Abstract
The levels of ribulose 1,5-bisphosphate (RuBP), 3-phosphoglyceric acid (PGA), glycolate, glycine, and serine were measured in soybean leaflets during photosynthesis in atmospheres ranging from 1 to 60% O2 and from 0 to 500 microliters per liter CO2.
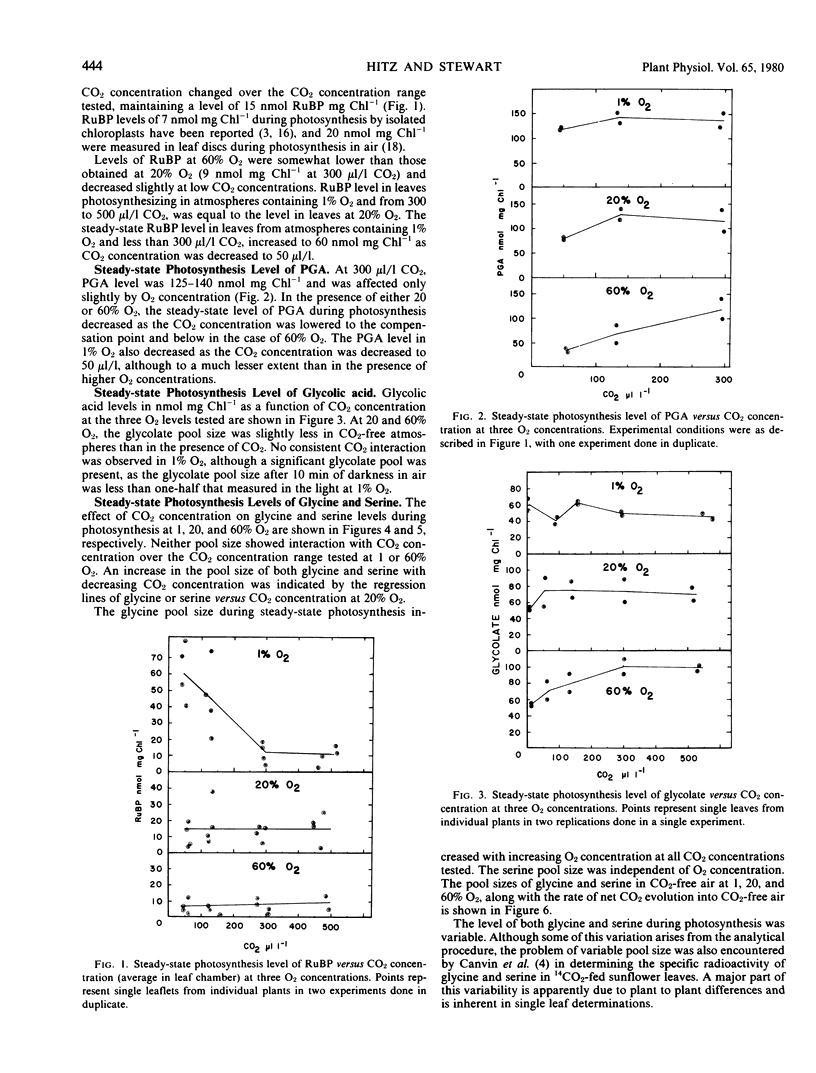
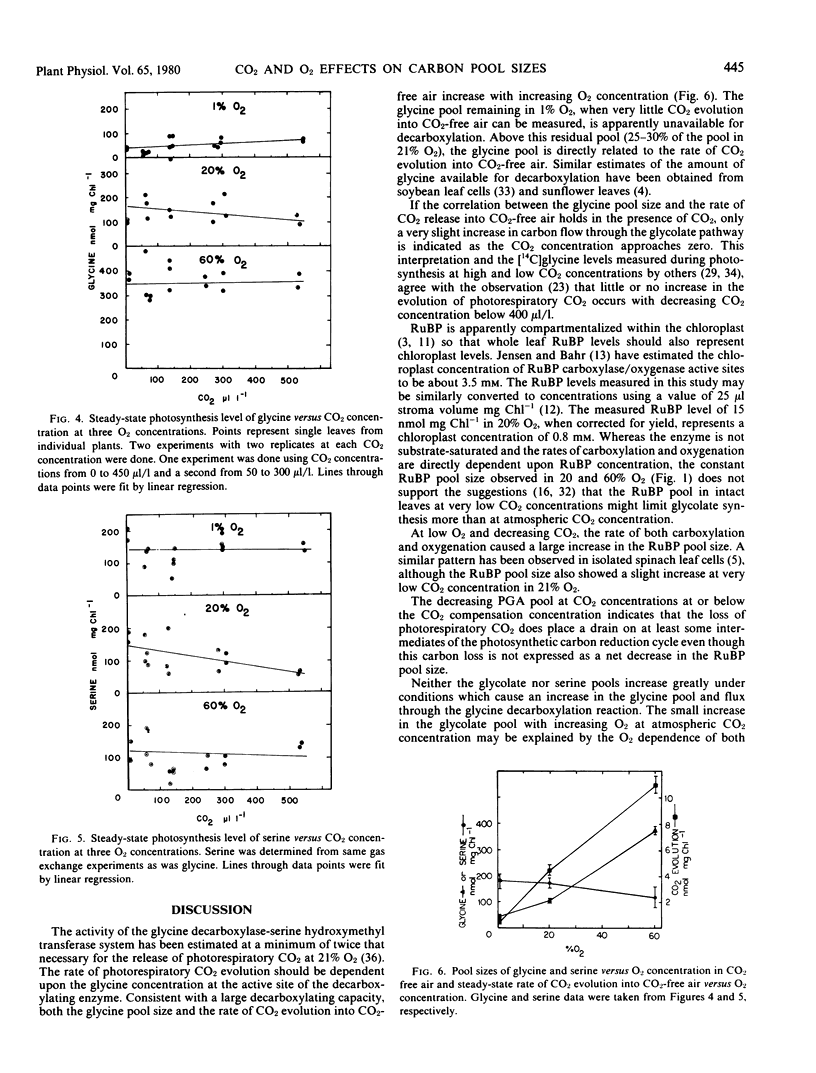
The RuBP level remained constant as CO2 concentration was decreased in atmospheres containing 20 or 60% O2, but increased as CO2 concentration was decreased in atmospheres containing 1% O2. PGA levels decreased at CO2 concentrations near or below the CO2 compensation point under all O2 concentrations. The glycolate pool at 300 microliters per liter CO2 increased slightly with increasing O2 concentration, but remained nearly constant at very low CO2. The serine pool showed no measurable change over the range of CO2 or O2 concentrations tested. The glycine pool did not change significantly with varying CO2 concentration but increased linearly with increasing O2 concentration.
Measured RuBP levels indicate an RuBP concentration less than the estimated concentration of RuBP carboxylase/oxygenase active sites. The constant RuBP pool size in 20% O2, however, indicates that RuBP level does not limit photosynthesis or photorespiration any more at 50 microliters per liter CO2 than at 450 microliters per liter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASSHAM J. A., KIRK M. The effect of oxygen on the reduction of CO2 to glycolic acid and other products during photosynthesis by Chlorella. Biochem Biophys Res Commun. 1962 Nov 27;9:376–380. doi: 10.1016/0006-291x(62)90019-0. [DOI] [PubMed] [Google Scholar]
- Bassham J. A., Kirk M., Jensen R. G. Photosynthesis by isolated chloroplasts. I. Diffusion of labeled photosynthetic intermediates between isolated chloroplasts and suspending medium. Biochim Biophys Acta. 1968 Jan 15;153(1):211–218. doi: 10.1016/0005-2728(68)90162-x. [DOI] [PubMed] [Google Scholar]
- CONNELL G. E., DIXON G. H., HANES C. S. Quantitative chromatographic methods for the study of enzymic transpeptidation reactions. Can J Biochem Physiol. 1955 May;33(3):416–427. [PubMed] [Google Scholar]
