 95% CI 0.84–2.59; P=0.18).
Conclusions
In conclusion, this meta-analysis suggests that PAI-1 4G/5G polymorphism might be associated with RPL development in Caucasians.
Keywords: Abortion, Habitual; Plasminogen Activator Inhibitor 1; Polymorphism, Genetic
Background
Recurrent pregnancy loss (RPL) is a common clinical problem that may occur during pregnancy. About 15% of clinically recognized pregnancies could suffer from RPL before the 20th week of gestation, with no known cause [1]. The pathogenesis of RPL remains unknown in many patients, and approximately 55% of patients are suspected to have thrombophilic defects [2].
Thrombophilia is a common cause of RPL and is found in 40–50% of cases [3]. Laude et al. found that levels of circulating procoagulant microparticles were higher in women with RPL when compared with controls [4]. Thrombophilia might cause a syncytiotrophoblast invasion of the maternal blood vessels, which could lead to the formation of microthrombosis at the site of implantation and result in RPL and implantation failure [5].
Plasminogen activator inhibitor-1 (PAI-1) is the urinary plasminogen activator and principal inhibitor of tissue. The main function of PAI-1 is converting plasminogen to the proteolytic enzyme plasmin [6]. Sun and colleagues found that PAI-1 4G/5G polymorphism was significant positive explanatory variable for polycystic ovary syndrome (PCOS) patients with spontaneous abortions [7]. In addition, PAI-1 4G/5G polymorphism was associated with increased PAI-1 concentrations and hypofibrinolysis and contributed to early pregnancy loss [7]. Many studies assessed the association between PAI-1 4G/5G polymorphism and RPL risk [8–29]. However, the result was still uncertain. A meta-analysis found that PAI-1 4G/5G polymorphism did not increase the risk of RPL. However, recent studies did not confirm this result [24,25,27]. Therefore, we conducted this meta-analysis to investigate the association between PAI-1 4G/5G polymorphism and RPL risk.
Material and Methods
Publication search
Relevant studies were searched for in PubMed, Web of Science, Embase, and Cochrane Library. The following terms and strategies were used for the search: (“Plasminogen activator inhibitor-1” OR “PAI-1”) AND (“single nucleotide polymorphism” OR “SNP” OR “genetic variation” OR “genetic polymorphism”) AND (“Recurrent pregnancy loss” OR “RPL”). To avoid possible missing of qualified trails, introduction and reference list of eligible trails identified through primary search were screened manually. No language restriction was applied when searching.
Inclusion and exclusion criteria
The following criteria were used to screen eligible studies for this meta-analysis: (1) a case–control study or cohort study that studied the association between PAI-1 4G/5G polymorphism and RPL risk; and (2) sufficient data were available for calculation of allele/genotype frequency. Only studies meeting both these criteria were included for analysis.
Data extraction
Two authors extracted the data independently. These data included: the first author, year, ethnicity, genotype distribution, and sample size. Disagreement in data extraction was resolved by group discussion by referring to original studies with a third reviewer.
Statistical analysis
The odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated to assess the association between PAI-1 4G/5G polymorphism and RPL risk. The recessive genetic model (4G/4G vs. 4G/5G+5G/5G) was chosen, because PAI-1 4G/4G genotype was significantly associated with increased PAI-1 production. The significance of pooled estimates was assessed with Z test. Hardy-Weinberg equilibrium (HWE) of genotype frequency in the control group was assessed by chi-square test. Between-studies heterogeneity was assessed by the chi-square-based Q test and I2. P<0.1 or I2>50% was considered as significant heterogeneity. If no significant heterogeneity was observed, fixed-effects model with Mantel-Haenszel method was used to make estimates. However, if significant heterogeneity observed, the sources of heterogeneity would be further analyzed by Galbraith plots. If there were no significant clinical or methodological differences in trails, the random effects model based on DerSimonian-Laird method was be used. Subgroup analysis was performed based on ethnicity of participants recruited in each study. A cumulative meta-analysis was conducted. Sensitivity analysis by another model, HWE, and sample size were also conducted. Publication bias was tested using Begg’s test and funnel plot (P<0.05 was considered as significant). Statistical analysis was conducted using Stata software 11.0 (StataCorp, College Station, Texas, USA).
