Abstract
Background:
Leishmaniasis is a parasitic infection that may lead to a variety of manifestations. In Iran, cutaneous leishmaniasis (CL) has a high prevalence. There are many treatment modalities for CL. The use of oral terbinafine in the treatment of CL has recently been considered. The aim of this study was to compare combination of oral terbinafine plus cryotherapy versus systemic meglumine antimoniate plus cryotherapy in CL.
Methods:
Patients with proven direct smear for CL were divided randomly in 2 groups of 40 cases. For the first group systemic glucantime prescribed (IM, 15 mg/kg/day) for 3 weeks. For the second group oral terbinafine as two folds of usual dose in the treatment of fungal diseases prescribed [125 mg/day for body weight (BW) <20 kg, 250 mg/day for BW 20–40 kg, 500 mg/day for BW>40 kg] for 4 weeks. Both groups received cryotherapy every 2 weeks for 4 weeks. The patients were followed monthly for 3 months after the treatment.
Results:
Partial (HR= 0.55, CI 95%= 0.3–1.1) and complete (HR= 0.53, CI 95%= 0.3–0.98) clinical improvement in terbinafine group was much slower than glucantime group, although at the end of treatment protocols no significant difference between groups were statistically observed (P=0.27).
Conclusion:
Considering more convenient suitable route of administration and approximately comparable results, it seems that terbinafine can be used as an alternative treatment, especially in the case of allergy or resistance to systemic glucantime.
Keywords: Leishmaniasis, Oral terbinafine, Systemic glucantime, Cryotherapy, Iran
Introduction
One of the most important public health problems is leishmaniasis, which is due to a range of diseases from self-limited cutaneous lesions to the fatal visceral one. Leishmaniasis is a vector born disease that is transmitted by sand fly. A parasite named Leishmanina causes the disease that has many types, which are endemic for special regions like L.amzonesis, L.donovani, and L.mexicana in the Latin America. L.tropica the cause of anthroponotic cutaneous leishmaniasis, ACL is particularly prevalent from (CL) patients in Kerman province, southeastern Iran (1–3).
Systemic pentavalent antimoniates have been used as the first line therapy of leishmaniasis for more than eighty years. Although, the drugs are effective (about 30%) (4), but their adverse effects such as fatal cardiotoxicity, myalgia, pancreatitis, and elevated serum amylase, lipase and liver enzyme levels etc. limit their usage. Besides, their painful injections are another limitation of the drug, especially in children. Hence, presenting new methods to cure this neglected disease is considered in many investigations.
One of the new modified treatments for CL is miltefosine.). It is an anti-neoplastic agent and a membrane active phospholipid derivative (5,6).The phosphocholine analogue demonstrated in vivo and in vitro activity against different species of Leishmania (5).
The discovery of using some antifungal agents as a leishmanicidal has been assessed. In 1990s, several studies focused on the effect of antifungal agents such as clotrimazole, ketoconazole and terbinafine on various types of Leishmania. A suppressive effect on Leishmania promastigotes and amastigotes growth has been appeared in vitro. Moreover, large multivesicular bodies have been found in the parasites by using the allylamine terbinafine and azole, ketoconazole(7).
Terbinafine inhibits the enzyme squalene epoxidase, which converts squalene-to-squalene epoxide, and this reduction in squalene epoxide leads to a decrease in ergosterol formation (8). Ergosterol is an essential component of Leishmania cell wall membranes. Furthermore, excess squalene damages cellular membranes and may cause the release of lytic enzymes from vacuoles. These actions may be responsible for the leishmanicidal effect of terbinafine in vitro (8).
Except of hepatitis, neutropenia, pancytopenia, Stevens-Johnson syndrome, loss of taste, erythema multiforme and toxic epidermal necrolysis, there were not any serious side effects for terbinafine (9).
The aim of this study was to evaluate the efficacy of oral terbinafine plus cryotherapy versus systemic glucantime plus cryotherapy in the treatment of CL due to L. tropica.
Material and Methods
Study population
A randomize clinical trial was performed at Afzalipour Hospital, Kerman University of Medical Sciences, Iran, in 2011–12. The study was registered on the Australian New Zealand Clinical Trials Registry (No. ACTRN12609-000114246).