- Ellyard P. W., Gibbs M. Inhibition of photosynthesis by oxygen in isolated spinach chloroplasts. Plant Physiol. 1969 Aug;44(8):1115–1121. doi: 10.1104/pp.44.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Koch B., Klucas R. Preparation of nitrogenase from nodules and separation into components. Methods Enzymol. 1972;24:470–476. doi: 10.1016/0076-6879(72)24092-7. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Laber L. J., Latzko E., Gibbs M. Photosynthetic path of carbon dioxide in spinach and corn leaves. J Biol Chem. 1974 Jun 10;249(11):3436–3441. [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig L. J., Canvin D. T. The Rate of Photorespiration during Photosynthesis and the Relationship of the Substrate of Light Respiration to the Products of Photosynthesis in Sunflower Leaves. Plant Physiol. 1971 Dec;48(6):712–719. doi: 10.1104/pp.48.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Ogren W. L., Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971 Mar 31;230(13):159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- Paul J. S., Bassham J. A. Effects of Sulfite on Metabolism in Isolated Mesophyll Cells from Papaver somniferum. Plant Physiol. 1978 Aug;62(2):210–214. doi: 10.1104/pp.62.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt S. G., Plaut Z., Bassham J. A. Steady-state photosynthesis in alfalfa leaflets: effects of carbon dioxide concentration. Plant Physiol. 1977 Aug;60(2):230–234. doi: 10.1104/pp.60.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M., Cotler D. N. Influence of pH upon the Warburg Effect in Isolated Intact Spinach Chloroplasts: I. Carbon Dioxide Photoassimilation and Glycolate Synthesis. Plant Physiol. 1977 Apr;59(4):530–534. doi: 10.1104/pp.59.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Chemical inhibition of the glycolate pathway in soybean leaf cells. Plant Physiol. 1977 Oct;60(4):461–466. doi: 10.1104/pp.60.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder F. W. Effect of CO(2) Concentration on Glycine and Serine Formation during Photorespiration. Plant Physiol. 1974 Mar;53(3):514–515. doi: 10.1104/pp.53.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]