Results
Study characteristics
After searching and screening with preset criteria, a total of 22 studies with 4306 cases and 3076 controls were included for this meta-analysis. Four studies were conducted in Asians and 18 in Caucasians. The characteristics and the HWE results of the included studies are listed in Table 1. Four studies were not in HWE.
Table 1.
Characteristics of the studies included in this meta-analysis.
| Year | Ethnicity | Case | Control | Case | Control | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 4G/4G | 4G/5G | 5G/5G | 4G/4G | 4G/5G | 5G/5G | |||||
| Wolf | 2003 | Caucasian | 49 | 102 | 17 | 25 | 7 | 32 | 50 | 20 | Yes |
| Buchholz | 2003 | Caucasian | 184 | 127 | 72 | 75 | 37 | 41 | 58 | 28 | Yes |
| Dossenbach-Glaninger | 2003 | Caucasian | 49 | 48 | 12 | 28 | 9 | 8 | 25 | 16 | Yes |
| Guan | 2005 | Asian | 127 | 117 | 58 | 52 | 17 | 20 | 59 | 38 | Yes |
| Coulam | 2006 | Caucasian | 150 | 20 | 11 | 117 | 22 | 2 | 13 | 5 | Yes |
| Coulam | 2008 | Caucasian | 550 | 41 | 39 | 396 | 115 | 6 | 22 | 13 | Yes |
| Goodman | 2009 | Caucasian | 120 | 84 | 38 | 57 | 25 | 23 | 48 | 13 | Yes |
| Al Sallout | 2010 | Caucasian | 100 | 100 | 16 | 44 | 40 | 16 | 48 | 36 | Yes |
| Ivanov | 2010 | Caucasian | 110 | 97 | 46 | 44 | 20 | 26 | 45 | 26 | Yes |
| Ivanov | 2011 | Caucasian | 52 | 125 | 14 | 23 | 15 | 26 | 45 | 26 | Yes |
| Aarabi | 2011 | Caucasian | 63 | 114 | 19 | 30 | 14 | 25 | 47 | 22 | Yes |
| Jeddi-Tehrani | 2011 | Caucasian | 100 | 100 | 9 | 31 | 60 | 1 | 27 | 72 | Yes |
| Idali | 2012 | Caucasian | 106 | 100 | 17 | 53 | 35 | 1 | 27 | 72 | Yes |
| Torabi | 2012 | Caucasian | 100 | 100 | 9 | 31 | 60 | 1 | 27 | 72 | Yes |
| Ozdemir | 2012 | Caucasian | 543 | 327 | 121 | 331 | 91 | 10 | 62 | 34 | No |
| Parveen | 2013 | Asian | 200 | 300 | 45 | 100 | 55 | 74 | 131 | 95 | No |
| Magdoud | 2013 | Caucasian | 304 | 371 | 37 | 128 | 139 | 10 | 104 | 257 | Yes |
| Subrt | 2013 | Caucasian | 157 | 74 | 59 | 75 | 23 | 10 | 54 | 10 | No |
| Jeon | 2014 | Asian | 308 | 227 | 129 | 132 | 47 | 71 | 117 | 39 | Yes |
| Khosravi | 2014 | Caucasian | 595 | 100 | 122 | 288 | 184 | 1 | 27 | 72 | Yes |
| Kim | 2014 | Asian | 227 | 304 | 73 | 123 | 31 | 102 | 154 | 48 | Yes |
| Lino | 2014 | Caucasian | 112 | 98 | 12 | 57 | 37 | 16 | 40 | 42 | Yes |
HWE – Hardy-Weinberg equilibrium.