A sample size of 40 participants per treatment group was planned, a probability of a type I error at alpha =0.05 and beta=0.1 to determine a 20% difference between topical terbinafine compared with control group.
A total of 80 participants with proven direct smear for CL were enrolled in this trial. Skin scraping was taken from the margin of active lesion, smeared on a glass slide, methanol fixed, Giemsa stained and microscopically checked for amastigote stage.
Inclusion criteria were as follows: They should not have received any other leishmanicidal treatment during last three months. The duration of their lesions should be less than six months. The participants between 2–60 years and without serious medical illness were included.
The participants with cardiac, hepatic, renal and other systemic diseases were excluded. Pregnant and nursing women and those who had hypersensitivity to trial drugs were dropped out. All participants were aware of the trial plans and all participants or their parents for those fewer than 18 years of old obtained informed consent.
Demographic data including age, sex, and the number, location, type and the size of lesions, the presence of indurations and ulcer, and the duration of lesions were recorded for each patient.
Review board and Ethics Committee of Kerman University of Medical Sciences approved the study, under number K-90/507.
Randomization
The randomization sequence was obtained by the use of a randomization table. A simple block randomization list with a block size of 4 was accumulated by a team member who was not involved in the enlistment and follow-up of the patients. The randomization allocation concealment was carried out by sending the randomization numbers in envelopes to a dermatologist who was responsible for giving the assigned treatment after each patient was enrolled. This study was an assessor blind trial in which the outcome assessor was unaware of the drugs used by the patients.
Interventions and follow-up
Group A was treated with 125 mg/kg Ibd (for less than 20 kg body weight), 250 mg/kg Ibd (20–40 kg body weight), 500 mg/kg Ibd (for more than 40 kg body weight) oral terbinafine (lamisil® manufactured in Novartis in Switzerland country with IRC number: 122803330) for 4 weeks and accompanied with cryotherapy for every 2 weeks.
The other group (B) was treated with 15 mg/kg/day IM systemic Meglumine antimoniate (glucantime® manufactured in Haupt Pharma in France country with IRC number: 1228027161) for 3 weeks and using cryotherapy the same as group A.
Cryotherapy involved the application of liquid nitrogen via a cotton swab for 10–25 seconds until the lesion and 1–2 mm of surrounding normal tissue appeared frozen in both groups.
Different modalities of treatment were not given to the same patient in different lesions (i.e. each patient received oral terbinafine plus cryotherapy, or combined cryotherapy and systemic glucantime).
Laboratory testing including complete blood count (CBC), urea, creatinine, LFT(alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, bilirubin total) were performed for all participants at base line, in the middle, and at the end of the treatment course.
The improving rate determined by measuring indurations at baseline, in the middle (day 10th for glucantime group and day14th for terbinafine group), and at the end of the study (day 21st for glucantime group and day 28th for terbinafine group). The size of indurations of the lesions was measured in two perpendicular directions based on Sokal method by an independent observer who was not aware of the treatment options. To confirm the complete improvement direct smear was done clinically improved for all lesions. Clinical response was determined based on the following criteria:
Complete improvement (decrease in induration size > 75%),
Partial improvement (decrease in induration size between 25% and 75%),
No improvement (decrease in induration size < 25%).
A masked dermatologist reported the overall treatment responses.
All participants were followed for 3 months after completing the project, they were examined every month to assess the recurrence of the lesions at the site of the previous lesions or around them and increasing the size of the lesions after primary partial improvement.
We decided to crossover the patients who did not respond to terbinafine to glucantime group. While, they did not respond to glucantime, other common remedies were used for them.
Statistical analysis
Having compared demographic and baseline data of subjects in two groups, the data file was converted to the long format in a way that each record showed the data subject in one session. Then, the size of each lesion was computed based on its dimensions. In our analysis, no response to treatment was defined if the size of a lesion decreased less than 25%, partial treatment if decreased between 25% and 75%, and complete treatment if decreased 75% (complete re-epithelialization), compared to its baseline area. Survival curves were calculated using the Kaplan–Meier method, and hazard ratios were estimated using the Cox proportional hazards model.