Meta-analysis results
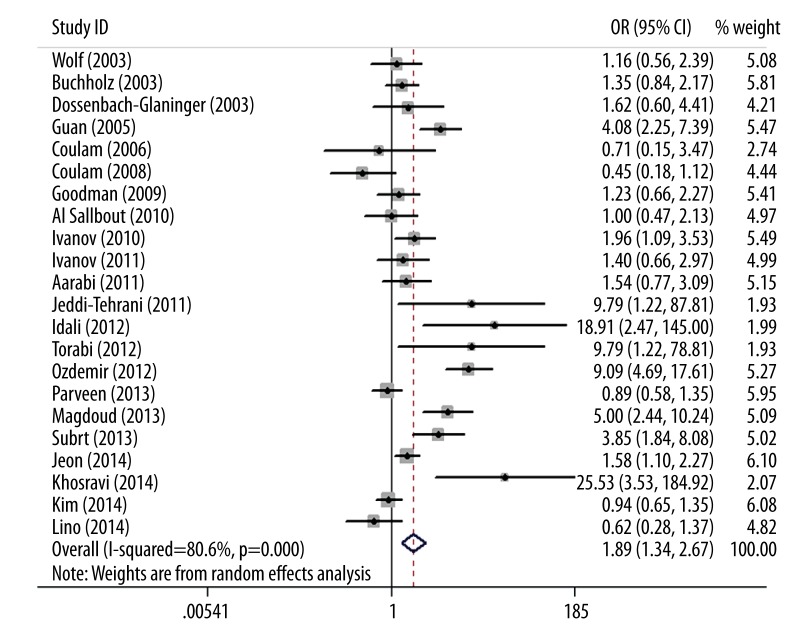
As shown in Figure 1, we found that PAI-1 4G/5G polymorphism was significantly associated with an increased RPL risk (OR=1.89; 95% CI 1.34–2.67; P=0.0003). In the subgroup analysis by race, PAI-1 4G/5G polymorphism was significantly associated with an increased RPL risk in Caucasians (OR=2.23; 95% CI 1.44–3.46; P=0.0003). However, no significant association was observed in Asians (OR=1.47; 95% CI 0.84–2.59; P=0.18). Results of the meta-analysis are listed in Table 2. In the sensitivity analysis, when the fixed-effects model was used, the result was still positive (OR=1.87; 95% CI 1.64–2.13; P<0.00001). When the studies without HWE were excluded, the result was not changed (OR=1.56; 95% CI 1.12–2.19; P=0.009). Furthermore, when the studies with small sample size were excluded, the result was also not changed (OR=1.98; 95% CI 1.25–3.13; P=0.004). The results of sensitivity analysis are listed in Table 3.
Figure 1.
Forest plot of RPL risk associated with PAI-1 4G/5G polymorphism.
Table 2.
Results of meta-analysis.
| Comparison | Subgroup | No. studies | OR (95% CI) | P Value | I2 (%) |
|---|---|---|---|---|---|
| 4G/4G vs. 4G/5G+5G/5G | Overall | 22 | 1.89 (1.34–2.67) | 0.0003 | 81 |
| 4G/4G vs. 4G/5G+5G/5G | Asian | 4 | 1.47 (0.84–2.59) | 0.18 | 86 |
| 4G/4G vs. 4G/5G+5G/5G | Caucasian | 18 | 2.23 (1.44–3.46) | 0.0003 | 79 |
Table 3.
Results of sensitivity analyses.