In order to compare subjects’ response to treatments in each group, we used the Cox regression model. Two types of models were constructed: 1) the event of interest was complete treatment; 2) the event of interest was partial treatment. Since the shape and location of lesions, before treatment was significantly different between the two groups, in Cox regression models, they were adjusted for, after computing the crude Hazard Ratio (HR). Similarly, the confounding effect of sex, the duration, and the size of lesions before treatment were evaluated by this approach. These analyses were done using Stata (version 11).
Results
Of eighty patients entered to the study, half were in the terbinafine group (18 males and 18 females; the sex of four patients was unknown) and the other half of participants were in the glucantime group (16 males and 18 females; the sex of six patients was unknown). - No major clinical or laboratory parameter adverse effect was observed in the both groups after treatment.
Participants’ mean age was 18.52 ± 17.3 years and the majority were female (n= 36, 51.43%). There was no significant difference between both groups regarding baseline data (Table 1).
Table 1.
Comparing main characteristics of subjects classified by their received treatments
| Variable | Terbinafine (n=40†) | Glucantim (n=40†) | P-value |
|---|---|---|---|
| Mean±SD of age (yr) | 16.3 ± 14.8 | 20.74 ± 19.4 | 0.26 |
| Mean±SD of duration of lesions (month) | 3.97 ± 0.3 | 3.81 ± 0.3 | 0.70 |
| Mean±SD of number of lesions | 1.85 ± 1.31 | 1.93 ± 1.16 | 0.78 |
| Mean±SD of the initial lesion size (mm2) | 4.15 ± 5.97 | 5.21 ± 10.65 | 0.46 |
| Sex n(%) | |||
| Male | 18 (52.9) | 16 (47.1) | 0.061 |
| Female | 18 (50) | 18 (50 ) | |
| Response to treatment * | |||
| No response | 15 | 12 | |
| Partial | 10 | 7 | 0.394 |
| Complete | 15 | 21 |
As total sample size in each group was less than 100, crude numbers without percent are reported.
Patients who had more than one lesion were completely treated if they had one or more lesions that showed symptoms of complete response to treatment; patients were partially treated if they had no symptom of complete treatment but showed symptoms of partial treatment in one or more lesions. Those who did not show evidence of partial or complete treatment at all were considered as cases with no response to treatment
Most participants had one lesion (terbinafine: n=25, 62.5% vs. glucantime: n=19, 47.5%; P=0.420), the most frequent location of the lesions was on patients’ face (terbinafine: n=99, 45.8% vs. glucantime: n=93, 50%; P=0.035). The lesions were most frequently in the shape of nodules (terbinafine: n=123, 55.4% vs. glucantime: n=78, 37.5%; P<0.0001). Figure 1 shows box plot of initial lesion size in glucan-time and terbinafine groups.
Fig. 1:
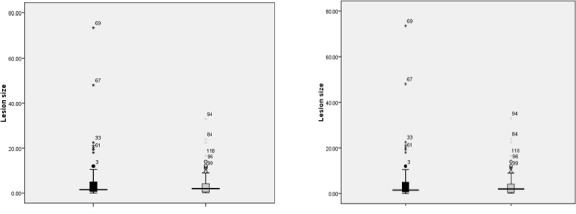
Median of initial lesion size in glucantime and terbinafine groups / * since each patient could have more than one lesion, lesion codes are more than 80 (the total number of subjects)
Having used Cox regression model, we found that the trend of partial treatment in terbinafine group was slower than glucantime group, but this was not statistically significant (HR= 0.55, CI 95%= 0.3–1.1; panel 1, left hand). Adjustment for the duration, location, size, and the number of the lesions did not change the pattern. After adjustment for lesion shape (papule), partial treatment in terbinafine group was significantly much slower than glucantime group (HR= 0.48, CI 95%= 0.24–0.98) (Table 2).
Table 2.
Comparing the crude and adjusted partial and complete response to treatments
| Hazard Ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Crude | Adjusted for the Lesion Shape (Papule) | |||
| Partial treatment | 0.55 (0.3–1.1) | 0.48 (0.24–0.98) | ||
| Sex | Ulcer size | Sex and Ulcer size | ||
| Complete treatment | 0.53 (0.27–0.99) | 0.35 (0.19–0.69) | 0.68 (0.37–1.28) | 0.55 (0.29–1.06) |
The trend of complete treatment in terbinafine group was significantly slower than glucantime group (HR= 0.53, CI 95%= 0.3–0.98; panel 1, right hand). Adjustment for the duration, location, shape, and the number of the lesions did not change the pattern. In terbinafine versus glucantime group, complete treatment was faster in females rather than males; therefore, after adjustment for sex, complete treatment in terbinafine group became significantly much slower than glucantime group (HR= 0.36, CI 95%= 0.19–0.69). In contrast, the larger lesions achieved the complete treatment slower; therefore, adjustment for ulcer size accelerated the speed of complete treatment in terbinafine versus glucantime group up to 15%, but it did not change the direction of the pattern at all. After adjustment for both confounders (sex and ulcer size), the HR did not differ from the crude one but it was not statistically significant anymore (Table 2).