| Comparison | Subgroup | No. studies | OR (95% CI) | P Value | I2 (%) |
|---|---|---|---|---|---|
| 4G/4G vs. 4G/5G+5G/5G | Fixed-effect model | 22 | 1.84 (1.61–2.09) | <0.00001 | 81 |
| 4G/4G vs. 4G/5G+5G/5G | HWE | 19 | 1.56 (1.12–2.19) | 0.009 | 67 |
| 4G/4G vs. 4G/5G+5G/5G | n<200 | 16 | 1.98 (1.25–3.13) | 0.004 | 84 |
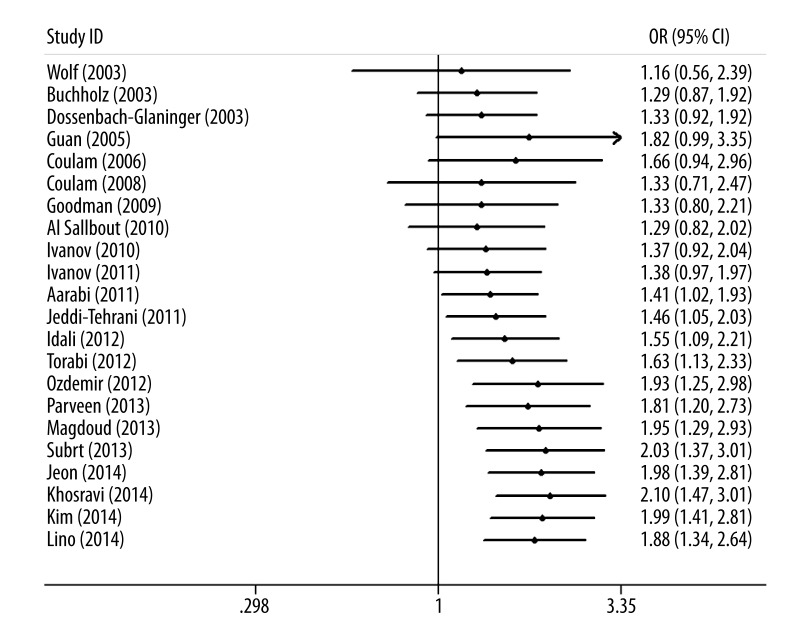
Results from the cumulative meta-analysis suggest that the evidence was consistent over time (Figure 2), indicating that the results of this meta-analysis are robust. Significant heterogeneity (I2=81%) was detected in the recessive genetic model. Thus, we used the Galbraith plot to explore the source of the heterogeneity. As shown in Figure 3, 10 studies were considered as outliers. When these studies were excluded, the heterogeneity was decreased. No obvious heterogeneity among the remaining studies could be found (I2=0%). Furthermore, the result was significant (OR=1.48, 95% CI 1.22–1.79, P<0.0001). No significant publication bias was found by Begg’s funnel plot (Figure 4) and Egger’s test (P=0.313).
Figure 2.
Cumulative meta-analysis of the association between PAI-1 4G/5G polymorphism and RPL risk.
Figure 3.
Galbraith plot of the association between PAI-1 4G/5G polymorphism and RPL risk.
Figure 4.
Funnel plot analysis to detect publication bias for PAI-1 4G/5G polymorphism and RPL risk.
Discussion
Our previous meta-analysis investigated the association between PAI-1 4G/5G polymorphism and RPL risk [30]. However, that meta-analysis did not find a positive association between PAI-1 4G/5G polymorphism and risk of RPL. Several new studies have been published since then. Thus, we did a new meta-analysis. When these new studies were included, we found that PAI-1 4G/5G polymorphism was significantly associated with risk of RPL, indicating that PAI-1 4G/5G polymorphism carriers might have increased RPL risk. In the subgroup analysis based on race, PAI-1 4G/5G polymorphism showed increased RPL risk in Caucasians. However, no positive association was found in Asians. Ethnic differences can occur in genotype frequencies, which might account for the different results. In addition, there were only four studies with Asians. More studies with Asian populations are needed to validate this result. We then did cumulative meta-analysis. We found that the result from our meta-analysis was stable. In the sensitivity analyses, we also found the results were not altered, indicating this meta-analysis was robust.
In human and animal models, PAI-1 was involved not only in fibrinolysis regulation, but also in extracellular proteolytic processes during ovulation, ovarian follicle growth, and embryo implantation [31,32]. Much evidence has suggested that abnormal levels of PAI-1 significantly increase miscarriage and complicated pregnancy rates [33]. Additionally, Glueck et al. suggested that PAI activity was a significant positive explanatory variable for the number of miscarriages [34]. Furthermore, Glueck et al. found that administration of metformin throughout pregnancy in women with PCOS reduced the risk of first-trimester spontaneous abortion [35]. Palomba and coworkers also confirmed that metformin reduced PAI-1 activity in women with PCOS and abortion risk [36]. Therefore, it might be possible that increased PAI-1 associated with high RPL risk and PAI-1 4G/5G polymorphism might influence RPL risk.