A Kaplan-Meier analysis indicated that the difference in complete and partial treatment between the treatment groups was not significant (Fig. 2).
Fig. 2:
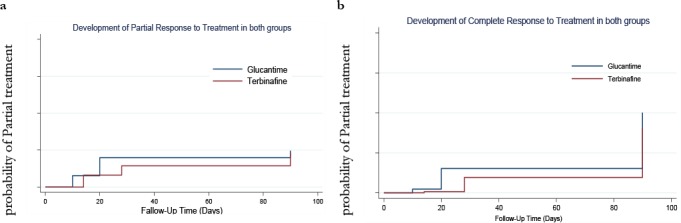
Development of Partial (Fig. a) and Complete (Fig. b) response to treatment in glucantime and terbinafine groups
Discussion
There are many modalities to treat CL including physical, topical, systemic, immuno-therapy etc. with different efficacies (10,11).
Miltefosine is an efficient drug for treatment of CL in Iran. Although feasibility of miltefosine versus glucantime is applicable, it is expensive compared to glucantime (12). The use of antifungal, to improve the CL lesions has been recently considered, as they are safer than the pentavalent antimoniates. The mechanism of action of antifungal drugs is inhibition of synthesis of ergosterol, which is found in large amounts in cell wall of Leishmania. One of the antifungals used in the treatment of CL patients is terbinafine. Terbinafine belongs to the allylamine group of antifungal drugs. The mechanism of action of terbinafine is based on the cell-wall of Leishmania destruction, which is rich in ergosterol(13).
Vanniersantons et al. demonstrated that antifungal, ketoconazole and terbinafine compound could be effective on Leishmania, in vitro (7). In an in vivo experimental study, 60 mice were exposed to L. major. They compared two groups (control and terbinafine groups) and terbinafine group was meaningfully treated (14). Our findings have supported their results.
Based on the literature there has not been any clinical trial conducted in the world about oral terbinafine on CL lesions until 2013. There has been only one pilot study on the efficacy of oral terbinafine to treat CL due to L.tropica in Saudi Arabia. In this study, they randomly enrolled 27 patients for a pilot study using terbinafine with a dose range of 250–500 mg/day for four weeks. They found that complete and partial response were 28.5% and 43%, respectively (15). Comparing to our findings, complete response in this trial is much more than the pilot study and also this is true about partial treatment, but the difference is more comparable in complete response that might be related to the sample size, as this trial had more participants than the pilot one. Another reason might be accompanying the treatment with cryotherapy, which was not performed in the pilot study. In another study, systemic terbinafine has been used in an HIV-positive patient suffering from New World CL (250mg/day for 2 weeks), as well (16).
We performed the first assessor blind-randomize-clinical trial to compare the efficacy of combination therapy of oral terbinafine and cryotherapy versus systemic glucantime and cryotherapy in CL patients. Our findings confirmed that consuming oral terbinafine in the dose of 125–500 mg/day ( depending on patient’s body weight) for four weeks was as effective as systemic glucantime in the dose of 15 mg/Kg for three weeks in matter of healing of the acute Old World dry type CL, caused by L. tropica. Hence, we suggest oral terbinafine as a second-line therapy, in patients who have hypersensitivity or resistance to systemic glucantime. As it is non-invasive treatment and painless it would be appropriate option especially for children.
On the other hand adverse effects of glucantime such as pain(16), burning sensation(17,18) lymphangitis (19), secondary infection(20), nausea, urticaria, necrosis, sporotrichoid lesions (20), vomiting, dizziness, dyspnea and anaphylactic shock(19–21), limits its use especially in children(22). Some studies have showed that terbinafine is a relatively safe drug (15, 23). A post marketing surveil-lance study regarding terbinafine side effects, finally conclude that terbinafine is a safe drug with limited side effects (24).
The main limitation is a lack of placebo arm to address the self-healing observed with time that is mainly due to the ethical limitations.
In addition, we found that oral terbinafine in the treatment of CL is less cost effective than antimoniate drugs. We suggest using oral terbinafine especially in children in endemic areas (25, 26). The other limitation is that we did not confirm the results using methods such as PCR.
Large sample size, randomization, parasitological and biochemical tests were the strengths of this study.
The clinical implications of our study are that using oral terbinafine indicated similar healing but slower than glucantime for lesions due to L. tropica. Oral terbinafine is non-invasive and safe. In contrast to glucantime, terbinafine is costly, slow in action. In our study no adverse effect in both groups were observed but according to the other studies using glucantime caused many adverse effect (topically or systemically)(22).
Investigation in different endemic areas can play a role for assessment the efficacy of terbinafine in other species of Leishmania.
Conclusion
The use of oral terbinafine may have approximately the same efficacy as glucantime. Hence, it seems that in case of hypersensitivity and/or resistance to pentavalent antimoniate, we may be able to use oral terbinafine. On the other hand the administration of pentavalent antimoniate could be associated with several side effects, although they are still the first-line drugs in the antileishmanial armamentarium.
Acknowledgements
We would like to thank the Kerman University of Medical Sciences, which accepted the financial support of this study and all participants and personnel of dermatology ward. The authors declare that there is no conflict of interests.
References
- 1.Sharifi F, Sharifi I, Zarean M, Hakimi Parizi M, Aflatoonian MR, Fasihi Harandi M, Zahmatkesh R, Mashayekhi M, Kermanizadeh AR.Spatial Distribution and Molecular Identification of Leishmania Species from Endemic Foci of South-Eastern Iran. Iran J Parasitol. 2012; 7 (1): 45– 52. [PMC free article] [PubMed] [Google Scholar]
- 2.Sharifi I, Nakhaei N, Aflatoonian MR, Hakimi Parizi M, Fekri AR, Safizadeh H, Shirzadi MR, Gooya MM, Khamesipour A, Nadim A.Cutaneous Leishmaniasis in Bam: A Comparative Evaluation of Pre- and Post-Earthquake Years (1999–2008). Iran J Public Health. 2011; 40 (2): 49– 56. [PMC free article] [PubMed] [Google Scholar]
- 3.Aflatoonian MR, Sharifi I, Hakimi Parizi M, Aflatoonian B, Sharifi M, Khosravi A, Fekri AR, Khamesipour A, Sharifi H.A Prospective Cohort Study of Cutaneous Leishmaniasis Risk and Opium Addiction in South Eastern Iran. PLOS ONE. 2014; 9 (2): e89043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esfandiarpour I, Afsáneh A.Evaluating the efficacy of allopurinol and meglumine antimoniate (glucantime) in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2002; 41: 521– 4. [DOI] [PubMed] [Google Scholar]
- 5.Lira R, Contreras LM, Rta RM, Urbina JA.Mechanism of action of anti-proliferative lysophospholipid analogues against protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. J Antimicrob Chemother. 2001; 47: 37– 46. [DOI] [PubMed] [Google Scholar]
- 6.Zuffery R, Mamoun CB.Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogs. Mol Biochem Parasitol. 2005; 125: 127– 34. [DOI] [PubMed] [Google Scholar]
- 7.Vanniersantons MA.Alternations induced by antifungal compound ketoconazole and terbinafin in leishmania. J Eukaryote Microbial. 1995; 42 (4): 337– 46. [DOI] [PubMed] [Google Scholar]
- 8.Ryder N.Terbinafine: mode of action and properties of the squalene epoxidase inhibition. Br J Dermatol. 2006; 126 (39): 2– 7. [DOI] [PubMed] [Google Scholar]