There were some limitations in this meta-analysis. First, lack of the original data limited the evaluation of gene-gene and gene-environment interactions. Second, Dawson et al. indicated that PAI-1 level was not only changed by PAI- 1 4G/5G polymorphism, but also by concentration of blood sugar, insulin, and triglycerides [37]. However, we did not adjust these factors in this meta-analysis due to lack of original data. Third, the studies included in our meta-analysis were small. Thus our meta-analysis had little statistical power to assess the association between the PAI-1 4G/5G polymorphism and RPL risk.
Conclusions
The results of this meta-analysis suggest that PAI-1 4G/5G polymorphism might be associated with RPL development in Caucasians. Large-scale studies are necessary to validate this result.
Footnotes
Source of support: Departmental sources
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cohn DM, Goddijn M, Middeldorp S, et al. Recurrent miscarriage and antiphospholipid antibodies: prognosis of subsequent pregnancy. J Thromb Haemost. 2010;8(10):2208–13. doi: 10.1111/j.1538-7836.2010.04015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middeldorp S. Pregnancy failure and heritable thrombophilia. Semin Hematol. 2007;44(2):93–97. doi: 10.1053/j.seminhematol.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Brenner B, Sarig G, Weiner Z, et al. Thrombophilic polymorphisms are common in women with fetal loss without apparent cause. Thromb Haemost. 1999;82(1):6–9. [PubMed] [Google Scholar]
- 4.Laude I, Rongières-Bertrand C, Boyer-Neumann C, et al. Circulating procoagulant microparticles in women with unexplained pregnancy loss: a new insight. Thromb Haemost. 2001;85(1):18–21. [PubMed] [Google Scholar]
- 5.Azem F, Many A, Ben Ami I, et al. Increased rates of thrombophilia in women with repeated IVF failures. Hum Reprod. 2004;19(2):368–70. doi: 10.1093/humrep/deh069. [DOI] [PubMed] [Google Scholar]
- 6.Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005;93(4):631–40. doi: 10.1160/TH05-01-0033. [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Lv H, Wei W, et al. Angiotensin-converting enzyme D/I and plasminogen activator inhibitor-1 4G/5G gene polymorphisms are associated with increased risk of spontaneous abortions in polycystic ovarian syndrome. J Endocrinol Invest. 2010;33(2):77–82. doi: 10.1007/BF03346557. [DOI] [PubMed] [Google Scholar]
- 8.Wolf CE, Haubelt H, Pauer HU, et al. Recurrent pregnancy loss and its relation to FV Leiden, FII G20210A and polymorphisms of plasminogen activator and plasminogen activator inhibitor. Pathophysiol Haemost Thromb. 2003;33:134–37. doi: 10.1159/000077821. [DOI] [PubMed] [Google Scholar]
- 9.Buchholz T, Lohse P, Rogenhofer N, et al. Polymorphisms in the ACE and PAI-1 genes are associated with recurrent spontaneous miscarriages. Hum Reprod. 2003;18:2473–77. doi: 10.1093/humrep/deg474. [DOI] [PubMed] [Google Scholar]
- 10.Dossenbach-Glaninger A, van Trotsenburg M, Dossenbach M, et al. Plasminogen activator inhibitor 1 4G/5G polymorphism and coagulation factor XIII Val34Leu polymorphism: impaired fibrinolysis and early pregnancy loss. Clin Chem. 2003;49:1081–86. doi: 10.1373/49.7.1081. [DOI] [PubMed] [Google Scholar]
- 11.Guan LX, Du XY, Wang JX, et al. Association of genetic polymorphisms in plasminogen activator inhibitor-1 gene and 5,10-methylenetetrahydrofolate reductase gene with recurrent early spontaneous abortion. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22(3):330–33. [PubMed] [Google Scholar]