- 9.Ottevanger V, Petersen CS.Terbinafine. A new allylamine derivative for local and peroral use in fungal infections. Ugeskrift for Laeger. 1991; 153 (48): 3421– 4. [PubMed] [Google Scholar]
- 10.Consigli J, Danielo C, Gallerano V, Papa M, Guidi A, et al. Cutaneous leishmaniasis: successful treatment with itraconazole. Int J Dermatol. 2006; 45 (1): 46– 9. [DOI] [PubMed] [Google Scholar]
- 11.Lee SA, Hasbun R.Therapy of cutaneous leishmaniasis. Int J Infect Dis. 2003; 7 (2): 86– 93. [DOI] [PubMed] [Google Scholar]
- 12.Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhoundi B, Rahnema A, Razaghian AR, Kabir MJ, Nadim A.Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Tropica. 2007; 103: 33– 40. [DOI] [PubMed] [Google Scholar]
- 13.Perez A.Terbinafine: broad new spectrum of indications in several subcutaneous and systemic and parasitic diseases. Mycoses. 1999; 42: 111. [DOI] [PubMed] [Google Scholar]
- 14.Zakai HA, Zimmo SK.Effects of itraconazole and terbinafine on Leishmania major lesions in BALB/c mice. Ann Trop Med Parasitol. 2000; 94 (8): 787– 91. [DOI] [PubMed] [Google Scholar]
- 15.Bahamdan KA, Tallab TM, Johargi H, Nourad MM, PliD, Karam Ibrahim, CES, El Sherbini AH, Karkashan E, Khare AK, Nauri MM.Terbinafine in the treatment of cutaneous leishmaniasis: a pilot study. Int J Dermatol. 2008; 36 (1): 59– 60. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalés-Rupérez J, Morlius M, Carazo A.Remission of localized cutaneous leishmaniasis in a HIV-positive patient using systemic terbinafine. Dermatol; 1997: 194. [DOI] [PubMed] [Google Scholar]
- 17.Alkhawajah AM, Larbi E, al-Gindan Y, Abahussein A, Jain S.Treatment of cutaneous antimony: intramuscular administration leishmaniasis with versus intralesional. Ann Trop Med Parasitol. 1997; 91 (8): 899– 906. [DOI] [PubMed] [Google Scholar]
- 18.Mujtaba G, Khalid M, Weekly VS.fortnightly intralesional meglumine antimoniate in cutaneous leishmaniasis. Int J Dermatol. 2001; 38 (8): 607– 9. [DOI] [PubMed] [Google Scholar]
- 19.Akhyani M, Tajsar S.Comparative study of cryotherapy and intralesional glucantime in the treatment of cutaneous leishmaniasis. J Med Univ. 1997; 34: 29– 34. [Google Scholar]
- 20.Masmoudi A, Maalej N, Boudaya S. Adverse effects of intralesional glucantime in the treatment of cutaneous leishmaniasis. Med Mal Infect. 2006; 36: 226– 228. [DOI] [PubMed] [Google Scholar]
- 21.Beheshti M, Ghotbi S, Amirizade S.Therapeutic and adverse effects of glucantime used for treatment of cutaneous leishmaniasis. Shiraz E Med J. 2007; 8: 155– 161. [Google Scholar]
- 22.Esfandiarpour I, Farajzadeh S, Rahnama Z, Fathabadi EA, Heshmatkhah A.Adverse effects of intralesional meglumine antimoniate and its influence on clinical laboratory parameters in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2012; 51 (10): 1221– 5. [DOI] [PubMed] [Google Scholar]
- 23.Gupta AK, Adamiak A , Cooper E.The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003; 17 (6): 627– 40. [DOI] [PubMed] [Google Scholar]
- 24.Michael H, Monka G, Krupp P, O'Sullivan D.Safety of Oral terbinafine Results of a Postmarketing Surveillance Study in 25 884 Patients. Arch Dermatol. 1997; 133 (10): 1213– 9. [DOI] [PubMed] [Google Scholar]
- 25.Reithinger R, Coleman PG.Treating cutaneous leishmaniasis patients in Kabul, Afghanistan: cost-effectiveness of an operational program in a complex emergency setting. BMC infectious diseases. 2007; 7 (1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vega JC, Sanchez BF, Montero LM, Montaña R, Del Pilar Mahecha M, Dueñes B, Baron AR, Reithinger R.Short communication: The cost-effectiveness of cutaneous leishmaniasis patient management during an epidemic in Chaparral. Colombia in 2004. Trop Med Int Health. 2007; 12 (12): 1540– 4. [DOI] [PubMed] [Google Scholar]