- 12.Coulam CB, Jeyendran RS, Fishel LA, Roussev R. Multiple thrombophilic gene mutations rather than specific gene mutations are risk factors for recurrent miscarriage. Am J Reprod Immunol. 2006;55(5):360–68. doi: 10.1111/j.1600-0897.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- 13.Coulam CB, Wallis D, Weinstein J, et al. Comparison of thrombophilic gene mutations among patients experiencing recurrent miscarriage and deep vein thrombosis. Am J Reprod Immunol. 2008;60(5):426–31. doi: 10.1111/j.1600-0897.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 14.Goodman C, Hur J, Goodman CS, et al. Are polymorphisms in the ACE and PAI-1 genes associated with recurrent spontaneous miscarriages? Am J Reprod Immunol. 2009;62:365–70. doi: 10.1111/j.1600-0897.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 15.Al Sallout RJ, Sharif FA. Polymorphisms in NOS3, ACE and PAI-1 genes and risk of spontaneous recurrent miscarriage in the Gaza Strip. Med Princ Pract. 2010;19:99–104. doi: 10.1159/000273067. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov P, Komsa-Penkova R, Ivanov I, et al. Plasminogen activator inhibitor type 1 activity in women with unexplained very early recurrent pregnancy loss. Akush Ginekol (Sofiia) 2010;49(5):3–8. [PubMed] [Google Scholar]
- 17.Ivanov P, Komsa-Penkova R, Konova E, et al. Combined thrombophilic factors among women with late recurrent spontaneous abortions. Akush Ginekol (Sofiia) 2011;50(3):8–12. [PubMed] [Google Scholar]
- 18.Aarabi M, Memariani T, Arefi S, et al. Polymorphisms of plasminogen activator inhibitor- 1, angiotensin converting enzyme and coagulation factor XIII genes in patients with recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24:545–48. doi: 10.3109/14767058.2010.511331. [DOI] [PubMed] [Google Scholar]
- 19.Jeddi-Tehrani M, Torabi R, Zarnani AH, et al. Analysis of plasminogen activator inhibitor-1, integrin beta3, beta fibrinogen, and methylenetetrahydrofolate reductase polymorphisms in Iranian women with recurrent pregnancy loss. Am J Reprod Immunol. 2011;66:149–56. doi: 10.1111/j.1600-0897.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 20.Idali F, Zareii S, Mohammad-Zadeh A, et al. Plasminogen activator inhibitor 1 and methylenetetrahydrofolate reductase gene mutations in iranian women with polycystic ovary syndrome. Am J Reprod Immunol. 2012;68(5):400–7. doi: 10.1111/aji.12002. [DOI] [PubMed] [Google Scholar]
- 21.Torabi R, Zarei S, Zeraati H, et al. Combination of thrombophilic gene polymorphisms as a cause of increased the risk of recurrent pregnancy loss. J Reprod Infertil. 2012;13(2):89–94. [PMC free article] [PubMed] [Google Scholar]
- 22.Ozdemir O, Yenicesu GI, Silan F, et al. Recurrent pregnancy loss and its relation to combined parental thrombophilic gene mutations. Genet Test Mol Biomarkers. 2012;16:279–86. doi: 10.1089/gtmb.2011.0191. [DOI] [PubMed] [Google Scholar]
- 23.Parveen F, Tuteja M, Agrawal S. Polymorphisms in MTHFR, MTHFD, and PAI-1 and recurrent miscarriage among North Indian women. Arch Gynecol Obstet. 2013;288(5):1171–77. doi: 10.1007/s00404-013-2877-x. [DOI] [PubMed] [Google Scholar]
- 24.Magdoud K, Herbepin VG, Touraine R, et al. Plasminogen activator inhibitor 1 4G/5G and -844G/A variants in idiopathic recurrent pregnancy loss. Am J Reprod Immunol. 2013;70:246–52. doi: 10.1111/aji.12116. [DOI] [PubMed] [Google Scholar]
- 25.Subrt I, Ulcova-Gallova Z, Cerna M, et al. Recurrent pregnancy loss, plasminogen activator inhibitor-1 (-675) 4G/5G polymorphism and antiphospholipid antibodies in Czech women. Am J Reprod Immunol. 2013;70:54–58. doi: 10.1111/aji.12099. [DOI] [PubMed] [Google Scholar]
- 26.Jeon YJ, Kim YR, Lee BE, et al. Genetic association of five plasminogen activator inhibitor-1 (PAI-1) polymorphisms and idiopathic recurrent pregnancy loss in Korean women. Thromb Haemost. 2013;110:742–50. doi: 10.1160/TH13-03-0242. [DOI] [PubMed] [Google Scholar]
- 27.Khosravi F, Zarei S, Ahmadvand N, et al. Association between plasminogen activator inhibitor 1 gene mutation and different subgroups of recurrent miscarriage and implantation failure. J Assist Reprod Genet. 2014;31:121–24. doi: 10.1007/s10815-013-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JJ, Choi YM, Lee SK, et al. The PAI-1 4G/5G and ACE I/D Polymorphisms and Risk of Recurrent Pregnancy Loss: A Case-Control Study. Am J Reprod Immunol. 2014;72(6):571–76. doi: 10.1111/aji.12302. [DOI] [PubMed] [Google Scholar]
- 29.Lino FL, Traina E, Barreto JA, et al. Thrombophilic Mutations and Polymorphisms, Alone or in Combination, and Recurrent Spontaneous Abortion. Clin Appl Thromb Hemost. 2014 doi: 10.1177/1076029613520465. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Su MT, Lin SH, Chen YC, Kuo PL. Genetic association studies of ACE and PAI-1 genes in women with recurrent pregnancy loss: a systematic review and meta-analysis. Thromb Haemost. 2013;109(1):8–15. doi: 10.1160/TH12-08-0584. [DOI] [PubMed] [Google Scholar]
- 31.Piquette GN, Simón C, el Danasouri I, et al. Gene regulation of interleukin-1 beta, interleukin-1 receptor type I, and plasminogen activator inhibitor-1 and -2 in human granulosa-luteal cells. Fertil Steril. 1994;62(4):760–70. doi: 10.1016/s0015-0282(16)57001-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Long J, Wang X, Sun Y. Association of the plasminogen activator inhibitor-1 (PAI-1) Gene -675 4G/5G and -844 A/G promoter polymorphism with risk of keloid in a Chinese Han population. Med Sci Monit. 2014;20:2069–73. doi: 10.12659/MSM.892397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kujovich JL. Thrombophilia and pregnancy complications. Am J Obstet Gynecol. 2004;191(2):412–24. doi: 10.1016/j.ajog.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Glueck CJ, Wang P, Fontaine RN, et al. Plasminogen activator inhibitor activity: an independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metabolism. 1999;48(12):1589–95. doi: 10.1016/s0026-0495(99)90250-0. [DOI] [PubMed] [Google Scholar]
- 35.Glueck CJ, Phillips H, Cameron D, et al. Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reduce first-trimester spontaneous abortion: a pilot study. Fertil Steril. 2001;75(1):46–52. doi: 10.1016/s0015-0282(00)01666-6. [DOI] [PubMed] [Google Scholar]
- 36.Palomba S, Orio F, Jr, Falbo A, et al. Plasminogen activator inhibitor 1 and miscarriage after metformin treatment and laparoscopic ovarian drilling in patients with polycystic ovary syndrome. Fertil Steril. 2005;84(3):761–65. doi: 10.1016/j.fertnstert.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Dawson S, Hamsten A, Wiman B, et al. Genetic variation at the plasminogen activator inhibitor-1 locus is associated with altered levels of plasma plasminogen activator inhibitor-1 activity. Arterioscler Thromb. 1991;11(1):183–90. doi: 10.1161/01.atv.11.1.183. [DOI] [PubMed] [Google Scholar]